One-pot Cu(I)-catalyzed Multicomponent Click Reaction Mediated by Secondary Amine-functional Polysiloxane
2015-03-20WANGCaiyunWANGDingZOUJinfengZHANGLijunZHENGZhanjiangYANGKefangXULiwenXUZheng
WANG Caiyun,WANG Ding,ZOU Jinfeng,ZHANG Lijun,ZHENG Zhanjiang,YANG Kefang,XU Liwen,XU Zheng
(1.Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education,Hangzhou Normal University,Hangzhou 311121,China;2.Key Laboratory of Chemical Utilization of Forestry Biomass of Zhejiang Province,Zhejiang A &F University,Lin'an 311300,China)
1 Introduction
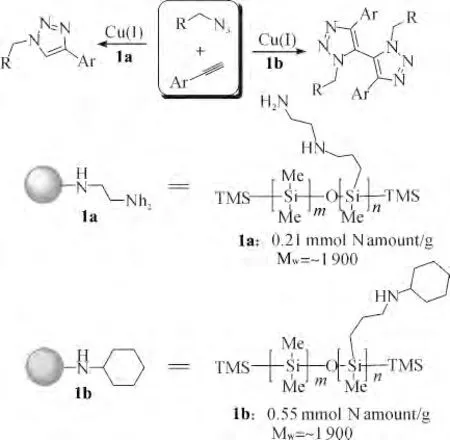
In the past decades,it is well known that the secondary and primary amine-functional polysiloxanes are usually used in textile industry because of extraordinary flexibility and superhydrophobility of the siloxane backbone[1-7].Interestingly and recently,the application of amine-functional polysiloxanes in catalysis has been attracted much attention,and it has been showed that the secondary and primary amine-functional polysiloxanes or polysiloxane-supported amines could be used as highly efficient macromolecular organocatalyst or supported ligand,even as an environmentally friend supporter in organocatalysts[8-11].In this context,we have developed a novel chiral amine-functional polysiloxane in 2008 to improve the stereoselectivity in enamine catalysis by the introduction super-hydrophobic long-chain silicone/polysiloxane as support/functional group for a model aldol reaction,in which the direct aldol reaction of cyclic ketones with different aromatic aldehydes catalyzed by polysiloxane derived primary amines was achieved with high yields and excellent enantioselectivities(up to 99%ee)[10].And recently,we have described that commercially available amino-functional polysiloxane was efficient organocatalyst in multicomponent Gewald reaction,α-allylic alkylation of aldehydes,and Knoevenagel condensation[11].The amine-functional polysiloxanes were also suitable nitrogen-containing ligand for metal-based catalysis.We have ever disclosed that both the secondary and primary amine-functional polysiloxanes were good ligand for copper-catalyzed Huisgen reaction of organic azide and alkynes.And the secondary amine-functional polysiloxanes mediated oxidative coupling of Huisgsen reaction of azides and alkynes efficiently,in which it provided the symmetrically 5,5’-coupled bis-triazole as the major product(Scheme 1)[12].These results supported the important role of the amine-functional polysiloxane in copper catalysis.On the basis of previous results in click chemistry[13-14],we continued to utilize the secondary and primary amine-functional polysiloxanes in copper-catalyzed transformations for the preparation of synthetically useful molecules.Herein,we report our new findings on the aminefunctional polysiloxanes accelerated copper-catalyzed multicomponent click coupling reaction of sodium azide,organic halides,and alkynes,in which the different catalytic activity of secondary amine-functional polysiloxane and primary amine-functional polysiloxane would be revealed in this work.
Scheme 1 The amine-functional polysiloxanes mediated copper-catalyzed Huisgen reaction
The 1,3-dipolar cycloadditions of azides and alkynes,broadly known as Huisgen cycloadditions,have gained much attention since the pioneering work of introducing copper(I)as catalyst by Medal and Sharpless[15-17].The reaction is widely investigated in the past decade owing to its high yielding,insensitive,mild reaction conditions and suitable for biomolecular ligation or even as a polymerization reaction to synthesis of special polymers[18-25].Notably,most of the reported click reactions are two component reactions(organic azide and alkyne)using Cu(II)source with addition of a reducing agent in large excess.On the other hand,to the best of our knowledge,there is few reports about the three component reactions(in situ generation of azides from alkyl halides and sodium azide)of the click reaction[26-35],and the strategies usually need the heteroginization of the Cu(I)catalyst on inorganic materials such as TD@nSiO2and activated carbon or organic supporter such as(acrylamide-imidazole)copolymer and N-heterocyclic carbene at high temperature or under microwave irradiation[26-42].Thus,it is highly desirable to develop the one-pot three component,easy to handle protocol for the click reaction using copper(I)catalyst under mild conditions.To avoid the use of potential hazards of organic azides,we report herein a simple method for the preparation of 1,2,3-triazoles via amine-functional polysiloxanes accelerated copper-catalyzed multicomponent Huisgen reaction.
