应激性抑郁样行为发生中眶额叶5-HT1A受体对谷氨酸和γ-氨基丁酸的调节*
2015-02-02李江娜安书成
李江娜 安书成 李 珍
(陕西师范大学生命科学学院,西安 710119)
1 问题提出
抑郁症是一种比较普遍,且危害极大的心理与精神障碍性疾病。单胺类递质失调学说是研究抑郁症发生机制及治疗的主要理论之一。基于此而被广泛使用的一些抗抑郁药虽能快速增加突触间隙单胺类递质的浓度,但由于其抗抑郁起效时间延迟,使得单胺类递质失调假说不足以解释抑郁发生的全部现象。单胺类递质及其受体,尤其是5-羟色胺(5-hydroxytryptamine,5-HT)及其受体失调可能是抑郁发生的基本原因(Carr &Lucki,2011)。而大脑谷氨酸(glutamic acid,Glu)能兴奋性系统与 γ-氨基丁酸(gamma-amino-butyric acid,GABA)能抑制性系统的失调可能是抑郁症发生的重要原因(Sanacora et al.,2004;何婷,乔卉,安书成,2011,11月)。因此,研究单胺类递质及其受体对 Glu兴奋性系统与GABA抑制性系统的调节作用,对探讨抑郁症的发生机制可能更有意义。
5-羟色胺能系统失调与抑郁症发生关系密切。研究发现,抑郁的发生与 5-HT能突触传递失调有关(Cubala &Landowski,2006),海马注射5-HT具有抗应激性抑郁的作用(Luo,An,&Zhang,2008)。激活中缝核的5-HT1A受体,能够降低大鼠强迫游泳不动时间(Almeida,Trovo,Tokumoto,Pereira,&Padovan,2013),眶额叶5-HT经5-HT1A受体发挥抗抑郁作用(Li,An,&An,2009)。也有研究发现,抑郁大鼠海马5-HT1A受体的结合位点数量明显下降,经电针治疗后可显著提高其数量(康汇婷,王朝伟,2010)。激活突触后膜上的5-HT1A受体能够改善慢性应激引起的抑郁样行为(Zhou et al.,2014)。抗抑郁药治疗后,突触后膜5-HT1A受体的信号传导能力增强(Savitz,Lucki,&Drevets,2009)。由此可见,5-HT及其1A受体在抑郁发生和抗抑郁治疗中具有重要作用。新一代的单胺能抗抑郁药,如选择性5-HT重摄取抑制剂(selective serotonin reuptake inhibitors,SSRIs)及具有双重作用的 5-HT/NE重摄取抑制剂,虽能改善抑郁病人的治疗效果和生活质量,却具有一定的临床局限性,表现在抗抑郁作用起效慢,引起认知障碍等副作用。临床试验表明,30%~40%的单相抑郁病人对基于单胺类递质的抗抑郁药没有反应(Kornstein &Schneider,2001);超过90%的双向抑郁患者会经历反复的狂躁发作(Sachs,2003)。越来越多的研究显示,氨基酸能系统在抑郁症病理生理和治疗中起着重要作用(Kendell,Krystal,&Sanacora,2005)。Cai等(2013)研究提示,抑郁发生可能由于5-HT对 Glu能兴奋性突触调节异常,使兴奋性突触过度激活所致。
Glu和GABA分别是中枢神经系统中主要的兴奋性递质和抑制性递质,二者的协调对于维持正常情感状态及行为表现至关重要,其中任何一种变化而使二者失调,可引起中枢神经系统多种疾病的发生(Kumar,Sharma,Kumar,&Deshmukh,2013)。研究显示,Glu、GABA在抑郁症中的变化不一致。重度抑郁患者血浆中GABA水平显著下降,Glu水平明显升高;经抗抑郁药物治疗后,能够反转此效果(Kücükibrahimoğlu et al.,2009)。与对照组相比,急性创伤应激后,大鼠前额叶中Glu、GABA均无显著变化,海马中 Glu、GABA虽均升高,但 Glu/GABA比值升高(Gao et al.,2014)。慢性应激动物模型研究表明,建模组大鼠内侧前额叶和海马Glu水平显著升高,GABA含量明显下降(唐亚梅等,2013)。应激引起眶额叶、海马Glu水平升高,而谷氨酸NMDA受体拮抗剂 MK-801具有抗应激性抑郁的作用(吴帅,安书成,陈慧彬,李菲,2014;余伶,安书成,廉婷,2010)。可见,Glu、GABA含量异常导致兴奋性系统和抑制系统失衡,是抑郁发生的原因之一。近10年来,Glu在抑郁治疗中的作用逐渐引起人们的注意,主要是因为其非竞争性 NMDA受体拮抗剂氯胺酮具有快速而相对持久的抗抑郁效果(Kendell et al.,2005)。氯胺酮的抗抑郁作用能够被5-HT耗竭而终止(Gigliucci et al.,2013)。表明其抗抑郁效果可能是 5-HT依赖的。因此,对于抑郁症的治疗策略可能需要将单胺能系统与Glu能系统共同思考。对 GABA的研究显示,中枢 GABA水平异常及GABA受体功能障碍与抑郁等多种神经和精神疾病发生有关(Quandt,Höfner,&Wanner,2013)。越来越多的研究表明,中枢 GABA功能缺陷参与抑郁症的发生(Frisardi,Panza,&Farooqui,2011)。研究发现,抑郁症患者前额叶皮层GAD65和GAD67水平均下调,GABA浓度显著降低,电休克疗法和抗抑郁药物均可使GABA水平恢复正常。此外,抑郁症患者眶额叶皮层GABA能神经元密度降低(Lussier,Romay-Tallón,Caruncho,&Kalynchuk,2013)。有报道认为,GABA对慢性应激性抑郁大鼠的认知功能有改善作用,与抗抑郁剂联用,这种改善效应更显著(姜英凤,2012)。选择性GABA再摄取抑制剂噻加宾也具有改善抑郁和焦虑的作用(高尚锋,2012)。这些结果提示,GABA神经递质参与抑郁症的发生。
