复合蛋白源替代鱼粉对大菱鲆生长、体组成和表观消化率的影响*
2015-03-20周慧慧麦康森
董 纯,周慧慧,麦康森,徐 玮,何 艮
(中国海洋大学水产学院农业部水产动物营养与饲料重点实验室,山东 青岛 266003)
复合蛋白源替代鱼粉对大菱鲆生长、体组成和表观消化率的影响*
董 纯,周慧慧,麦康森,徐 玮,何 艮**
(中国海洋大学水产学院农业部水产动物营养与饲料重点实验室,山东 青岛 266003)
本实验旨在研究谷朊粉、宠物级鸡肉粉、脱脂肉骨粉、豆粕和玉米蛋白粉复合替代0%(对照组,CON)、35%(FM35)、50%(FM50)、65%(FM65)、80%(FM80)鱼粉对大菱鲆(Scophthalmusmaximus)生长、体组成和表观消化率的影响。设计5组等氮等能(粗蛋白52%,总能19kJ/g)的饲料,养殖鱼初重(8.63±0.01)g,养殖周期9周。结果显示,替代组体末重、增重率和特定生长率显著低于CON(P<0.05),而摄食率和成活率各处理组之间没有显著变化(P>0.05)。替代组饲料效率和蛋白质效率随着替代水平的升高而降低,FM65和FM80饲料效率和FM80蛋白质效率显著低于CON(P<0.05)。复合蛋白替代鱼粉对鱼体水分、粗蛋白和粗脂肪没有显著影响(P>0.05),但替代组鱼体灰分显著高于CON(P<0.05)。除FM35干物质表观消化率与CON没有显著差异(P>0.05),其他替代组的干物质和粗蛋白表观消化率均显著低于CON(P<0.05)。研究表明,该比例复合蛋白源替代鱼粉水平应不超过35%。
大菱鲆;鱼粉;复合蛋白;生长;体组成;表观消化率
大菱鲆(Scophthalmusmaximus)肉质鲜美,在中国北方广泛养殖,是一种重要的商业肉食性鱼类,主要投喂高蛋白商业饲料[1-2]。鱼粉蛋白含量高、氨基酸平衡、适口性好、抗营养因子少,含有一些未知促生长因子,是大菱鲆商业饲料的首选蛋白源[3]。但随着野生渔业资源的不断减少,养殖业规模的不断扩大,鱼粉资源供不应求,价格持续上涨。因此,寻求新型高效蛋白源替代鱼粉成为水产动物营养与饲料学的研究重点。
目前常用新型蛋白源主要是植物蛋白源如豆粕、玉米蛋白粉、花生粕等。植物蛋白源资源丰富、价格低廉,是一种理想的蛋白源,但抗营养因子[4]、氨基酸不平衡[5]等因素限制其广泛应用,如豆粕替代鱼粉水平大于20%就会显著降低黑海比目鱼(Scophthalmusmaeoticus)的生长和营养利用[6]。动物蛋白源如鸡肉粉、肉骨粉、血粉等,富含游离氨基酸、牛磺酸、鹅肌肽等[7],这些水溶性小分子含氮化合物具有促摄食作用,可以改善饲料的味道[8],鱼粉替代率较高,如宠物级鸡肉粉替代60%鱼粉对军曹鱼(Rachycentroncanadum)的生长没有显著影响[9],肉骨粉替代45%鱼粉而不影响大黄鱼(PseudosciaenacroceaRichardson)的生长[10]。复合蛋白源是将2种以上的植物蛋白源和(或)动物蛋白源以一定的配比混合,由于可以平衡营养物、补充氨基酸、掩盖差的适口性[11-13],近年来在新型蛋白源的研究中广受关注,如发酵豆粕和鱿鱼副产物替代36%鱼粉对牙鲆(Paralichthysolivaceus)的生长不造成影响[14],混合植物蛋白替代39%鱼粉,对大菱鲆生长无显著影响[15]。
