Synthesis and characterization of a layered organic-inorganic hybrid(C4H9NH3)2NiCl4
2014-10-11ZHAOFeiyanGAOYanyang
ZHAO Feiyan,GAO Yanyang
(School of Science,North University of China,Taiyuan030051,Shanxi,China)
Layered organic-inorganic hybrids with molecular formula(CnH2n+1NH3)2MX4(M represents divalent metal ions and X represents halide ions)feature a very ordered two-dimensional structure which consists of corner-sharing octahedral anion([MX6]4-)inorganic layer,alternating with the organic cations(CnH2n+1NH+3)bilayer,and they are increasingly attracting attention[1].Nowadays,enormous attention has been paid to layered organic-inorganic hybrids,not only due to their unique layered structure but also because of their useful and potential properties in electronics,magnetism and optics[2-8].However,few reports are currently available about(CnH2n+1NH3)2NiCl4layered organic-inorganic hybrids,because this type of hybrid material cannot be obtained by liquid method,the most useful access to synthesizing this type of organic-inorganic hybrid[9].As an exception,GUO et al[10]successfully obtained(CnH2n+1NH3)2MX4(M =Cu,Zn,Co,Mn,Fe andn=2,4,6,8,10,12)by liquid method,but they failed to obtain the series of Ni-containing hybrids.
In this paper,we report the synthesis of(C4H9NH3)2NiCl4(1)by a solid-state chemistry route involving the reaction of nickel chloride with a short chain alkyl ammonium salt.
1 Experimental
1.1 Materials and physical measurement
C4H9NH2·HCl powder was prepared according to previous literature[11]and confirmed by infrared(IR)spectrometric analysis,the other reagents were obtained from commercial resources and used without further purification.Elemental analyses(C,H and N)were performed with a Germany Elementar Vario ELⅢelemental analyzer.X-ray diffraction(XRD)patterns were taken with a Germany Bruker D8-ADVANCE X-ray diffractometer equipped with Cu-Kαradiation(λ=0.154 18nm).An IR-8400Sspectrometer(Shimadzu Corporation,Japan)was performed to record the IR spectrum of as-synthesized product palletized with KBr over the wavenumber range from 4 000to 400cm-1.Thermogravimetric(TG)analyses were carried out with a ZCT-A TG/DTA system (Beijing High-tech Instrument Company,China).
1.2 Synthesis of 1
NiCl2·6H2O (0.36g,1.51mmol)and C4H9NH2·HCl(0.33g,3.03mmol)were separately ground and then mixed intimately in an agate mortar.The aqua solid mixture was transferred to a petridish and heated at 80℃for 6h.Upon completion of heating,the powder was ground again and heated at 150℃for additional 3h.Anal.Calcd for C8H24N2Cl4Ni(%):C,27.35,H,6.88,N,8.03.Found(%):C,27.46,H,6.63,N,8.11.
2 Results and discussion
2.1 XRD patterns
Fig.1shows the XRD patterns of NiCl2·6H2O,C4H9NH2·HCl and hybrid1.It is seen that the crystal structure of1is different from that of the raw materials.Namely,hybrid1shows regular diffraction peaks at 2θ=5.5°,8.2°,11.0°,13.4°,16.4°,19.6°,25.3°.These diffraction peaks correspond to the([h00],h=2,3,4,5,6,7,9)reflections and indicate that hybrid1has a layered structure.The presence of these high ordered[h00]peaks suggests that hybrid1is well stacked along thea-axis and the alternating organic-inorganic layers parallel to substrate surface.The value of interlayer spacingdis calculated to be 3.220 7nm from the diffraction peaks.