2 Results and Discussion
In the previous work on the amine-functional polysiloxane accelerated copper-catalyzed Huisgen coupling reaction[12],we have demonstrated an interesting example of divergent catalysis with copper(I)catalyst controlled by amine-functional macromolecular polysiloxanes.The remarkable catalytic ability of the secondary amine-functional polysiloxane(1b)was successfully revealed in the CuCl-mediated Huisgen reactions of organic azides and alkynes,in which the secondary amine-functional polysiloxane led to bistriazoles with good yields while the use of diamine-functional polysiloxane(1a)as ligand led to classic 1,2,3-triazoles via general Huisgen cycloaddition.All these results suggested that the secondary aminefunctional polysiloxane(1b)-changed the mechanism of copper-catalyzed Huisgen coupling reaction.Thus we hypothesized that secondary amine-functional polysiloxane would be useful for the one-pot multicomponent Huisgen coupling reactions.
Initially,we carried out the one-pot three-component Huisgen coupling reaction of sodium azide and benzyl bromide with phenylacetylene in the presence of various copper salts and amine-functional polysiloxane(1b).We expected the one-pot multicomponent cycloaddition could be carried out in DMF to give the desired product in the presence of copper salt and amine-functional polysiloxane.As shown in Tab.1,the secondary amine-functional polysiloxane(1b)was proved to effective ligand in the CuCl-catalyzed multicomponent cycloaddition in DMF(Entry 1).Surprisingly,other copper salts such as Cu(OAc)2,Cu(OTf)2and CuI,gave inferior activity in this reaction(Entries 2~5).And Cu2O was also not suitable catalyst in the one-pot multicomponent cycloaddition.Interestingly,when the DCM was used instead of DMF,good yield was achieved with the same amine-functional polysiloxane and CuCl in this case(Entry 6,81%yield).Notably,poor yield of the desired product was achieved under solvent-free conditions(Entry 8).And the use of primary amine-functional polysiloxane(1a)resulted in almost no reaction(Entry 9).These data thus show that the catalytic activity of Cu(I)is significantly enhanced in the presence of secondary amine-functional polysiloxane(1b).

Tab.1 Catalyst screening in the copper-catalyzed three-component Huisgen coupling reaction in the presence of amine-functional polysiloxane
Intrigued by these results,the reaction was carried out in different solvents.When solvents such as acetonitrile,DCE,MeOH,THF,toluene,and water,the desired product,1,2,3-triazole 4a,was obtained in only moderate yields(30%~67%yield).As shown in Fig.1,the effect of organic solvents evaluated in this work on the copper-catalyzed multicomponent cycloaddition supported the privileged role of DCM in this reaction.Although water was also suitable and green solvent in the amine-functional polysiloxane mediated click reaction,we utilized DCM as better solvent for the next investigation.

Fig.1 The effect of solvents on the amine-functional polysiloxane-accelerated copper-catalyzed Huisgen reaction of sodium azide and benzyl bromide with phenylacetylene
As reported in the previous work[43-49],various organic amines,including imidazole and related ligands,have been found to superior accelerating ligands for the copper(I)-catalyzed Huisgen azide-alkyne cycloaddition.Thus we carried out the experiments with various organic amines to investigate the accelerating effect of small molecular amines in this reaction.As shown in Tab.2,commercially available secondary amines,such as diethylamine and dimethylamine,resulted in moderate yields in this reaction(49%and 64%respectively).And many primary amines,such as ethylenediamine,2-dimethylaminoethylamine,cyclohexylamine,3-ethyladamantan-1-amine,led to poorer yields(10% ~28% yield).Although the tertiary amines,such as ethyldiisopropylamine and tributylamine gave promising activity in the CuCl-catalyzed Huisgen azide-alkyne cycloaddition,the corresponding yields were still good enough in comparison to that of secondary amine-functional polysiloxane(1b).Notably,the heterocycle-based organic base,such as pyrrole or imidazole,the CuCl-catalyzed multicomponent cycloaddition was not satisfied in yield of the desired product 4a.Thus under the same reaction conditions except the use of amine-based ligand,the secondary amine-functional polysiloxane(1b)exhibited superior activity the CuCl-catalyzed multicomponent cycloaddition of sodium azide and benzyl bromide with phenylacetylene.Similarly to previous findings on the amine-functional polysiloxane mediated organic transformations[8-14],we suggested the main chain of polysiloxane would be beneficial to stability of activated Cu(I)catalyst that was important for the catalytic cycle of copper-promoted Huisgen cycloaddition of organic azide and alkyne.