本研究选择适口性好的复合蛋白源谷朊粉、宠物级鸡肉粉、脱脂肉骨粉、豆粕和玉米蛋白粉,依照氨基酸平衡的原则设计配方(1:1:4:1:3),以不同水平替代鱼粉,研究该配比复合蛋白源对大菱鲆生长、体组成和表观消化率的影响,为大菱鲆新型蛋白源的开发研究提供参考。
1 材料与方法
1.1 饲料原料和饲料配方
实验用蛋白源为红鱼粉、谷朊粉、宠物级鸡肉粉、脱脂肉骨粉、豆粕和玉米蛋白粉,脂肪源为鱼油和棕榈油,糖源为小麦粉。其中肉骨粉脂肪较高,易发生氧化酸败,因此对其进行脱脂。原料的营养和必需氨基酸组成见表1。
实验设计5组等氮等能(粗蛋白52%,总能19kJ/g)的饲料,以62%鱼粉组作为对照(CON),谷朊粉、宠物级鸡肉粉、脱脂肉骨粉、豆粕和玉米蛋白粉(1:1:4:1:3)复合替代35%(FM35)、50%(FM50)、65%(FM65)、80%(FM80)鱼粉。根据鱼粉对照组必需氨基酸组成,添加晶体氨基酸L-组氨酸、L-赖氨酸和DL-蛋氨酸平衡各处理组必需氨基酸。添加微晶纤维素平衡能量。具体饲料配方见表2。
制作饲料时,首先将所有原料粉碎后过80目筛,依配方表(见表2)从小到大逐一混匀,再将鱼油、棕榈油和大豆卵磷脂搓散搅拌均匀后加到原料中,所有原料与油彻底混匀,然后加水搓均匀。用双螺杆制粒机(F-26(Ⅱ),华南理工大学)制粒,45℃烘箱干燥12h,最后用塑料袋装好密封保存于-20℃冰箱备用。
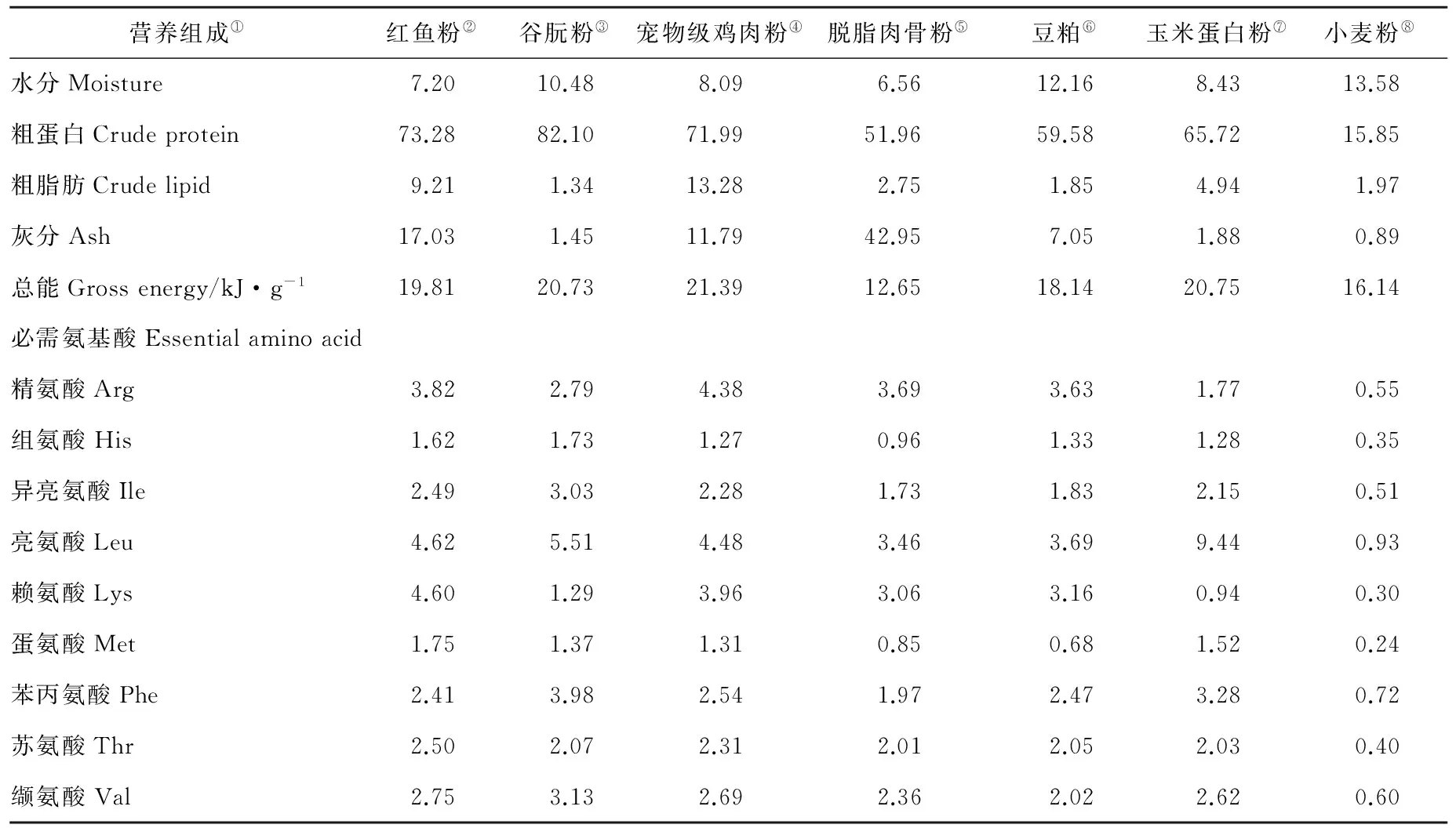
表1 饲料原料营养和必需氨基酸组成(干物质)Table 1 Nutrition and essential amino acid composition of dietary ingredient (dry matter) /%
Note:①Nutrient composition;②Brown fishmeal;③Wheat gluten meal;④Pet food-grade poultry by-product meal;⑤Defatted meat and bone meal;⑥Soybean meal;⑦Corn gluten meal;⑧Wheat flour
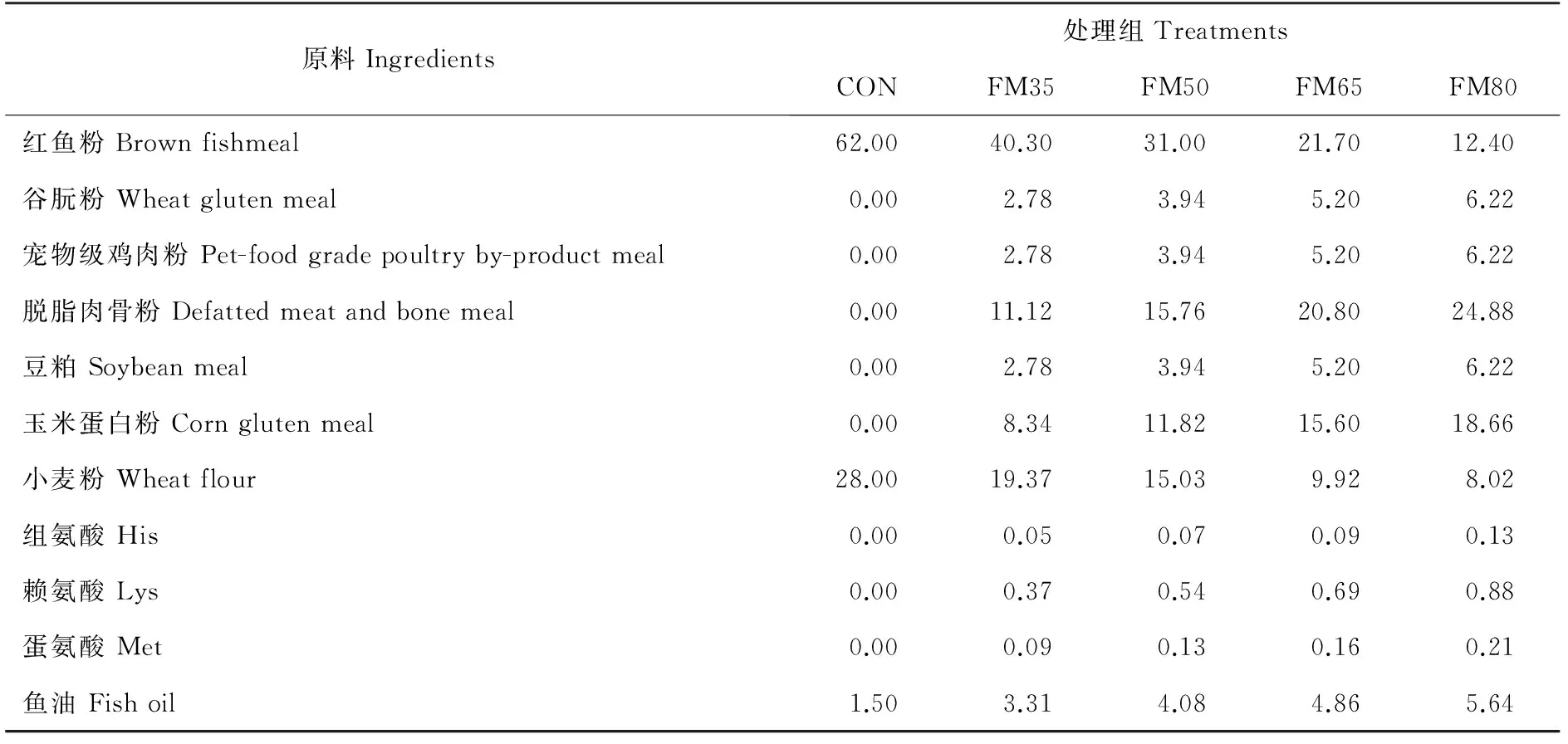
表2 实验饲料配方和主要营养成分(干物质)Table 2 Formulation and proximate chemical composition of the tested diets (dry matter)
续表2
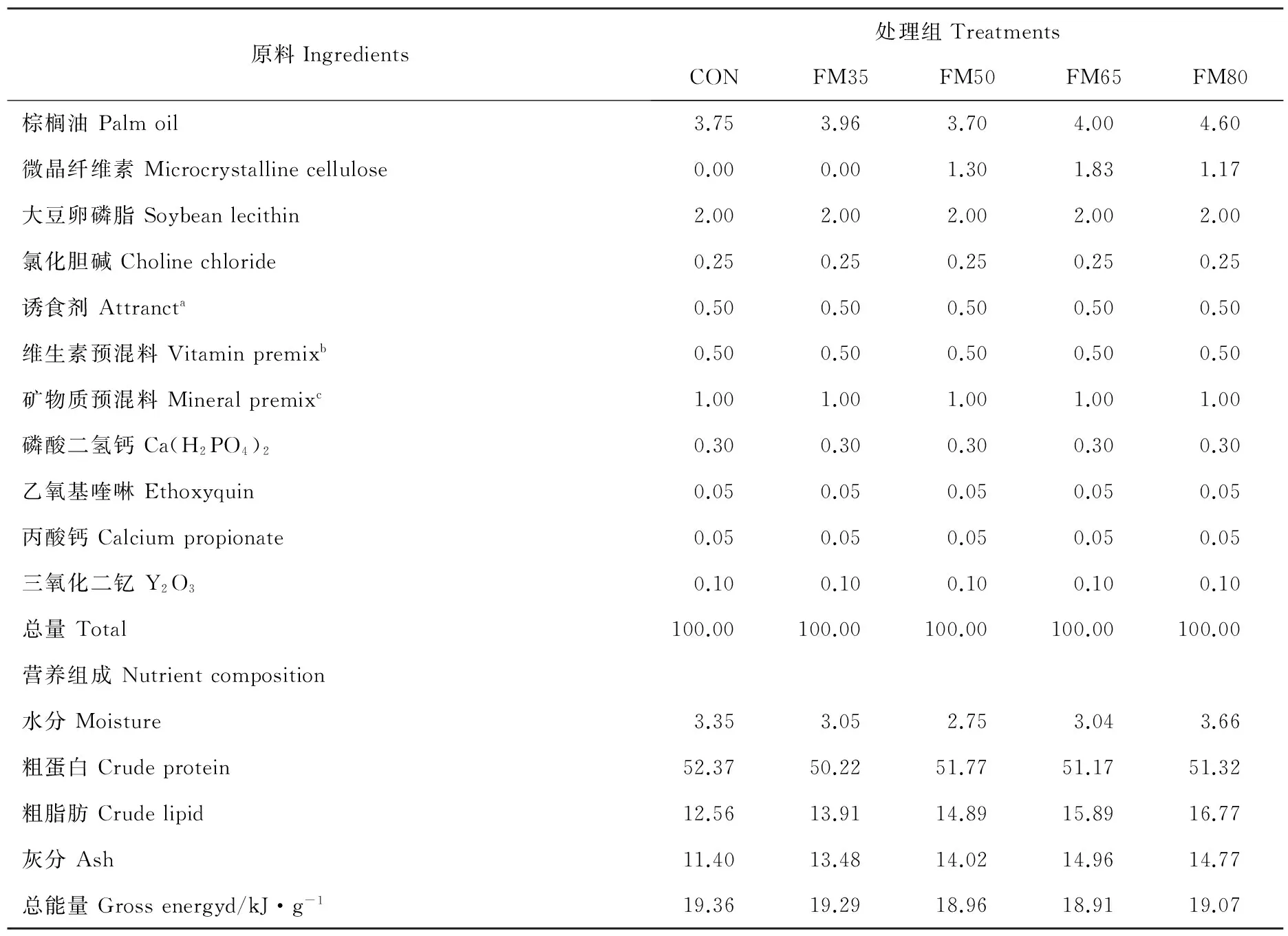
原料Ingredients处理组TreatmentsCONFM35FM50FM65FM80棕榈油Palmoil3.753.963.704.004.60微晶纤维素Microcrystallinecellulose0.000.001.301.831.17大豆卵磷脂Soybeanlecithin2.002.002.002.002.00氯化胆碱Cholinechloride0.250.250.250.250.25诱食剂Attrancta0.500.500.500.500.50维生素预混料Vitaminpremixb0.500.500.500.500.50矿物质预混料Mineralpremixc1.