5-HT对GABA具有调节作用。抑郁患者5-HT缺乏会导致GABA能功能紊乱,SSRIs通过使5-HT受体脱敏上调5-HT水平,从而改变5-HT能系统对GABA能系统的调节作用,可能是其抗抑郁的途径之一(Zhong &Yan,2004)。新型抗抑郁药Vortioxetine属于SSRIs的一种,能够通过5-HT系统调节GABA能神经传递(Pehrson,Li,Haddjeri,Gulinello,&Sanchez,2013)。应激与行为研究发现,前额叶5-HT参与调控杏仁核 GABA能神经元,束缚应激引起基底外侧杏仁核 GABA释放增加,而选择性耗竭内侧前额叶5-HT,能够减弱应激引起的GABA变化(Andolina,Maran,Valzania,Conversi,&Puglisi-Allegra,2013)。这些研究提示,在应激引起的行为变化中5-HT能系统对GABA传递具有调控作用。5-HT1A受体参与腹外侧眶额叶引起的镇痛作用,而GABA型受体拮抗剂能够减缓5-HT1A受体介导的此效果(Huo,Qu,Li,Tang,&Jia,2008)。提示,OFC区5-HT1A受体与GABA能神经元有一定关系。
眶额叶(orbitofrontal cortex,OFC)是前额叶的一个重要亚区,它与人的情绪、认知等高级脑功能密切相关。Taylor等(2007)研究发现,抑郁症患者OFC体积减小,结构和功能发生改变(Zhang,Chen,Jia,&Gong,2014)。谷氨酸能神经元在OFC大量分布,ɣ-氨基丁酸能神经元为OFC的中间神经元。中缝核内的5-HT能神经元投射到OFC,且在谷氨酸能神经元及中间神经元上分布有5-HT1A受体(Huo et al.,2009;Simpson,Lubman,Slater,&Deakin,1996)。研究表明,应激后大鼠OFC区 Glu含量升高(吴帅等,2014),眶额叶5-HT经5-HT1A受体发挥抗抑郁作用(Li et al.,2009)。
综上所述,Glu水平过高或GABA水平的变化是应激诱发抑郁的重要原因之一。应激会引起OFC区Glu水平升高,眶额叶5-HT经1A受体能发挥抗抑郁作用,然而,应激性抑郁样行为发生中眶额叶5-HT水平有何变化,是5-HT减少,还是其水平不能很好地调节Glu或GABA,5-HT是否通过对Glu和 GABA能神经元调节而发挥抗抑郁作用,以及调节的受体途径等并不清楚。
为了解决以上问题,本研究主要通过建立慢性不可预见性温和应激(chronic unpredictable mild stress,CUMS)动物模型,采用微量注射的方法分别向OFC注射5-HT1A受体激动剂8-OH-DPAT和拮抗剂 WAY100635,通过行为学检测,并结合高效液相色谱法(high-performance liquid chromatography,HPLC)检测OFC区5-HT、Glu和GABA的含量,以此来探讨慢性应激性抑郁发生中OFC区5-HT 1A受体与Glu、GABA之间的关系。
2 材料和方法
2.1 实验动物及分组
健康成年雄性Sprague-Dawley (SD)大鼠32只(250~300 g,约90日龄),由西安交通大学医学院提供。实验前动物 4~5只一笼,自由进食饮水,适应环境一周。随后将动物随机分为四组:A组为正常对照组(Control),正常饲养,在实验的第 1、7、14和21天,双侧OFC均微量注射生理盐水各1 µl;B组为CUMS模型组,即CUMS处理的同时,在上述时间内双侧OFC均微量注射生理盐水各1 µl;C组为 WAY100635组,即正常饲养,在相同的时间里双侧OFC均微量注射5-HT1A受体拮抗剂WAY100635各 1 µl (30 nmol) (问黎敏,安书成,刘慧,2012);D组为 CUMS+8-OH-DPAT组,即 CUMS 处理的同时,在同一时间双侧OFC均微量注射5-HT1A受体激动剂 8-OH-DPAT各 1 µl (10 nmol) (问黎敏等,2012)。A~D 组均进行行为学测试,并采用 HPLC检测OFC 5-HT、Glu和GABA水平。
2.2 实验试剂和仪器
8-OH-DPAT (5-HT1A 受体选择性激动剂)、WAY100635 (5-HT1A受体选择性拮抗剂)均为美国sigma-Aldrich公司产品;5-HT标准品(sigma-Aldrich公司);Glu、GABA标准品(sigma公司);2,4二硝基氟苯(DNFB) (sigma公司);甲醇、乙腈(美国Fisher公司);脑立体定位仪(KOPF型)为美国Stoelting公司产品;旷场实验Video Mot 2黑白多目标动物行为监测分析系统(302050-BWM)为德国TSE公司;微量注射器(1 µl)为上海安亭微量进样器厂产品。高效液相色谱仪为日本SHIMADZU公司产品及自动进样器、UV检测器和色谱数据处理系统。