Tab.2 The effect of organic amines on the copper-catalyzed Huisgen coupling reaction
Based on the interesting result,we investigated the generality of this three-component cylcoaddition of sodium azide,organic halides,and alkynes under the optimized reaction conditions.As shown in Tab.3,various benzyl bromides with simple nonfunctionlized alkynes,together with sodium azide were subjected to the present reaction conditions to evaluate the scope and limitation of the multicomponent cycloaddition reaction.The benzyl bromides bearing electron donating groups including methyl,methoxy andtert-butyl groups,reacted with sodium azide cleanly in the one-pot multicompoenent cycloaddition reaction,providing the desired triazoles in good yields(Entries 1~9).The best yield could be up to 99%.However,benzyl bromides containing the electron withdrawing groups like NO2and fluorine,as well as the bromide substituent,resulted in only low to moderate yields of the corresponding products(Entries 10~14).It is possibly due to the poor yield of benzyl azide in the initial step of nucleophilic addition.Interestingly,when the 1,4-bis(bromomethyl)benzene or 1,3-bis(bromomethyl)benzene was used with 2 equiv of phenylacetylene and Na N3,the desired bistriazole was obtained in promising yield(Entries 18 and 20 respectively).The experimental results in Tab.1 clearly indicates that the secondary amine-functional polysiloxane(1b)is a good macromolecular ligand in the copper(I)-catalyzed multicomponent cycloaddition of organic halides,sodium azide,and alkynes.

Tab.3 Secondary amine-functional polysiloxane-accelerated CuCl-catalyzed multicomponent cycloaddition reaction
3 Conclusions
In summary,we have described an interesting finding that the secondary amine-functional polysiloxane effectively accelerated copper(I)-catalyzed multicomponent cycloaddition of sodium azide,organic halide,and alkynes.With the mediation of secondary amine functional polysiloxane,the one-pot threecomponent click reaction could be performed smoothly under mild conditions to give various 1,2,3-triazoles in moderate to good yields.The ease of preparation of various 1,2,3-triazoles together with the simple conditions and work up,suggest this multicomponent click reaction could have wide potential.
4 Experimental
4.1 Materials
All reagents and solvents were commercially available and used as received unless otherwise stated.Secondary Amine-functional Polysiloxane(AFP,1b)was purchased from Hangzhou Bald Silicone Co.,Ltd.(0.55 mmol/g polysiloxane,Mw~1 900).IR:2 962,2 905,1 412,1 260,1 092,1 020,884,800 cm-1;GPC:Mw:1 900;Mw/Mn=1.94.
4.2 Methods and equipments
Flash column chromatography was performed over silica(200~300 mesh).1H-NMR and13C-NMR spectra were recorded at 400 and 100 MHz,respectively on Advance(Brucker)400 MHz Nuclear Magnetic Resonance Spectromer,and were referenced to the internal solvent signals.Thin layer chromatography was performed using silica gel;F254TLC plates and visualized with ultraviolet light.GC/MS spectrometer.Data are reported in the form of(m/z).The product was known and confirmed by GC-MS,and usual spectral methods(1H-NMR,13C-NMR).
4.3 General Procedure for one-pot multicomponent click reaction
A catalytic amount of N—H oil(10 mol%)and CuCl(5 mol%)in DCM (3 m L)were stirred at RT for 20 min,and then NaN3(1.0 mmol)and benzyl bromide(1.0 mmol)added at room temperature,the reaction mixture was stirred for 1 h.Subsequently,the Phenylacetylene(1.0 mmol)was added to above solution at room temperature,and the reaction continued to stir for 2 d.Without working up,the crude product was purified by silica gel column chromatography to furnish the desired product and confirmed by GC-MS,NMR.
4a:1H NMR(400 MHz,CDCl3)δ7.74(d,J=7.9 Hz,2 H),7.62(s,1 H),7.37~7.17(m,7 H),5.47(s,2 H).13C NMR (100 MHz,CDCl3)δ138.20,134.80,130.64,129.15,128.83,128.76,128.17,128.06,125.74,119.66,54.19.GC-MS calculated for C15H13N3,235.11;found 235.1.
4b:1H NMR(400 MHz,CDCl3)δ7.80~7.68(m,2 H),7.51(s,1 H),7.41~7.08(m,7 H),5.53(s,2H),2.27(s,3H).13C NMR(100 MHz,CDCl3)δ137.11,132.62,131.20,130.57,129.54,129.31,128.91,128.30,126.82,125.84,119.48,52.61,19.10.GC-MS calculated for C16H15N3,249.13;found 249.1.