001.001.001.001.00磷酸二氢钙Ca(H2PO4)20.300.300.300.300.30乙氧基喹啉Ethoxyquin0.050.050.050.050.05丙酸钙Calciumpropionate0.050.050.050.050.05三氧化二钇Y2O30.100.100.100.100.10总量Total100.00100.00100.00100.00100.00营养组成Nutrientcomposition水分Moisture3.353.052.753.043.66粗蛋白Crudeprotein52.3750.2251.7751.1751.32粗脂肪Crudelipid12.5613.9114.8915.8916.77灰分Ash11.4013.4814.0214.9614.77总能量Grossenergyd/kJ·g-119.3619.2918.9618.9119.07
注:a诱食剂:甜菜碱:二甲基-丙酸噻亭:甘氨酸:丙氨酸:5-磷酸肌苷=4:2:2:1:1。b维生素预混料(mg/kg):维生素A,32;维生素D,5;维生素E,240;维生素K,10;维生素B1,25;维生素B2,45;维生素B6,20;维生素B12,10;泛酸钙,60;烟酸,200;叶酸,20;生物素,60;肌醇,800;维生素C磷酸酯,2000;微晶纤维素,1473。c矿物质预混料(mg/kg):MgSO4·7H2O,1200;CuSO4·5H2O;10;FeSO4·H2O,80;ZnSO4·H2O,50;MnSO4·H2O,45;CoCl2·6H2O(1%),50;Na2SeO3(1%),20;碘酸钙,60;沸石粉,8485。d能量通过蛋白质、脂肪和糖类的平均产热量计算得出,产热量分别为23.6,39.5和17.2 kJ/g。糖类物质为100-(蛋白质+脂肪+灰分+水分)。aAttractant:betaine:dimethyl-propiothetin:glycine: alanine:5-phosphate inosine = 4:2:2:1:1.bVitamin premix(mg/kg diet): retinal palmitate, 32; cholecalciferol, 5; DL-ɑ-tocopherol acetate, 240; menadione, 10; thiamin-HCl, 25; riboflavin, 45; pyridoxine-HCl, 20; cyanocobalamin, 10; D-calcium pantothenate, 60; amine nicotinic acid, 200; folic acid, 20; biotin, 60; mesoinositol, 800; ascorbyl polyphosphate(contained 35% ascorbic acid) , 2000; microcrystalline cellulose, 1473.cMineral premix(mg/kg diet): MgSO4·7H2O, 1200; CuSO4·5H2O, 10; FeSO4·H2O, 80; ZnSO4·H2O, 50; MnSO4·H2O, 45; CoCl2·6H2O(1%), 50; Na2SeO3(1%), 