2.2 IR spectrum
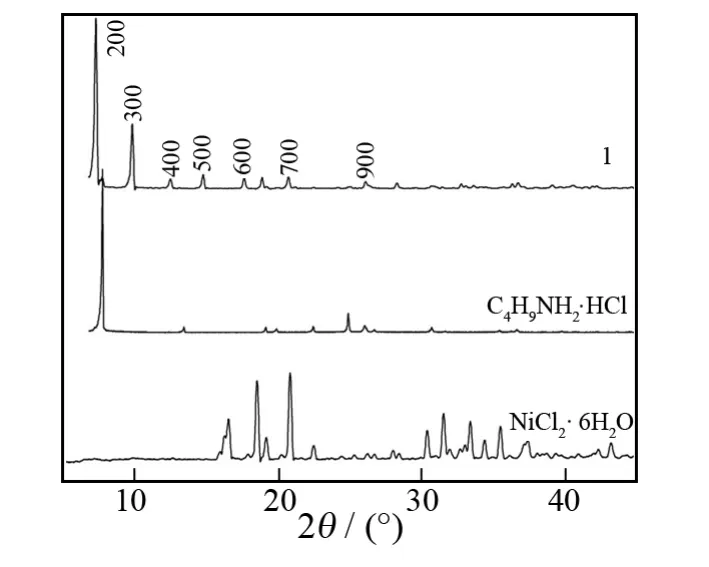
Fig.1 XRD patterns of NiCl2·6H2O,C4H9NH2·HCl and hybrid 1
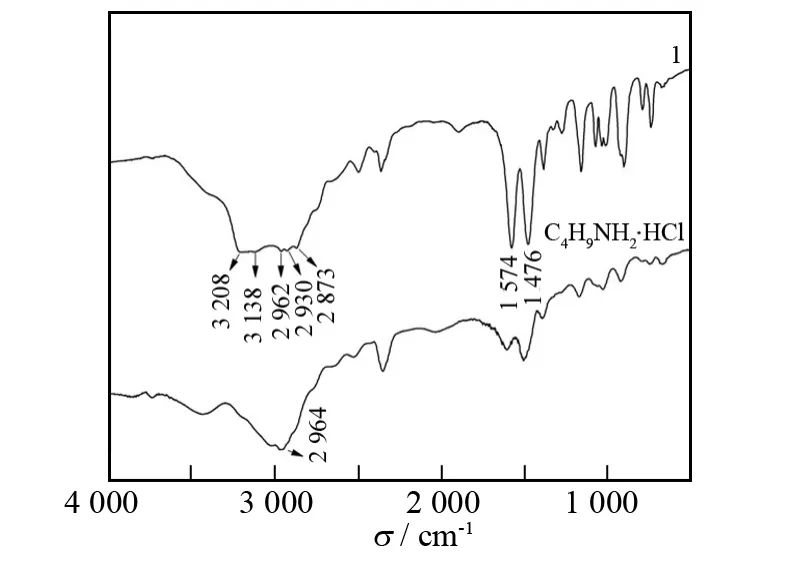
Fig.2 IR spectra of C4H9NH2·HCl and hybrid 1
Fig.2shows the IR spectra of starting material C4H9NH2·HCl and hybrid1,respectively.It is seen that the major characteristic vibrations of hybrid1emerge over the wavenumber range from 3 100to 3 300 cm-1.The strong peaks at 3 208and 3 138cm-1are attributed to the asymmetric/symmetric N-H stretching vibration.These peaks of NH+3groups of1shift to higher wavenumber(3 208and 3 138cm-1)as compared to that of C4H9NH2·HCl(2 964cm-1)but they are lower than that of butylamine (3 332 cm-1)[12],which can be ascribed to the change of chemical environment of NH+3groups after they self-assemble into the layered perovskite structure.This suggests that N-H…Cl hydrogen bonds have formed in hybrid1,and the intensity of such hydrogen bonds in hybrid1is stronger than that of C4H9NH2·HCl but weaker than that of butylamine.
In Fig.2,the locations and assignments of other peaks are:2 962(νas,-CH3),2 930(νas,-CH2-),2 873(νa,-CH3),1 574(δ,-NH3)and 1 476(δ,-CH3)cm-1.