2.3 实验方法
2.3.1 脑立体定位及OFC微量注射
用2%的戊巴比妥钠40 mg/Kg腹腔注射麻醉大鼠,参照大鼠脑图谱(Paxinos &Watson,1998)进行脑立体定位(见图1中A2),在OFC区(AP 3.7 mm;RL 1.4 mm;H 5.0 mm)之上1.5 mm处植入两根直径为0.9 mm,长度为1.4 cm的不锈钢套管。脑内注射采用微量注射器(1 µl)匀速给药1 min,停针1 min防止药物溢出。注射时注射器伸出套管外1.5 mm,到达OFC。所用药物均用生理盐水溶解稀释至所需浓度。在实验的第1、7、14和21天,对各组大鼠OFC进行微量注射药物。实验结束后,进行定位检测,见图1中A1,不准确者剔除。具体方法见(慈蕾,安书成,2007)。
2.3.2 CUMS模型建立
共9种刺激,3种强刺激(夹尾1 min,4℃冰泳5 min,45℃热泳5 min),6种弱刺激(禁水24 h、禁食24 h、潮湿24 h、水平摇晃5 min、昼夜颠倒12 h、倾斜24 h)随机安排在21天内,每天一种刺激,同种刺激不能连续出现,避免动物出现适应。建模21天后,进行行为学检测。
2.4 行为学检测
各组动物的行为学基线测试结果显示,行为基线稳定而正常,组间无显著性差异。
2.4.1 糖水测试(吴帅等,2014)
实验前在安静的房间内训练动物含糖饮水,每只笼内放置同样体积的水瓶。第一个24 h,两瓶均装有 1%的蔗糖溶液(称为两糖适应);第二个 24 h,两瓶液体量相等,一瓶为清水,一瓶仍为 1%的蔗糖溶液(称为一糖一水适应);第三个 24 h,禁水;第四个24 h,进行糖水消耗测试,量取等量的一瓶1%的蔗糖溶液,一瓶清水(一糖一水)进行测试。24 h后测量糖水消耗量,即同时轻轻取下两瓶,用量筒分别测量各自剩余量,计算出清水和糖水各自消耗量,并计算动物糖水偏爱[糖水偏爱率(%)=糖水消耗量/总液体消耗量×100%]。
2.4.2 旷场实验
测试当天,先将动物置于测试环境中适应至少半个小时。实验开始时,将大鼠放置在一个四周和底面均涂黑的无盖方箱(60 cm × 60 cm × 40 cm)箱底中心。用Video Mot 2黑白多目标动物行为监测系统记录其5 min的活动情况。主要指标:动物的水平穿格次数、直立次数、理毛次数,以此计算大鼠的水平运动得分、竖直运动得分及修饰得分。每只大鼠观察结束后,都要清理箱内残留物,酒精去除异味,防止影响其它鼠的测试结果。

图1 OFC药物注射点(A1)及其示意图(A2)注:A和A’箭头所指的点分别代表左右两侧打药的位置Fig.1 The injection point sites within OFC (A1) and its schematic diagram (A2).The spot of arrowA and A’ point to the left and right locations of injecting drug,respectively.
2.4.3 悬尾实验(问黎敏等,2012)
实验在悬尾暗箱中进行,距大鼠尾根部 1/3处用医用胶布固定于悬尾箱内,记录大鼠悬尾后,5 min内的不动时间。
2.5 HPLC实验(刘慧,问黎敏,乔卉,安书成,2013)
配制不同浓度的 5-HT、Glu和GABA标准品溶液,测其峰面积,绘制标准曲线。制备OFC组织样品,检测其峰面积。根据标准曲线将样品的峰面积换算为其浓度。5-HT进样条件为,A水相:柠檬酸-乙酸钠缓冲体系(PH=3.8);B有机相(甲醇),使用荧光检测器进行检测,发射波长330 nm,激发波长280 nm。Glu、GABA进样条件,A水相:醋酸钠缓冲液(PH=6);B有机相(乙腈水V/V=1:1),进行梯度洗脱,使用紫外检测器进行检测,检测波长为360 nm。
2.6 数据处理
实验数据均以平均值±标准误(Mean±SEM
)表示,采用 SPSS 20.0软件进行数据分析,组间差异检验用单因素方差分析(one-wayANOVA
),组间多重比较行LSD检验。p
<0.05时认为差异具有统计学意义,p
<0.01表示有极显著性差异。3 实验结果
3.1 行为学结果
3.1.1 糖水偏爱率

3.1.2 旷场实验


图2 各组大鼠总液体消耗(A)与糖水偏爱率(B)Fig.2 Effects of different treatment on the total fluid intake (A) and the sucrose preference (B).Results are expressed as the means± SEM (n =7~8).## p <0.01 vs Control group (n =8);** p <0.01 vs CUMS group (n =8).