20; calcium iodine, 60; zoelite, 8485.dGross energy calculated using combustion values for protein, lipid and carbohydrate of 23.6, 39.5 and 17.2 kJ·g-1, respectively. Carbohydrate was calculated by the difference: 100-(protein + lipid + ash + moisture).
1.2 实验用鱼和实验条件
实验大菱鲆幼鱼购买于莱州养殖厂(山东烟台)。养殖实验在山东海阳黄海水产有限公司进行,实验开始前,鱼暂养1周以适应养殖环境,期间投喂商业饲料。驯化结束后,禁食24h,然后挑选规格均一、体格健壮的大菱鲆幼鱼(初重(8.63±0.01)g)随机分配到15个216 L纤维玻璃缸中,每个处理3个重复,每个重复30尾鱼。实验用海水经水泵持续抽送到过滤池中,经沙滤以大约1.5 L/min的速度流到实验桶。养殖9周期间,每天于07:00和19:00饱食投喂,每次摄食0.5h后吸污、换水以保证水质。整个养殖期间,持续曝气,水温控制在19~22℃,pH=7.5~8.0,盐度30~33。
1.3 样品收集和分析
实验开始前,随机收集20尾初始鱼保存于-20℃冰箱以备全鱼分析。养殖实验结束时,停喂24h,记录每桶实验鱼的尾数和体重,每桶随机选择5尾作为全鱼分析,保存于-20℃。从每桶中随机选3尾实验鱼,测量体长并称重以计算肥满度,之后取出全肝称重计算肝体比。
实验原料、饲料和实验鱼在105℃烘箱中烘至恒重计算水分含量;采用凯氏定氮法检测样品粗蛋白含量;采用索氏抽提法检测粗脂肪含量;采用马福炉在550℃环境下灼烧12h计算灰分含量。
实验饲料中添加1%的三氧化二钇(Y2O3)作为指示剂测定干物质和粗蛋白表观消化率。实验进行4周后,用虹吸法收集粪便,保存于-20℃。待收集充足的粪便后,按上述方法测定粪便中的干物质和粗蛋白。采用Frukawa和Tsukabatra的方法测定饲料和粪便中钇的含量,即用高氯酸消解后,使用电感耦合等离子体原子发射光谱仪(ICP-OES,Vista-mpx,Varian,美国)分析饲料和粪便中的钇含量。
1.4 计算和统计方法
存活率(Survival rate,SR)=终末尾数/初始尾数×100%。
增重率(Weight gain rate,WGR)=(鱼体末重-鱼体初重)/鱼体初重×100%。
特定生长率(Specific growth rate,SGR)=(ln(鱼体末重)-ln(鱼体初重))/养殖天数×100%。
摄食率(Feed intake,FI)=(摄食饲料量/((鱼体初重+鱼体末重)/2))/养殖天数×100%。
饲料效率(Feed efficiency ratio,FER)=鱼体增重/摄食饲料量×100%。
蛋白质效率(Protein efficiency ratio,PER)=鱼体增重/摄入蛋白量×100%。
肥满度(Condition factor,CF)=鱼体重(g)/鱼体长(cm)3×100%。
肝体比(Hepatosomatic index,HSI)=肝重/体重×100%。