4c:1H NMR(400 MHz,CDCl3)δ7.88~7.74(m,2H),7.67(s,1H),7.45~7.02(m,7H),5.53(s,2H),2.35(s,3H).13C NMR(100 MHz,CDCl3)δ148.30,139.16,134.74,130.73,129.66,129.1,128.91,128.27,125.85,125.27,119.62,54.38,21.44.GC-MS calculated for C16H15N3,249.13;found 249.2.
4d:1H NMR(400 MHz,CDCl3)δ7.80(d,J=7.3 Hz,2H),7.68(s,1 H),7.33(m,4 H),6.94~6.71(m,3 H),5.52(s,2 H),3.78(d,J=0.9 Hz,3 H).13C NMR (100 MHz,CDCl3)δ 160.28,148.31,136.26,130.68,130.32,128.89,128.25,125.81,120.34,119.64,114.35,113.78,55.41,54.26.GC-MS calculated for C16H15N3O,265.12;found 265.0.
4e:1H NMR(400 MHz,CDCl3)δ7.89~7.10(m,10H),5.52(s,2H),2.36(s,3H).13C NMR(100 MHz,CDCl3)δ148.26,138.84,131.78,130.74,129.92,128.89,128.23,125.82,119.51,54.16,21.27.GC-MS calculated for C16H15N3,249.1;found 249.1.
4f:1H NMR(400 MHz,CDCl3)δ7.87~7.77(m,2H),7.68(s,1 H),7.46~7.20(m,7 H),5.55(s,2H),1.34(s,9 H).13C NMR (100 MHz,CDCl3)δ152.07,148.24,131.78,130.73,128.90,128.24,128.02,126.19,125.84,119.61,54.08,34.77,31.38.GC-MS calculated for C19H21N3,291.17;found 291.0.
4g:1H NMR(400 MHz,CDCl3)δ8.21~8.08(m,2H),7.99(s,1H),7.81~7.55(m,5H),7.32~7.20(m,2H),5.86(s,2H),4.17(s,3H).13C NMR(100 MHz,CDCl3)δ160.10,148.23,130.74,129.77,128.81,128.22,126.77,125.81,119.40,114.65,114.07,55.46,53.89.GC-MS calculated for C16H15N3O,265.12;found 265.1.
4h:1H NMR(400 MHz,CDCl3)δ7.74~7.49(m,7 H),7.42~7.03(m,6 H),5.53(s,2 H).13C NMR(100 MHz,CDCl3)δ133.26,132.13,130.62,129.19,128.84,128.19,127.91,127.37,126.76,125.75,125.35,119.75,54.37.GC-MS calculated for C19H15N3,285.13;found 285.1.
4i:1H NMR(400 MHz,CDCl3)δ7.88~7.59(m,3H),7.48~7.14(m,3H),6.44(s,3H),5.47(s,2H),3.75(s,6H).13C NMR (100 MHz,CDCl3)δ161.48,148.31,136.92,130.67,128.90,128.25,125.81,119.64,106.21,100.58,55.53,54.38.GC-MS calculated for C17H17N3O2,295.13;found 295.1.
4j:1H NMR(400 MHz,CDCl3)δ8.20~8.05(m,2 H),7.74(d,J=7.1 Hz,3 H),7.60~7.40(m,2 H),7.40~7.16(m,3H),5.61(s,2H).13C NMR (100 MHz,CDCl3)δ148.69,136.96,133.97,130.35,128.98,128.52,125.85,123.81,122.93,119.85,53.24.GC-MS calculated for C15H12N4O2,280.10;found 280.0.
4k:1H NMR(400 MHz,CDCl3)δ8.15(d,J=8.7 Hz,2H),7.75(m,3H),7.48~7.17(m,5H),5.63 (s,2H).13C NMR (100 MHz,CDCl3)δ148.30,141.99,130.36,129.11,128.72,125.95,124.49,53.36.GC-MS calculated for C15H12N4O2,280.10;found 280.0.
4l:1H NMR(400 MHz,CDCl3)δ7.89~7.60(m,3H),7.47~7.19(m,5H),7.06(m,2H),5.52(s,2H).13C NMR (100 MHz,CDCl3)δ164.25,161.78,130.81,130.43,130.05,128.95,128.38,125.85,119.56,116.37,116.15,53.60.GC-MS calculated for C15H12FN3,253.10;found 253.2.
4m:1H NMR(400 MHz,CDCl3)δ7.93~7.81(m,2 H),7.79(s,1 H),7.58~7.22(m,7 H),5.58(s,2 H).13C NMR (100 MHz,CDCl3)δ148.42,136.97,132.04,131.08,130.80,130.35,128.95,128.44,126.66,125.87,123.21,119.78,53.56.GC-MS calculated for C15H12Br N3,313.02;found 315.1.