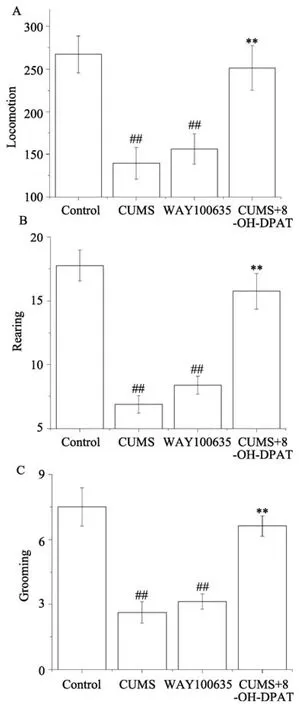
图3 各组大鼠旷场行为表现Fig.3 Effects of different treatments on locomotion (A),rearing (B) and grooming (C) in the open field test.Results are expressed as the means±SEM (n =8).## p <0.01 vs Control group (n =8);** p <0.01 vs CUMS group (n =8).
3.1.3 悬尾实验结果

3.2 高效液相色谱实验结果
3.2.1 5-HT含量变化
测定5-HT标准品溶液峰面积,确定5-HT的出峰时间为9.275 min,绘制标准曲线。检测其线性范围为:2 ng/mL~300 ng/mL;其线性回归方程为:Y=25867X + 72385 (R
=0.9996)。四组大鼠5-HT含量没有显著性差异(p
=0.588 >0.05)。见图5C。3.2.2 Glu、GABA含量变化

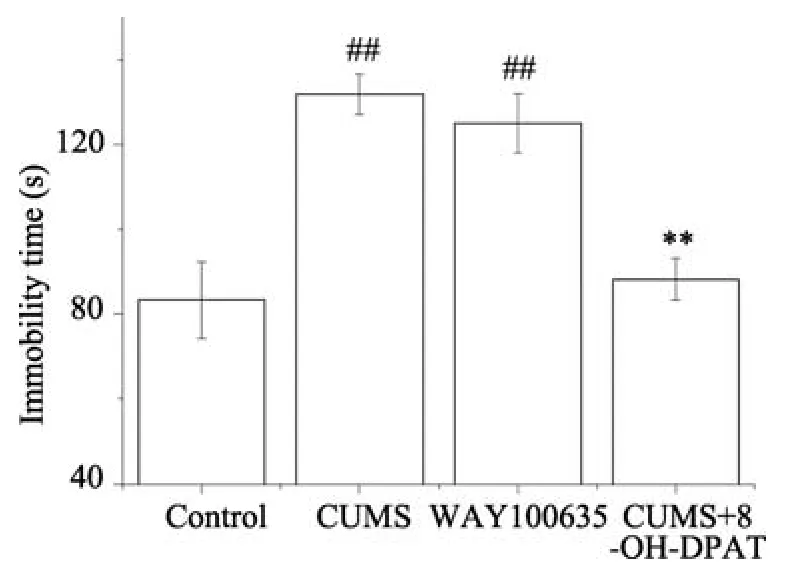
图4 各组大鼠在悬尾实验中的不动时间Fig.4 Effects of different treatments on immobility time in the tail suspension test.Results are expressed as the means±SEM (n=8).## p<0.01 vs Control group (n=8);** p <0.01 vs CUMS group (n=8).

图5 各组大鼠OFC区Glu (A)、GABA (B)和5-HT(C)浓度Fig.5 Effects of different treatments on orbital frontal cortex Glu (A),GABA (B) and 5-HT (C) concentrations.Results are expressed as the means± SEM (n =8).## p <0.01 vs Control group (n =8);* p <0.05 vs CUMS group (n =8).
4 讨论
慢性不可预见性温和应激抑郁动物模型因能模拟出人类抑郁的核心症状,而被广泛采纳。本研究建立的 CUMS模型,动物表现出糖水偏爱率下降,运动缓慢,探究能力下降,自我关注程度下降以及行为绝望度提高,所测的各项行为学指标均能代表抑郁的典型症状。
单胺类递质尤其是5-HT失调是抑郁症发生的经典假说。有研究报道,慢性不可预见性应激能够显著降低中缝核5-HT能神经元的自发放电活动及自发放电神经元的数目,从而产生抑郁样行为(Bambico,Nguyen,&Gobbi,2009)。Drevets等(2007)研究发现,与健康人相比,抑郁症病人颞叶内侧区及中缝核5-HT1A受体的结合力及该受体的功能均下降。而长期注射5-HT1A受体激动剂能够缓解抑郁以及因抑郁引起的痛觉迟钝(Jiang,Qi,Wang,&Luo,2014)。 然而,另有研究发现,抑郁症中5-HT的含量并未发生显著性变化(Laugeray et al.,2010;Venzala,García-García,Elizalde,&Tordera,2013),但外源性给予5-HT却具有抗抑郁作用(Luo et al.,2008),其原因到底是 5-HT水平降低引起抑郁,还是抑郁产生是因 5-HT水平不能满足需要所致,具体机制并不清楚。而本实验结果显示,给予慢性不可预见性温和应激之后,大鼠表现出抑郁样行为,但其OFC中5-HT的含量并未发生显著性变化。然而,当在OFC外源性微量注射5-HT1A受体激动剂8-OH-DPAT后,大鼠的抑郁样行为得到显著改善,主要表现为糖水偏爱率显著提高及悬尾不动时间显著减少。反之,当正常大鼠外源性给予 5-HT1A受体拮抗剂 WAY100635后,大鼠糖水偏爱率显著降低,悬尾不动时间显著增加。这表明,尽管应激引起抑郁样行为发生时OFC区5-HT水平并未降低,但5-HT1A受体的激活可以起到抗抑郁作用,而阻断该受体能够使大鼠产生抑郁样行为。以上结果与之前的研究报道相一致(Li et al.,2009;Laugeray et al.,2010;Venzala et al.,2013)。由此推测,应激性抑郁发生的主要原因可能不是OFC区5-HT含量的变化,而很有可能是应激时 5-HT水平不能满足需要所致。当然,也有应激时突触后膜5-HT1A受体脱敏所致(Mahar,Bambico,Mechawar,&Nobrega,2014)的可能。
抑郁症病人 OFC形态异常在神经影像学中被广泛报道。与健康对照组相比,重度抑郁症患者OFC灰质体积明显减小(Lacerda et al.,2004)。尸检结果发现,抑郁症病人大脑 OFC中神经元及神经胶质细胞的密度减少,内侧眶额叶体积显著减小(Bremner et al.,2002)。由此可见,OFC在抑郁症的发生中具有重要作用。OFC主要接受来自中缝背核5-HT能神经元投射(Roberts,2011),5-HT1A受体在OFC及其它边缘系统和中脑中缝核分布密度最高(Muller &Jacobs,2009),其在OFC主要作为突触后膜异源性受体发挥作用(Stein,Miczek,Lucion,&de Almeida,2013)。免疫组化和原位杂交结果表明,大鼠(Santana,Bortolozzi,Serrats,Mengod,&Artigas,2004)、人和猴(de Almeida &Mengod,2008)的前额叶锥体神经元和腹外侧眶额叶 GABA能神经元(Huo et al.,2009)上均存在5-HT1A受体。也有研究发现,慢性不可预见性应激通过改变5-HT1A受体功能而可能影响内侧前额叶Glu能突触传递(Mahar et al.,2014)。由此可见,OFC中 5-HT调节Glu及GABA能神经元具有解剖学基础。