表观消化率(Apparent digestibility coefficients,ADC)=(1-(饲料中钇含量%/粪便中钇含量)×(粪便营养物/饲料营养物))×100%。
1.5 数据统计与分析
使用软件SPSS 17.0对所得数据进行单因素方差分析(One-way ANOVA),若差异显著,则进行Tukey 多重比较(Tukey HSD test),显著水平为P<0.05。实验所得数据表示为平均值±标准误(mean ±S.E.,n=3)。
2 结果
2.1 复合蛋白替代鱼粉对大菱鲆幼鱼生长性能和饲料利用的影响
复合蛋白替代组与全鱼粉对照组相比,体末重显著降低(P<0.05),其中,FM35、FM50和FM65之间没有显著差异(P>0.05)。增重率和特定生长率的变化趋势与体末重相同。摄食率和成活率各处理组之间没有显著变化(P>0.05)。当复合蛋白替代鱼粉水平不超过50%时,对大菱鲆幼鱼的饲料效率和蛋白质效率影响不显著(P>0.05)。具体数据见表3。

表3 复合蛋白替代鱼粉对大菱鲆幼鱼生长性能和饲料利用的影响Table 3 Effect of replacement of fishmeal by compound proteins on growth parameters and feed utilization of juvenile turbot
注:数据为平均值±标准误,n=3;同一行标有不同的上标表示显著性差异(P<0.05)。Values show with mean ± standard error,n=3. Values in the same row with different small letter superscript mean significant difference(P<0.05).
2.2 复合蛋白替代鱼粉对大菱鲆幼鱼体组成和形体指标的影响
根据表4可知,该复合蛋白替代鱼粉对大菱鲆鱼体水分、粗蛋白和粗脂肪没有显著影响(P>0.05),但粗蛋白有先上升后下降的趋势。灰分含量随复合蛋白替代水平的增加而升高,各替代组灰分均显著高于对照组(P<0.05)。替代对鱼体肥满度和肝体比没有影响,各处理组之间没有显著差异(P>0.05)。
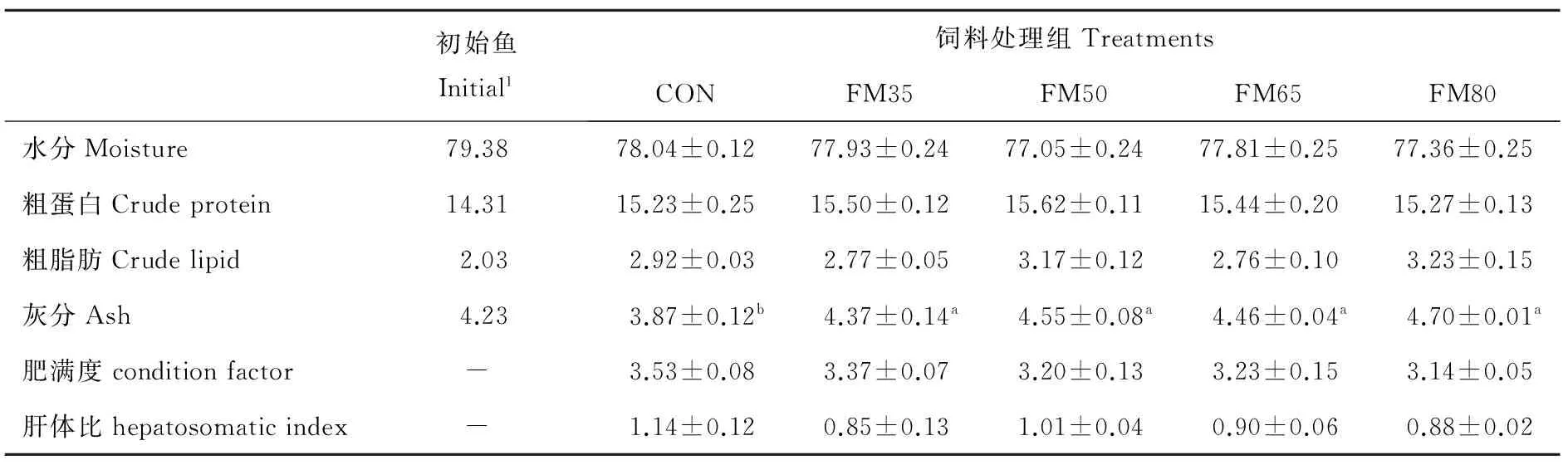
表4 复合蛋白替代鱼粉对大菱鲆幼鱼体组成(湿重)和形体指标的影响Table 4 Effect of replacement of fishmeal by compound proteins on proximate composition (wet weight) of the whole body and somatic parameters of juvenile turbot /%
注:数据为平均值±标准误,n=3;同一行标有不同的上标表示显著性差异(P<0.05)。Values show with mean ± standard error,n=3; Values in the same row with different small letter superscript mean significant difference(P<0.05).1初始鱼没有进行统计分析。1Initial values are not included in the statistical analysis.