4n:1H NMR(400 MHz,CDCl3)δ7.89~7.72(m,2H),7.67(s,1H),7.59~7.06(m,7H),5.52(s,2H).13C NMR (100 MHz,CDCl3)δ133.87,132.47,130.53,129.77,128.97,128.41,125.87,123.09,119.58,53.66.GC-MS calculated for C15H12Br N3,313.02;found 315.1.
4o:1H NMR(400 MHz,CDCl3)δ7.78(m,3H),7.44~7.18(m,5H),7.00~6.86(m,2H),5.57(s,2H),3.87(s,3H).13C NMR(100 MHz,CDCl3)δ130.35,128.80,128.02,125.76,121.06,119.88,110.92,55.60,49.22.GC-MS calculated for C16H15N3O,265.12;found 265.0.
4p:1H NMR(400 MHz,CDCl3)δ7.90~7.71(m,3H),7.48~7.21(m,3H),6.07(m,1H),5.36(t,J=13.6 Hz,2H),5.02(d,J=6.1 Hz,2 H).13C NMR(100 MHz,CDCl3)δ131.47,130.77,128.96,128.28,125.86,120.32,119.51,52.90.GC-MS calculated for C11H11N3,185.10;found 185.1.
4q:1H NMR(400 MHz,CDCl3)δ7.91(dd,J=5.4,4.2 Hz,1H),7.84~7.77(m,2 H),7.66~7.59(m,2 H),7.48~7.32(m,5 H),7.28~7.10(m,3 H),5.91(s,2H).13C NMR(100 MHz,CDCl3)δ148.17,148.17,148.17,134.12,132.74,175.89,130.55,129.35,128.76,128.71,128.51,128.10,127.50,126.58,125.65,123.05,119.58,52.55.GC-MS calculated for C19H15N3,285.13;found 285.0.
4r:1H NMR(400 MHz,CDCl3)δ8.78~8.71(m,3H),8.63(s,2H),8.40~8.12(m,8H),6.51(s,2H),5.40(s,2H).13C NMR(100 MHz,CDCl3)δ148.49,139.08,135.58,130.63,129.81,129.57,128.96,128.63,128.37,128.07,125.89,119.67,54.01,32.71.
4s:1H NMR(400 MHz,CDCl3)δ7.89~7.60(m,3 H),7.47~7.19(m,5 H),7.06(m,2 H),5.52(s,2H).13C NMR (100 MHz,CDCl3)δ164.25,161.78,130.81,130.43,130.05,128.95,128.38,125.85,119.56,116.37,116.15,53.60.GC-MS calculated for C15H12FN3,253.10;found 253.0.
4t:1H NMR(400 MHz,CDCl3)δ9.26(m,3H),8.99~8.61(m,9 H),7.05(s,2 H),5.96(s,2H).13C NMR(100 MHz,CDCl3)δ148.44,138.63,135.06,130.56,129.92,128.95,128.45,125.87,119.67,53.90,32.63.
4u:1H NMR(400 MHz,CDCl3)δ7.87(s,1 H),7.83~7.73(m,2 H),7.42~7.15(m,3 H),5.13(s,2H),4.20(q,J=7.1 Hz,2H),1.23(t,J=7.1 Hz,3H).13C NMR(100 MHz,CDCl3)δ166.34,148.19,130.48,128.86,128.26,125.83,121.15,62.42,50.97,14.07.GC-MS calculated for C12H13N3O2,231.10;found 231.1.
4v:1H NMR(400 MHz,CDCl3)δ7.75(m,3 H),7.57~7.22(m,7 H),5.70(s,2H),2.52(s,3H).13C NMR(100 MHz,CDCl3)δ138.59,134.87,130.55,129.25,129.13,128.70,128.15,126.49,122.92,54.31,21.49.GC-MS calculated for C16H15N3,249.13;found 249.1.
4w:1H NMR(400 MHz,CDCl3)δ7.68~7.56(m,3 H),7.34~7.16(m,7 H),5.47(s,2 H).13C NMR(100 MHz,CDCl3)δ147.16,134.62,133.89,129.08,128.12,126.99,119.72,54.30.GC-MS calculated for C15H12Cl N3,269.07;found 269.1.
4x:1H NMR(400 MHz,CDCl3)δ7.77(d,J=8.0 Hz,2 H),7.43(t,J=6.1 Hz,3 H),7.38~7.23(m,5 H),5.62(s,2H),2.71~2.63(m,2 H),1.74~1.59(m,2H),1.38(m,4H),0.95(t,J=6.9 Hz,3 H).13C NMR (100 MHz,CDCl3)δ143.23,134.93,129.25,128.91,128.16,125.75,54.34,35.83,31.58,31.16,22.65,14.13.GC-MS calculated for C20H23N3,305.19;found 305.1.