众多研究表明,Glu能突触传递异常在抑郁症的病理机制及治疗中起着重要的作用(Hashimoto,2009;Musazzi,Treccani,Mallei,&Popoli,2013)。与健康对照组相比,抑郁症病人脑脊液(Levine et al.,2000)、额叶(Hashimoto,Sawa,&Iyo,2007)及眶额叶(吴帅等,2014)中Glu水平均显著增加。而抗抑郁药能够减少Glu释放及其突触传递(Musazzi et al.,2013)。本实验结果也显示,CUMS大鼠OFC区Glu含量显著高于正常对照组,大鼠表现出抑郁样行为。为了证明应激引起眶额叶Glu水平升高是否与5-HT1A受体有关,本实验通过微量注射抗抑郁药5-HT1A受体激动剂 8-OH-DPAT后,CUMS大鼠OFC区Glu水平显著降低,且能显著改善大鼠的抑郁样行为。此外,临床前研究发现,非竞争性的NMDA受体拮抗剂氯胺酮能够产生快速、持久的抗抑郁效果,但此抗抑郁效果会因 5-HT的耗竭而被终止(Gigliucci et al.,2013)。Cai Xiang等研究提出,抑郁发生可能由于5-HT对Glu能兴奋性突触调节异常,使兴奋性突触过度激活所致(Cai et al.,2013)。而兴奋性突触的过度激活,很有可能打破了 OFC区Glu和GABA原有的平衡,导致抑郁。另有研究报道,在治疗抑郁症及其相关的认知紊乱中,5-HT可通过5-HT1A受体对Glu进行调节(Ciranna,2006;Pehrson &Sanchez,2014)。慢性不可预见性应激改变5-HT1A受体功能,可能影响内侧前额叶Glu能突触传递(Mahar et al.,2014)。本实验结果还显示,微量注射5-HT1A受体拮抗剂WAY100635后,正常大鼠OFC区 Glu含量显著升高,动物也表现出抑郁样行为。以上研究结果均表明,5-HT1A受体参与了对Glu水平的调节,而且慢性应激诱发抑郁样行为,可能存在着5-HT1A受体对Glu水平控制失调,使得Glu水平过高所致。然而,尽管有研究提出腹外侧眶额叶GABA能神经元(Huo et al.,2009)上存在5-HT1A受体,但在本研究整个实验的各个项目中,并未发现眶额叶 GABA含量的变化。该结果提示,OFC区 5-HT可能主要是通过 Glu能神经元上的5-HT1A受体抑制 Glu水平过度升高,而未明显影响眶额叶GABA能神经元,这可能与相对于Glu能神经元上5-HT1A受体,GABA能神经元上5-HT1A受体敏感度较低有关(Pehrson &Sanchez,2014)。
综上所述,CUMS在引发大鼠抑郁样行为的同时,并未降低OFC中5-HT的水平,GABA水平也没有明显变化,而是显著升高Glu含量。外源性注射 5-HT1A受体激动剂产生抗抑郁作用的同时,OFC中Glu水平显著降低。以上结果提示,应激性抑郁样行为发生,可能是 5-HT水平不能起到对Glu能神经元的有效调控,因而使OFC区Glu水平过高所致。但也有应激使OFC区Glu能神经元上5-HT1A受体功能降低,导致其对 Glu释放的抑制作用减弱,从而使Glu水平异常升高的可能。研究结果对解释基于单胺类递质的抗抑郁药物延迟现象也可能具有一定的启示。
Almeida,P.V.G.,Trovo,M.C.,Tokumoto,A.M.,Pereira,A.C.,&Padovan,C.M.(2013).Role of serotonin 1A receptors in the median raphe nucleus on the behavioral consequences of forced swim stress.Journal of Psychopharmacology,27
(12),1134–1140.Andolina,D.,Maran,D.,Valzania,A.,Conversi,D.,&Puglisi-Allegra,S.(2013).Prefrontal/amygdalar system determines stress coping behavior through 5-HT/GABA connection.Neuropsychopharmacology,38
(10),2057–2067.Bambico,F.R.,Nguyen,N.-T.,&Gobbi,G.(2009).Decline in serotonergic firing activity and desensitization of 5-HT1A autoreceptors after chronic unpredictable stress.European Neuropsychopharmacology,19
(3),215–228.Bremner,J.D.,Vythilingam,M.,Vermetten,E.,Nazeer,A.,Adil,J.,Khan,S.,...Charney,D.S.(2002).Reduced volume of orbitofrontal cortex in major depression.Biological Psychiatry,51
(4),273–279.Cai,X.,Kallarackal,A.J.,Kvarta,M.D.,Goluskin,S.,Gaylor,K.,Bailey,A.M.,...Thompson,S.M.(2013).Local potentiation of excitatory synapses by serotonin and its alteration in rodent models of depression.Nature Neuroscience,16
(4),464–472.Carr,G.V.,&Lucki,I.(2011).The role of serotonin receptor subtypes in treating depression: A review of animal studies.Psychopharmacology,213
(2–3),265–287.Ci,L.,&An,S.C.(2007).Effect and mechanisms of dopamine in orbitofrontal cortex on the regulation of gastric motility.Journal of Shaanxi Normal University(Natural Science Edition),35
(1),99–102.[慈蕾,安书成.(2007).眶额叶区多巴胺对胃运动的影响及其机制研究.陕西师范大学学报(自然科学版),35(1),99–102.]
Ciranna,L.(2006).Serotonin as a modulator of glutamateand GABA-mediated neurotransmission: implications in physiological functions and in pathology.Current Neuropharmacology,4