2.3 复合蛋白替代鱼粉对饲料干物质和粗蛋白表观消化率的影响
表5显示,该复合蛋白替代鱼粉显著影响粗蛋白表观消化率,随着替代水平的增加,粗蛋白表观消化率显著降低(P<0.05),FM35和FM50显著低于对照组(P<0.05),显著高于FM65和FM80(P<0.05)。只有FM35替代组的干物质表观消化率与对照组相比没有显著变化(P>0.05),其他替代组均显著低于对照组(P<0.05)。

表5 饲料干物质和粗蛋白的表观消化率(ADC)Table 5 Apparent digestibility coefficients (ADC) for dry matter and crude protein of the test diets /%
注:数据为平均值±标准误,n=3;同一行标有不同的上标表示显著性差异(P<0.05)。 Values show with mean ± standard error,n=3. Values in the same row with different small letter superscript mean significant difference(P<0.05).
3 讨论
本实验条件下,复合蛋白源替代不同水平的鱼粉对大菱鲆摄食率没有显著影响,这与之前鱼粉替代的部分研究结果一致[6,16],但与Fournier等的研究结果不同,随着植物蛋白替代鱼粉水平的增加,大菱鲆摄食率显著降低[17]。本实验较好的摄食率结果说明复合蛋白源或诱食剂提高了饲料适口性。研究显示,在没有诱食剂的情况下,土豆浓缩蛋白替代50%鱼粉,与全鱼粉对照组相比,虹鳟(Oncorhynchusmykiss)摄食率显著下降,然而即使添加促摄食物质贻贝粉或氨基酸混合物(1丙氨酸:1甘氨酸:1甜菜碱),摄食率也并未升高,诱食物并未改善虹鳟饲料适口性[18]。Silva-Neto等发现添加商业诱食剂或螺旋藻促摄食物的鱼粉替代组与投喂商业饲料和没有添加诱食物的替代组相比,凡纳滨对虾(Litopenaeusvannamei)的摄食均没有显著升高[19]。这说明诱食剂只是在一定程度上改善饲料适口性,当鱼粉替代水平增加,替代组适口性差的缺点随之突出,即使添加诱食剂,饲料适口性依然下降。另有研究指出,塞内加尔鳎(Soleasenegalensis)能有效利用植物蛋白饲料,但其生长和营养利用取决于蛋白源混合物的选择,而非替代水平[20]。因此,高比例替代鱼粉关键在于替代蛋白源的选择和其适口性。本实验复合蛋白源是根据前期适口性实验做出的选择,摄食率结果也进一步证实该复合蛋白源对大菱鲆有着较好的适口性,80%鱼粉替代组的摄食率依然没有显著降低。然而该复合蛋白源替代35%鱼粉显著降低大菱鲆体末重、增重率和特定生长率。因此,就本实验而言,大菱鲆生长降低与该复合蛋白的适口性关系不大,较低的蛋白利用率可能是导致大菱鲆生长缓慢的主要原因。
有研究表明,谷朊粉替代67%鱼粉而不影响大菱鲆生长[21]。豆粕和谷朊粉100%替代鱼粉对西伯利亚鲟的生长没有显著影响[22]。在理想蛋白模式下,宠物级鸡肉粉100%替代鱼粉,对杂交条纹鲈(Moronechrysops♀ ×M.saxatilis♂)的生长影响不显著[23]。占本实验复合蛋白比重较大的植物蛋白源玉米蛋白粉替代鱼粉33%,对大菱鲆的生长和饲料效率也没有造成负面影响[24]。然而本实验条件下复合蛋白源替代水平低于以上蛋白源单一替代,这可能是由于各组分之间没有起到补充营养物,提高蛋白利用等作用,反而掩盖了谷朊粉、宠物级鸡肉粉高蛋白、适口性好的优点[25-26],导致替代鱼粉35%大菱鲆生长显著降低。首先,复合蛋白源替代组较低水平的消化率可能是导致大菱鲆低生长性能的主要因素,除了FM35替代组的干物质表观消化率与对照组没有显著差异,其他替代组的干物质和粗蛋白表观消化率均显著低于对照组。有文献报道,单宁不仅降低饲料适口性,而且影响蛋白质消化率[27]。而复合蛋白源中的豆粕含有单宁、植酸、胰蛋白酶抑制因子、凝集素等[4],这些抗营养因子降低蛋白的吸收利用,而肉食性鱼类的生长需要高蛋白[1,28-29],大菱鲆得不到生长所需蛋白质而生长缓慢。此外,饲料灰分含量与蛋白质消化率存在负相关关系[30-31],占复合蛋白比重最大的脱脂肉骨粉灰分含量高。因此,高比例的脱脂肉骨粉可能导致蛋白质表观消化率较低,从而影响到大菱鲆幼鱼的生长。玉米蛋白粉的偏酸性也会降低蛋白质消化率[32]。同时,复合蛋白源质量和粪便收集方法的不同也会不同程度的影响蛋白质消化率[33-34]。
关于蛋白源替代鱼粉对水生动物体成分的影响,不同的实验得到的结果不一样。发酵豆粕和鱿鱼副产物复合替代鱼粉,牙鲆鱼体成分没有显著变化[14]。混合动物蛋白替代鱼粉75%甚至100%,对西伯利亚鲟的体组成也都没有产生显著影响[35]。随着复合植物蛋白替代鱼粉水平的增加,大菱鲆鱼体粗蛋白和灰分降低,粗脂肪和水分升高[17]。而本实验动植物复合蛋白源替代鱼粉,对大菱鲆体水分、粗蛋白和粗脂肪没有显著影响,但粗蛋白有先升高后下降的趋势。可能由于高比例、高灰分脱脂肉骨粉的存在,随替代水平的增加,鱼体灰分显著升高。
综上,本实验条件下,复合蛋白源谷朊粉、宠物级鸡肉粉、脱脂肉骨粉、豆粕和玉米蛋白粉(1:1:4:1:3)对大菱鲆有较好的适口性,不影响各替代水平的摄食率。而35%鱼粉替代组大菱鲆生长显著降低,所以以大菱鲆生长性能作为评价指标,该复合蛋白源替代鱼粉水平应低于35%。推测主要是因为较低的干物质和蛋白质表观消化率,大菱鲆对饲料蛋白质没有充分吸收利用所导致。因此,为达到利用蛋白源优点,降低缺点的目的,复合蛋白源配比的选择不仅要注重饲料适口性的改善,而且还应提高各蛋白源的消化吸收,总体提高鱼粉替代水平。
[1] Kroeckel S, Harjes A G E, Roth I, et al. When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetiaillucens) as fish meal substitute-growth performance and chitin degradation in juvenile turbot (Psettamaxima) [J]. Aquaculture, 2012, (364-365): 345-352.