4y:1H NMR(400 MHz,CDCl3)δ7.78(d,J=8.0 Hz,2H),7.72(s,1H),7.37(m,5H),7.27(d,J=7.8 Hz,2 H),5.61(s,2 H),2.69~2.61(m,2 H),1.78~1.64(m,2 H),1.00(t,J=7.3 Hz,3 H).13C NMR(100 MHz,CDCl3)δ142.95,134.85,129.19,128.90,128.10,125.71,54.29,37.87,24.51,13.83.GC-MS calculated for C18H19N3,277.16;found 277.1.
4z:1H NMR(400 MHz,CDCl3)δ7.76(d,J=8.1 Hz,2H),7.68(s,1H),7.47~7.23(m,7 H),5.59(s,2 H),2.70(q,J=7.6 Hz,2 H),1.29(t,J=7.6 Hz,3H).13C NMR(100 MHz,CDCl3)δ144.46,134.91,129.21,128.71,128.38,128.11,125.80,119.34,54.26,28.75,15.57.GC-MS calculated for C17H17N3,263.14;found 263.1.
4aa:1H NMR(400 MHz,CDCl3)δ7.79~7.64(m,3H),7.36(m,7 H),5.62(s,2H),2.43(s,3H).13C NMR(100 MHz,CDCl3)δ148.40,138.09,134.88,129.58,129.22,128.83,128.15,127.86,125.73,119.28,54.28,21.35.GC-MS calculated for C16H15N3,249.13;found 249.0.
4bb:1H NMR(400 MHz,CDCl3)δ7.80~7.49(m,3 H),7.44~7.23(m,5 H),6.98~6.81(m,2H),5.53(s,2H),3.81(s,3H).13C NMR(100 MHz,CDCl3)δ159.70,148.16,134.91,129.19,128.78,128.10,127.09,123.41,118.83,114.31,55.38,54.23.GC-MS calculated for C16H15N3O,265.12;found 265.1.
[1]Skinner M W,Qian C B,Grigoras S,etal.Fundamental aspects of aminoalkyl siloxane softeners by molecular modeling and experimental methods[J].Text Res J,1999,69:935-943.
[2]Kang T J,Kim M S.Effects of silicone treatments on the dimensional properties of wool fabric[J].Text Res J,2001,71:295-300.
[3]Ni S C,Kuo P L.Effect of amino and polysiloxane groups on the chemical adsorption of polymers containing thiophosphate at the oil/metal interface under extreme pressure[J].J Polym Sci Ploym Phy,2002,40:1795-1803.
[4]Kaneko Y,Iyi N,Matsumoto T,etal.Synthesis of ion-exchangeable layered polysiloxane by sol-gel reaction of aminoalkyltrialkoxysilane:a new preparation method for layered polysiloxane materials[J].J Mater Chem,2003,13(9):2058-2060.
[5]Colilla M,Darder M,Aranda P,etal.Amino-polysiloxane hybrid materials as carbon composite electrodes for potentiometric detection of anions[J].J Mater Chem,2005,15(35/36):3844-3851.
[6]Colilla M,Salinas A J,Vallet-Regi M.Amino-polysiloxane hybrid materials for bone reconstruction[J].Chem Mater,2006,18(24):5676-5683.
[7]Xu Y J,Yin H,Zheng H F,etal.Application performance and surface morphologies of amino polysiloxanes with different amino values and amino types[J].J Appl Polym Sci,2011,119(4):2326-2333.
[8]DeClue M S,Siegel J S.Polysiloxane-bound ligand accelerated catalysis:a modular approach to heterogeneous and homogeneous macromolecular asymmetric dihydroxylation ligands[J].Org Biomol Chem,2004,2(16):2287-2298.
[9]Grunlan M A,Regan K R,Bergbreiter D E.Liquid/liquid separation of polysiloxane-supported catalysts[J].Chem Commun,2006(16):1715-1717.
[10]Xu L W,Ju Y D,Li L,etal.Homogeneous silicone modified primary amine-Bronsted acid salt catalyzed aldol reaction:unexpected synergistic effect of polysiloxane with remarkable improvement of efficiency and stereoselectivity[J].Tetrahedron Lett,2008,49(49):7037-7041.
[11]Zheng Z J,Liu L X,Gao G,etal.Amine-functional polysiloxanes(AFPs)as efficient polymeric organocatalyst for amino catalysis:efficient multicomponent Gewald reaction,α-allylic alkylaion of aldehydes,and Knoevenagel condensation[J].RSC Advances,2012,2(7):2895-2901.