(2),101–114.Cubala,W.J.,&Landowski,J.(2006).Serotoninergic system and limbic-hypothalamic-pituitary-adrenal axis (LHPA axis)in depression.Psychiatria Polska,40
(3),415–430.de Almeida,J.,&Mengod,G.(2008).Serotonin 1A receptors in human and monkey prefrontal cortex are mainly expressed in pyramidal neurons and in a GABAergic interneuron subpopulation: Implications for schizophrenia and its treatment.Journal of Neurochemistry,107
(2),488–496.Drevets,W.C.,Thase,M.E.,Moses-Kolko,E.L.,Price,J.,Frank,E.,Kupfer,D.J.,&Mathis,C.(2007).Serotonin-1A receptor imaging in recurrent depression: Replication and literature review.Nuclear Medicine and Biology,34
(7),865–877.Frisardi,V.,Panza,F.,&Farooqui,A.A.(2011).Late-life depression and Alzheimer's disease: The glutamatergic system inside of this mirror relationship.Brain Research Reviews,67
(1–2),344–355.Gao,J.,Wang,H.,Liu,Y.,Li,Y.Y.,Chen,C.,Liu,L.M.,...Yang,C.(2014).Glutamate and GABA imbalance promotes neuronal apoptosis in hippocampus after stress.Medical Science Monitor,20
,499–512.Gao,S.F.(2012).The pathological significance of GABA and NO in depressive patients and stress models
(Unpublished doctorial dissertation).Zhejiang University,Hangzhou.[高尚锋.(2012).神经递质GABA和NO在抑郁症患者和应激模型中的病理意义(博士学位论文).浙江大学,杭州.]
Gigliucci,V.,O'Dowd,G.,Casey,S.,Egan,D.,Gibney,S.,&Harkin,A.(2013).Ketamine elicits sustained antidepressantlike activity via a serotonin-dependent mechanism.Psychopharmacology,228
(1),157–166.Hashimoto,K.(2009).Emerging role of glutamate in the pathophysiology of major depressive disorder.Brain Research Reviews,61
(2),105–123.Hashimoto,K.,Sawa,A.,&Iyo,M.(2007).Increased levels of glutamate in brains from patients with mood disorders.Biological Psychiatry,62
(11),1310–1316.He,T.,Qiao,H.,&An,S.C.(2011,November).Glu,GABA and their receptors in depression induced by chronic unpredictable mild stress.
Paper presented at the meeting of Academic Conferences on Digestion,Endocrine Secretion and Reproduction in 2011,Beihai,Guangxi,China.[何婷,乔卉,安书成.(2011,11月).慢性应激性抑郁发生与海马Glu和GABA水平变化及其受体机制.中国生理学会消化内分泌生殖代谢生理专业委员会2011年消化内分泌生殖学术会议,中国广西北海.]
Huo,F.Q.,Chen,T.,Lv,B.C.,Wang,J.,Zhang,T.,Qu,C.L.,...Tang,J.S.(2009).Synaptic connections between GABAergic elements and serotonergic terminals or projecting neurons in the ventrolateral orbital cortex.Cerebral Cortex,19
(6),1263–1272.Huo,F.Q.,Qu,C.L.,Li,Y.Q.,Tang,J.S.,&Jia,H.(2008).GABAergic modulation is involved in the ventrolateral orbital cortex 5-HTreceptor activation-induced antinociception in the rat.Pain,139
(2),398–405.Jiang,Y.F.(2012).The effects of Shuyu capsule and bupleurum extract serum on GABAR-mediated signal pathway of ERK and AC/cAMP/CREB in primary cultured rat hippocampus neurons
(Unpublished master’s thesis).Shandong University of Traditional Chinese Medicine,Jinan.[姜英凤.(2012).舒郁胶囊及柴胡提取物含药血清对大鼠海马神经元GABAR介导的ERK通路和AC/cAMP/CREB通路的影响(硕士学位论文).山东中医药大学,济南.]