[2] Turker A, Yigit M, Ergun S, et al. Potential of poultry by-product meal as a substitute for fishmeal in diets for Black Sea TurbotScophthlmusMaeoticus: growth and nutrient utilization in winter [J]. The Israeli Jounal of Aquaculture-Bamidgeh, 2005, 57(1): 49-61.
[3] Bonaldo A, Parma L, Mandrioli L, et al. Increasing dietary plant proteins affects growth performance and ammonia excretion but not digestibility and gut histology in turbot (Psettamaxima) juveniles [J]. Aquaculture, 2011, 318(1): 101-108.
[4] Francis G, Makkar H P S, Becker K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish [J]. Aquaculture, 2001, 199(3): 197-227.
[5] Hansen A C, Rosenlund G, Karlsen Ø, et al. Total replacement of fish meal with plant proteins in diets for Atlantic cod (GadusmorhuaL.) I-effects on growth and protein retention [J]. Aquaculture, 2007, 272(1): 599-611.
[6] Ergun S, Yigit M, Turker A, et al. Incorporation of soybean meal and hazelnut meal in diets for Black Sea turbot (Scophthalmusmaeoticus) [J]. The Israeli Jounal of Aquaculture-Bamidgeh, 2008, 60(1): 27-36.
[7] Aksnes A, Hope B, Albrektsen S. Size-fractionated fish hydrolysate as feed ingredient for rainbow trout (Oncorhynchusmykiss) fed high plant protein diets. II: flesh quality, absorption, retention and fillet levels of taurine and anserine [J]. Aquaculture, 2006, 261: 318-326.
[8] Fuke S, Konosu S. Taste-active components in some foods: a review of Japanese research [J]. Physiology and Behavior, 1991, 49(5): 863-868.
[9] Zhou Q C, Zhao J, Li P, et al. Evaluation of poultry by-product meal in commercial diets for juvenile cobia (Rachycentroncanadum) [J]. Aquaculture, 2011, (322-323): 122-127.
[10] Ai Q, Mai K, Tan B, et al. Replacement of fish meal by meat and bone meal in diets for large yellow croaker,Pseudosciaenacrocea[J]. Aquaculture, 2006, 260(1): 255-263.
[11] Kader M A, Koshio S. Effect of composite mixture of seafood by-products and soybean proteins in replacement of fishmeal on the performance of red sea bream,Pagrusmajor[J]. Aquaculture, 2012, 368-369: 95-102.
[12] Guo J, Wang Y, Bureau D. Inclusion of rendered animal ingredients as fishmeal substitutes in practical diets for cuneate drum,Nibeamiichthioides(Chu, Lo et Wu) [J]. Aquaculture Nutrition, 2007, 13(2): 81-87.
[13] Tidwell J H, Coyle S D, Bright L A, et al. Evaluation of plant and animal source proteins for replacement of fish meal in practical diets for the largemouth bassMicropterussalmoides[J]. Journal of the World Aquaculture Society, 2005, 36(4): 454-463.
[14] Kader A, Koshio S, Ishikawa M, et al. Can fermented soybean meal and squid by-product blend be used as fishmeal replacements for Japanese flounder (Paralichthysolivaceus) [J]. Aquaculture Research, 2012, 43: 1427-1438.
[15] Bonaldo A, Parma L, Mandrioli L, et al. Increasing dietary plant proteins affects growth performance and ammonia excretion but not digestibility and gut histology in turbot (Psettamaxima) juveniles [J]. Aquaculture, 2011, 318: 101-108.
[16] Cheng Z, Ai Q, Mai K, et al. Effects of dietary canola meal on growth performance, digestion and metabolism of Japanese seabass,Lateolabraxjaponicus[J]. Aquaculture, 2010, 305(1): 102-108.
[17] Fournier V, Huelvan C, Desbruyeres E. Incorporation of a mixture of plant feedstuffs as substitute for fish meal in diets of juvenile turbot (Psettamaxima) [J]. Aquaculture, 2004, 236(1): 451-465.
[18] Tusche K, Berends K, Wuertz S, et al. Evaluation of feed attractants in potato protein concentrate based diets for rainbow trout (Oncorhynchusmykiss) [J]. Aquaculture, 2011, 321(1-2): 54-60.
[19] Silva Neto J F, Nunes A J P, Sabry Neto H, et al. Spirulina meal has acted as a strong feeding attractant for Litopenaeus vannamei at a very low dietary inclusion level [J]. Aquaculture Research, 2012, 43(3): 430-437.
[20] Cabral E, Bacelar M, Batista S, et al. Replacement of fishmeal by increasing levels of plant protein blends in diets for Senegalese sole (Soleasenegalensis) juveniles [J]. Aquaculture, 2011, 322-323: 74-81.
[21] Dietz C, Kroeckel S, Schulz C, et al. Energy requirement for maintenance and efficiency of energy utilization for growth in juvenile turbot (Psettamaxima, L.): The effect of strain and replacement of dietary fish meal by wheat gluten [J]. Aquaculture, 2012, 358-359: 98-107.