[12]Zheng Z J,Ye F,Zheng L S,etal.Copper-catalyzed huisgen and oxidative Huisgen coupling reactions controlled by polysiloxane-supported amines(AFPs)for the divergent synthesis of triazoles and bistriazoles[J].Chem Eur J,2012,18(44):14094-14099.
[13]Wang C Y,Zou J F,Zheng Z J,etal.BINOL-linked 1,2,3-triazoles:an unexpected fluorescent sensor with anion-πinteraction for iodide ions[J].RSC Advances,2014,4(97):54256-54262.
[14]Song T,Li L,Zhou W,etal.Enantioselective copper-catalyzed azide-alkyne click cycloaddition to desymmetrization of maleimide-based bis(alkynes)[J].Chem Eur J,2014,21(2):554-558.
[15]Kolb H C,Finn M G,Sharpless K B.Click chemistry:diverse chemical function from a few good reactions[J].Angew Chem Int Ed,2001,40(11):2004-2021.
[16]Rostovtsev V V,Green L G,Fokin V V,etal.A stepwise Huisgen cycloaddition process:copper(I)-catalyzed regioselective“ligation”of azides and terminal alkynes[J].Angew Chem,2002,114(14):2596-2599.
[17]Tornoe C W,Christensen C,Meldal M.Peptidotriazoles on solid phase:[1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides[J].J Org Chem,2002,67(9):3057-3064.
[18]Lutz J F.1,3-Dipolar cycloadditions of azides and alkynes:a universal ligation tool in polymer and materials science[J].Angew Chem Int Ed,2007,46(7):1018-1025.
[19]Kasteren S I,Kramer H B,Gamblin D P,etal.Site-selective glycosylation of proteins:creating synthetic glycoproteins[J].Nat Protoc,2007,21(2):3185-3194.
[20]Pedersen D S,Abell A.1,2,3-Triazoles in peptidomimetic chemistry[J].Eur J Org Chem,2011,2011(13):2399-2411.
[21]Li Y,Cai C Z.Click chemistry-based functionalization on non-oxidized silicon substrates[J].Chem Asian J,2011,6(10):2592-2605.
[22]Davis A R,Maegerlein J A,Carter K R.Electroluminescent networks via photo“click”chemistry[J].J Am Chem Soc,2011,133(50):20546-20551.
[23]Strukil V,Margetic D,Igrc M D,etal.Desymmetrisation of aromatic diamines and synthesis of non-symmetrical thiourea derivatives by click-mechanochemistry[J].Chem Commun,2012,48(78):9705-9707.
[24]Ghorai A,Padmanaban E,Mukhopadhyay C,etal.Design and synthesis of regioisomeric triazole based peptidomimetic macrocycles and their dipole moment controlled self-assembly[J].Chem Commun,2012,48(98):11975-11977.
[25]Wojcik F,O'Brien A G,Gotze S,etal.Synthesis of carbohydrate-functionalised sequence-defined oligo(amidoamine)s by photochemical thiol-ene coupling in a continuous flow reactor[J].Chem Eur J,2013,19(9):3090-3098.
[26]Jiang Y Q,Kong D Y,Zhao J L,etal.A simple,efficient thermally promoted protocol for Huisgen-click reaction catalyzed by CuSO4·5H2O in water[J].Tetrahedron Lett,2014,55(15):2410-2414.
[27]Radatz C S,Liliana L A,Vieira E R,etal.Recoverable Cu/SiO2composite-catalysed click synthesis of 1,2,3-triazoles in water media[J].New J Chem,2014,38(4):1410-1417.
[28]Mukherjee N,Ahammed S,Bhadra S,etal.Solvent-free one-pot synthesis of 1,2,3-triazole derivatives by the‘click’reaction of alkyl halides or aryl boronic acids,sodium azide and terminal alkynes over a Cu/Al2O3surface under ball-milling[J].Green Chem,2013,15(2):389-397.
[29]Zarei A,Khazdooz L,Hajipour A R,etal.Microwave-assisted click chemistry synthesis of 1,2,3-triazoles from aryldiazonium silica sulfates in water[J].Synthesis,2012,44(21):3353-3360.
[30]Quan Z J,Xu Q,Zhang Z,etal.Copper-catalyzed click synthesis of functionalized 1,2,3-triazoles with 3,4-dihydropyrimidinone or amide group via a one-pot four-component reaction[J].Tetrahedron,2013,69(2):881-887.
[31]Alonso V F,Moglie Y,Radivoy G,etal.Copper-catalysed multicomponent click synthesis of 5-alkynyl 1,2,3-triazoles under ambient conditions[J].Synlett,2012,23(15):2179-2182.