Jiang,Z.C.,Qi,W.J.,Wang,J.Y.,&Luo,F.(2014).Chronic administration of 5-HT1A receptor agonist relieves depression and depression-induced hypoalgesia.Scientific World Journal,2014
,405736.Kang,H.T.,&Wang,C.W.(2010).Effect of electro-acupuncture on 5-HTreceptor of hippocampus of the rat model with chronic stress depression.Henan Traditional Chinese Medicine,30
(1),38–40.[康汇婷,王朝伟.(2010).电针对慢性应激抑郁模型大鼠海马5-HT受体的影响.河南中医,30(1),38–40.]
Kendell,S.F.,Krystal,J.H.,&Sanacora,G.(2005).GABA and glutamate systems as therapeutic targets in depression and mood disorders.Expert Opinion on Therapeutic Targets,9
(1),153–168.Kornstein,S.G.,&Schneider,R.K.(2001).Clinical features of treatment-resistant depression.The Journal of Clinical Psychiatry,62
(Suppl.16),18–25.Küçükibrahimoğlu,E.,Saygın,M.Z.,Çalişkan,M.,Kaplan,O.K.,Ünsal,C.,&Gören,M.Z.(2009).The change in plasma GABA,glutamine and glutamate levels in fluoxetine- or S-citalopram-treated female patients with major depression.European Journal of Clinical Pharmacology,65
(6),571–577.Kumar,K.,Sharma,S.,Kumar,P.,&Deshmukh,R.(2013).Therapeutic potential of GABAreceptor ligands in drug addictio-n,anxiety,depression and other CNS disorders.Pharmacology Biochemistry and Behavior,110
,174–184.Lacerda,A.L.T.,Keshavan,M.S.,Hardan,A.Y.,Yorbik,O.,Brambilla,P.,Sassi,R.B.,...Soares,J.C.(2004).Anatomic evaluation of the orbitofrontal cortex in major depressive disorder.Biological Psychiatry,55
(4),353–358.Laugeray,A.,Launay,J.-M.,Callebert,J.,Surget,A.,Belzung,C.,&Barone,P.R.(2010).Peripheral and cerebral metabolic abnormalities of the tryptophan-kynurenine pathway in a murine model of major depression.Behavioural Brain Research,210
(1),84–91.Levine,J.,Panchalingam,K.,Rapoport,A.,Gershon,S.,McClure,R.J.,&Pettegrew,J.W.(2000).Increased cerebrospinal fluid glutamine levels in depressed patients.Biological Psychiatry,47
(7),586–593.Li,H.P.,An,F.L.,&An,S.C.(2009).Orbitofrontal cortex action of 5-hydroxytryptamine and its receptor in an acute forced swimming stress-induced depression model.Neural Regeneration Research,4
(7),530–535.Liu,H.,Wen,L.M.,Qiao,H.,&An,S.C.(2013).Modulation of hippocampal glutamate and NMDA/AMPA receptor by homocysteine in chronic unpredictable mild stress-induced rat depression.Acta Physiologica Sinica,65
(1),61–71.
[刘慧,问黎敏,乔卉,安书成.(2013).高半胱氨酸对慢性应激性抑郁大鼠海马谷氨酸及其受体的调节.生理学报,65(1),61–71.]
Luo,D.D.,An,S.C.,&Zhang,X.(2008).Involvement of hippocampal serotonin and neuropeptide Y in depression induced by chronic unpredicted mild stress.Brain Research Bulletin,77
(1),8–12.Lussier,A.L.,Romay-Tallón,R.,Caruncho,H.J.,&Kalynchuk,L.E.(2013).Altered GABAergic and glutamatergic activity within the rat hippocampus and amygdala in rats subjected to repeated corticosterone administration but not restraint stress.Neuroscience,231
,38–48.Mahar,I.,Bambico,F.R.,Mechawar,N.,&Nobrega,J.N.(2014).Stress,serotonin,and hippocampal neurogenesis in relation to depression and antidepressant effects.Neuroscience and Biobehavioral Reviews,38
,173–192.Muller,C.P.,&Jacobs,B.(2009).Handbook of the behavioral neurobiology of serotonin
(Vol.21).Amsterdam: Academic Press.Musazzi,L.,Treccani,G.,Mallei,A.,&Popoli,M.(2013).The action of antidepressants on the glutamate system:Regulation of glutamate release and glutamate receptors.Biological Psychiatry,73
(12),1180–1188.Paxinos,G.,&Watson,C.(1998).The rat brain in stereotaxic coordinates
(4th ed.).San Diego: Academic Press.Pehrson,A.,Li,Y.,Haddjeri,N.,Gulinello,M.,&Sanchez,C.(2013).P.1.g.014 Vortioxetine,a novel multimodal antidepressant,modulates GABA and glutamate neurotransmission via serotonergic mechanisms.European Neuropsychopharmacology,23
(Suppl.2),S196-S197.Pehrson,A.L.,&Sanchez,C.(2014).Serotonergic modulation of glutamate neurotransmission as a strategy for treating depression and cognitive dysfunction.CNS spectrums,19