2.3 TG curve
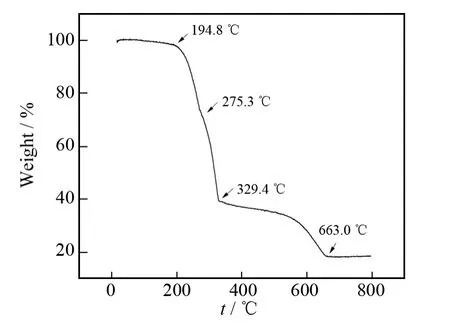
Fig.3 TG curve of hybrid 1
Fig.3shows the TG curve of hybrid1under air atmosphere.Minor weight loss(2.06%)is observed in the TG scan at a temperature below 194.8℃,which is due to the evaporation of moisture absorbed by hybrid1.Generally,hybrid1shows two obvious stepwise mass losses.The first drastic weight loss which occurs at 194.8 ℃ and stops at 329.4 ℃is attributed to the loss of C4H9NH2and HCl from hybrid1,which corresponds to a weight loss of 60.65%and agrees exactly with the organic component (theoretical value of 62.81%)in hybrid1.When the temperature scope is between 329.4and 663.0℃,NiCl2begins to discompose and is oxidized into nickelic oxide partly.The final amount of residue is 18.77%.
[1]WORTHAM E,ZORKO A,ARCON D,et al.Organic-inorganic perovskites for magnetic nanocomposites[J].Phys B,2002,318:387-391.
[2]TABUCHI Y,ASAI K,RIKAKUWA M,et al.Preparation and characterization of natural low dimensional layered perovsike-type compounds[J].J Phys Chem Solids,2000,61:837-845.
[3]MITZI D B.Templating and structural engineering in organic-inorganic perovskites[J].Dalton Trans,2001(1):1-12.
[4]NOBUAKI K,MASAMI A,WATANABE Y.Excitons in organic-inorganic hybrid compound(CnH2n+1NH3)2PbBr4(n=4,5,7and 12)[J].Thin Solid Films,2010,518:3199-3203.
[5]LIU Yao,LIU Peipei,MENG Jian.Synthesis,crystal structure and optical properties of a novel organic-inorganic hybrid material(C9H14N)2PbCl4[J].Solid State Sci,2011,13:1036-1040.
[6]KITARAWA N,AONO M,WATANABE Y.Synthesis and luminescence properties of lead-halide based organic-inorganic layered perovskite compounds(CnH2n+1NH3)2PbI4[J].J Phys Chem Solids,2011,72:1467-1471.
[7]CHONDROUDIS K,MITZI D B,BROCK P.Effect of thermal annealing on the optical and morphological properties of(AETH)PbX4(X=Br,I)perovskite films prepared using single source thermal ablation[J].Chem Mater,2000,12:169-175.
[8]MITZI D B.Synthesis,structure,and properties of organic-inorganic perovskites and related material[M].Washington,D C:John Wiley &Sons,1999.
[9]MIN Yulin,XIA Hongyu,GAO Suning,et al.Simple method to synthesize Ni-tryptophan layered hybrid complexes and their luminescence properties[J].Solid State Sci,2012,14:1040-1044.
[10]GUO Liling,XU Shengdong,ZHAO Guanghui,et al.The structural significance of the interlayer distances of the layered hybrids(CnH2n+1NH3)2MCl4[J].J Phys Chem Solids,2012,73:688-695.
[11]CHENG Ziyong,HAN Yanchun.Layered organic-inorganic perovskite-type materials fabricated by spray pyrolysis route[J].J Cryst Growth,2005,285:352-357.
[12]XIAO Zelong,CHEN Hongzheng,SHI Minmin,et al.Preparation and characterization of organic-inorganic hybrid perovskite(C4H9NH3)2CuCl4[J].Mater Sci Eng B,2005,117:313-316.