[32]Albadi J,Keshavarz M,Shirini F,etal.Copper iodide nanoparticles on poly(4-vinyl pyridine):a new and efficient catalyst for multicomponent click synthesis of 1,4-disubstituted-1,2,3-triazoles in water[J].Catal Commun,2012,27:17-20.
[33]Wang Y,Liu J H,Xia C G.Insights into supported copper(II)-catalyzed azide-alkyne cycloaddition in water[J].Adv Synth Catal,2011,353(9):1534-1542.
[34]Beneteau V,Olmos A,Boningari T,etal.Zeo-click synthesis:CuI-zeolite-catalyzed one-pot two-step synthesis of triazoles from halides and related compounds[J].Tetarhedron Lett,2010,51(28):3673-3677.
[35]Appukkuttan P,Dehaen W,Fokin V V,etal.A microwave-assisted click chemistry synthesis of 1,4-disubstituted 1,2,3-triazoles via a copper(I)-catalyzed three-component reaction[J].Org Lett,2004,6(23):4223-4225.
[36]Sharghi H,Beyzavi M H,Safavi A,etal.Immobilization of porphyrinatocopper nanoparticles onto activated multi-walled carbon nanotubes and a study of its catalytic activity as an efficient heterogeneous catalyst for a click approach to the three-component synthesis of 1,2,3-triazoles in water[J].Adv Synth Catal,2009,351(14/15):2391-2410.
[37]Alonso F,Moglie Y,Radivoy G,etal.Click chemistry from organic halides,diazonium salts and anilines in water catalysed by copper nanoparticles on activated carbon[J].Org Biomol Chem,2011,9(18):6385-6395.
[38]Wang W L,Wu J L,Xia C G,etal.Reusable ammonium salt-tagged NHC-Cu(I)complexes:preparation and catalytic application in the three component click reaction[J].Green Chem,2011,13(12):3440-3445.
[39]Yamada Y M A,Sarkar S M,Uozumi Y.Amphiphilic self-assembled polymeric copper catalyst to parts per million levels:click chemistry[J].J Am Chem Soc,2012,134(22):9285-9286.
[40]Zhang C,Huang B,Chen Y,etal.Porous copper catalyzed click reaction in water[J].New J Chem,2013,37(9):2606-2609.
[41]Purohit V B,Karad S C,Patel K H,etal.Cu(N-heterocyclic carbene)chloride:an efficient catalyst for multicomponent click reaction for the synthesis of 1,2,3-triazoles in water at room temperature[J].RSC Advances,2014,4(86):46002-46007.
[42]Nasr-Esfahani M,Mohammadpoor-Baltork I,Khosropour A R,etal.Copper immobilized on nanosilica triazine dendrimer(Cu(II)-TD@nSiO2)-catalyzed regioselective synthesis of 1,4-disubstituted 1,2,3-triazoles and bis-and tris-triazoles via a one-pot multicomponent click reaction[J].J Org Chem,2014,79(3):1437-1443.
[43]Rodionov V O,Presolski S I,Gardinier S,etal.Benzimidazole and related ligands for Cu-catalyzed azide-alkyne cycloaddition[J].J Am Chem Soc,2007,129(42):12696-12704.
[44]Donnelly P S,Zanatta S D,Zammit S C,etal.‘Click’cycloaddition catalysts:copper(I)and copper(II)tris(triazolylmethyl)amine complexes[J].Chem Commun,2008(21):2459-2461.
[45]Li F W,Hor T S A.Facile synthesis of nitrogen tetradentate ligands and their applications in CuI-catalyzed N-arylation and azide-alkyne cycloaddition[J].Chem Eur J,2009,15(40):10585-10592.
[46]Wallyn S,Lammens M,O'Reilly R K,etal.Highly active,thermo-responsive polymeric catalytic system for reuse in aqueous and organic Cu AAC reactions[J].J Polym Sci Polym Chem,2011,49(13):2878-2885.
[47]Michaels H A,Zhu L.Ligand-assisted,copper(II)acetate-accelerated azide-alkyne cycloaddition[J].Chem Asian J,2011,6(10):2825-2834.
[48]Wang W,Hong S L,Tran A,etal.Sulfated ligands for the copper(I)-catalyzed azide-alkyne cycloaddition[J].Chem Asian J,2011,6(10):2796-2802.
[49]Connell T U,Schieber C,Silvestri I P,etal.Copper and silver complexes of tris(triazole)amine and tris(benzimidazole)amine ligands:evidence that catalysis of an azide-alkyne cycloaddition(“click”)reaction by a silver tris(triazole)amine complex arises from copper impurities[J].Inorg Chem,2014,53(13):6503-6511.