(2),121–133.Quandt,G.,Höfner,G.,&Wanner,K.T.(2013).Synthesis and evaluation of N-substituted nipecotic acid derivatives with an unsymmetrical bis-aromatic residue attached to a vinyl ether spacer as potential GABA uptake inhibitors.Bioorganic&Medicinal Chemistry,21
(11),3363–3378.Roberts,A.C.(2011).The importance of serotonin for orbitofrontal function.Biological Psychiatry,69
(12),1185–1191.Sachs,G.S.(2003).Unmet clinical needs in bipolar disorder.Journal of Clinical Psychopharmacology,23
(3 Suppl.1),S2-S8.Sanacora,G.,Gueorguieva,R.,Epperson,C.N.,Wu,Y.T.,Appel,M.,Rothman,D.L.,… Mason,G.F.(2004).Subtype-specific alterations of γ-aminobutyric acid and glutamate in patients with major depression.Archives of General Psychiatry,61
(7),705–713.Santana,N.,Bortolozzi,A.,Serrats,J.,Mengod,G.,&Artigas,F.(2004).Expression of serotoninand serotoninreceptors in pyramidal and GABAergic neurons of the rat prefrontal cortex.Cerebral Cortex,14
(10),1100–1109.Savitz,J.,Lucki,I.,&Drevets,W.C.(2009).5-HTreceptor function in major depressive disorder.Progress in Neurobiology,88
(1),17–31.Simpson,M.D.C.,Lubman,D.I.,Slater,P.,&Deakin,J.F.W.(1996).Autoradiography with [H]8-OH-DPAT reveals increases in 5-HTreceptors in ventral prefrontal cortex in schizophrenia.Biological Psychiatry,39
(11),919–928.Stein,D.J.,Miczek,K.A.,Lucion,A.B.,&de Almeida,R.M.M.(2013).Aggression-reducing effects of F15599,a novel sele-ctive 5-HTreceptor agonist,after microinjection into the ventral orbital prefrontal cortex,but not in infralimbic cortex in male mice.Psychopharmacology,230
(3),375–387.Tang,Y.M.,Zhao,H.S.,Qin,L.X.,Zhang,R.S.,Chen,R.H.,&Liu,Y.(2013).Glutamate and GABA in brain tissue of chronic unpredicted mild stress-induced depression rats.Guangdong Medical Journal,34
(20),3098–3101.[唐亚梅,赵宏深,秦立新,张仁生,陈若虹,刘勇.(2013).慢性轻度不可预见性应激抑郁模型大鼠脑组织谷氨酸和γ-氨基丁酸浓度的变化.广东医学,34(20),3098–3101.]
Taylor,W.D.,Macfall,J.R.,Payne,M.E.,McQuoid,D.R.,Steffens,D.C.,Provenzale,J.M.,&Krishnan,K.R.(2007).Orbitofrontal cortex volume in late life depression:Influence of hyperintense lesions and genetic polymorphisms.Psychological Medicine,37
(12),1763–1773.Venzala,E.,García-García,A.L.,Elizalde,N.,&Tordera,R.M.(2013).Socialvs
.environmental stress models of depression from a behavioural and neurochemical approach.European Neuropsychopharmacology,23
(7),697–708.Wen,L.M.,An,S.C.,&Liu,H.(2012).Role of hippocampal 5-HTreceptor and its modulation to NMDA receptor and AMPA receptor in depression induced by chronic unpredictable mild stress.Acta Psychologica Sinica,44
(10),1318–1328.[问黎敏,安书成,刘慧.(2012).应激性抑郁样行为发生中海马 5-羟色胺 1A受体的作用及其对 NMDA受体和AMPA受体的调节.心理学报,44(10),1318–1328.]
Wu,S.,An,S.C.,Chen,H.B.,&Li,F.(2014).Orbital frontal cortex D1 dopamine receptor modulate glutamate and NMDA receptor in depression induced by chronic unpredictable mild stress.Acta Psychologica Sinica,46
(1),1–10.[吴帅,安书成,陈慧彬,李菲.(2014).慢性应激性抑郁发生中大鼠眶额叶多巴胺D1受体对谷氨酸及其NMDA受体的调节.心理学报,46(1),1–10.]
Yu,L.,An,S.C.,&Lian,T.(2010).Involvement of hippocampal NMDA receptor and neuropeptide Y in depression induced by chronic unpredictable mild stress.Acta Physiologica Sinica,62
(1),14–22.[余伶,安书成,廉婷.(2010).海马NMDA受体与神经肽Y在慢性应激性抑郁发生中的作用及其关系.生理学报,62(1),14–22.]
Zhang,H.W.,Chen,Z.Q.,Jia,Z.Y.,&Gong,Q.Y.(2014).Dysfunction of neural circuitry in depressive patients with suicidal behaviors: A review of structural and functional neuroimaging studies.Progress in Neuro-Psychopharmacology and Biological Psychiatry,53
,61–66.Zhong,P.,&Yan,Z.(2004).Chronic antidepressant treatment alters serotonergic regulation of GABA transmission in prefrontal cortical pyramidal neurons.Neuroscience,129
(1),65–73.Zhou,J.S.,Cao,X.,Mar,A.C.,Ding,Y.Q.,Wang,X.P.,Li,Q.,&Li,L.J.(2014).Activation of postsynaptic 5-HTreceptors improve stress adaptation.Psychopharmacology,231
(10),2067–2075.