[22] Yun B, Xue M, Wang J, et al. Fishmeal can be totally replaced by plant protein blend at two protein levels in diets of juvenile Siberian sturgeon,AcipenserbaeriiBrandt [J]. Aquaculture Nutrition, 2014, 20(1): 69-78.
[23] Gaylord T G, Rawles S D. The modification of poultry by-product meal for use in hybrid striped bassMoronechrysops×M.saxatilisDiets [J]. Journal of the World Aquaculture Society, 2005, 36(3): 363-374.
[24] Regost C, Arzel J, Kaushik S. Partial or total replacement of fish meal by corn gluten meal in diet for turbot (Psettamaxima) [J]. Aquaculture, 1999, 180(1): 99-117.
[25] Tibaldi E, Tulli F, Piccolo G, et al. Wheat gluten as a partial substitute for fish meal protein in sea bass (D.labrax) diets [J]. Italian Journal of Animal Science, 2011, 2(1): 613-615.
[26] Rawles S, Riche M, Gaylord T, et al. Evaluation of poultry by-product meal in commercial diets for hybrid striped bass (Moronechrysops♀×M.saxatilis♂) in recirculated tank production [J]. Aquaculture, 2006, 259(1): 377-389.
[27] McCurdy S, March B. Processing of canola meal for incorporation in trout and salmon diets [J]. Journal of the American Oil Chemists Society, 1992, 69(3): 213-220.
[28] Hevroy E M, El-Mowafi A, Taylor R, et al. Effects of a high plant protein diet on the somatotropic system and cholecystokinin in Atlantic salmon (SalmosalarL.) [J]. Comp Biochem Physiol A Mol Integr Physiol, 2008, 151(4): 621-627.
[29] Bromley P. Effect of dietary protein, lipid and energy content on the growth of turbot (ScophthalmusmaximusL.) [J]. Aquaculture, 1980, 19(4): 359-369.
[30] Kureshy N, Davis D A, Arnold C. Partial replacement of fish meal with meat and bone meal, flash dried poultry by-product meal, and enzyme digested poultry by-product meal in practical diets for juvenile red drum [J]. North American Journal of Aquaculture, 2000, 62(4): 266-272.
[31] 王裕玉, 石野, 杨雨虹, 等. 肉骨粉在水产饲料中的应用 [J]. 中国饲料, 2012(2): 32-35.
[32] Masumoto T, Ruchimat T, Ito Y, et al. Amino acid availability values for several protein sources for yellowtail (Seriolaquinqueradiata) [J]. Aquaculture, 1996, 146(1): 109-119.
[33] Cabral E, Bacelar M, Batista S, et al. Replacement of fishmeal by increasing levels of plant protein blends in diets for Senegalese sole (Soleasenegalensis) juveniles [J]. Aquaculture, 2011, 322: 74-81.
[34] Tibbetts S M, Milley J E, Lall S P. Apparent protein and energy digestibility of common and alternative feed ingredients by Atlantic cod,Gadusmorhua(Linnaeus, 1758) [J]. Aquaculture, 2006, 261(4): 1314-1327.
[35] Xue M, Yun B, Wang J, et al. Performance, body compositions, input and output of nitrogen and phosphorus in Siberian sturgeon,AcipenserbaeriiBrandt, as affected by dietary animal protein blend replacing fishmeal and protein levels [J]. Aquaculture Nutrition, 2012, 18(5): 493-501.
责任编辑 朱宝象
Replacement of Fishmeal in Juvenile Turbot Diets with Compound Proteins: Effects on Growth Performance, Whole Body Composition and Apparent Digestibility Coefficient
DONG Chun, ZHOU Hui-Hui, MAI Kang-Sen, XU Wei, HE Gen
(The Key Laboratory of Aquaculture Nutrition and Feeds of Ministry of Agriculture, Ocean University of China, Qingdao 266003, China)
A 9-week feeding trial was conducted to determine the effect of a mixture of wheat gluten meal, pet-food grade poultry by-product meal, defatted meat and bone meal, soybean meal and corn gluten meal as a partial replacement of fishmeal in juvenile turbot (Scophthalmusmaximus) diets on its growth performance, whole body composition and apparent digestibility coefficient. The fish initially weighed (8.63±0.01)g. Five isonitrogenous (52% crude protein) and isoenergetic (19kJ/g gross energy) diets were formulated by replacing 0 (CON), 35% (FM35), 50% (FM50), 65% (FM65) and 80% (FM80) fishmeal, respectively. Results indicated that the final body weight, weight gain and specific growth rate were significantly lower than those of CON (P<0.05). Feed intake and survival rate of turbot had no significant difference among tested diets (P>0.05). Feed efficiency rate and protein efficiency rate of tested diets decreased with the increase of replacement level. Feed efficiency rate of FM65 and FM80 and protein efficiency rate of FM80 were significantly lower than those of CON (P<0.05). The blend proteins substituted fishmeal had no influence on moisture, crude protein and crude lipid (P>0.05). However, the ash content of all treatments was significantly higher than that of CON (P<0.05). The apparent digestibility coefficient of dry matter of FM35 had no significant difference from that of CON (P>0.05). In addition, other replacement diets significantly decreased in the apparent digestibility coefficient of dry matter and protein (P<0.05). The results showed that compound proteins reduced the growth performance, whole body composition and apparent digestibility coefficient of juve-nile turbot. Based on these findings, the level of fishmeal substitution with compound proteins should be less than 35%.
turbot; fishmeal; compound protein; growth; whole body composition; apparent digestibility coefficient
公益性(农业)行业科研专项(201303053)资助
2014-03-02;
2014-04-30
董 纯(1986-),女,硕士生,研究方向:水生动物生理学。
** 通讯作者: E-mail:hegen@ouc.edu.cn
S963.3
A
1672-5174(2015)04-027-08
10.16441/j.cnki.hdxb.20140049
