Synthesis and crystal structure of an organic-inorganic composite phosphotungstate[HTA]3[α-PW12O40]·6H2O
2014-10-11QIXiuliZHAOHaozheZHANGJingliCHENLijuan
QI Xiuli ,ZHAO Haozhe,ZHANG Jingli ,CHEN Lijuan*
(1.College of Chemistry and Chemical Engineering,Henan University,Kaifeng475004,Henan,China;2.Basic Experimental Teaching Center,Henan University,Kaifeng475004,Henan,China)
Polyoxometalates(POMs),especially those with organic groups,are very attractive and extremely versatile inorganic building blocks,due to their structural diversity and potential applications ranging from photochemistry,catalysis,electromagnetism,sorption,analytical chemistry to materials science[1-5].This is why the preparation of inorganic-organic hybrid POM-based materials has increasingly gained attention.In the past two decades,a number of inorganic-organic POM derivatives have been synthesized and characterized[6-7],and most of them are based on Keggin-type polyoxotungstate anions such as [PW12O40]3-,[BW12O40]5-,[CoW12O40]6-and[SiW12O40]4-.For example,in 2004,NIU and co-workers reported the preparation,properties and single crystal structure of a two-dimensional extended borotungstate(dmaH)2[Nd(dmf)4(H2O)][α-BW12O40]·H2O[8].In 2007,two inorganic-organic hybrid frameworks constructed from Keggin tungstocobaltate units and cobalt-organic complexes,K[Co(phen)2(H2O)]2[HCoW12O40]·2H2O (phen = 1,10-phenanthroline)and [Co(2,2′-bipy)3]1.5{[Co(2,2′-bipy)2(H2O)][HCoW12O40]·0.5H2O (bipy= bipyridine),were discovered[9].In 2009,WANG et al synthesized a supramolecular compound(C10H8N2)3.2·H3PW12O4025.6H2O and investigated its oxidative degradation in the presence of chitosan and H2O2[10].Very recently,SHAMS′s group fabricated a carbon paste electrode with an inorganic-organic hybrid(NB)2H2SiW12O40(NB= Nile blue)and evaluated its electrocatalytic activity toward nitrite reduction[11].Herein,we report the synthesis of an organic-inorganic composite phosphotungstate[HTA]3[α-PW12O40]·6H2O (CCDC:971209)viathe reaction of Na3[α-PW12O40]·6H2O and 1,2,4-triazole as well as its structural characterization.Our focus is placed on the strong hydrogen-bonding and electrostatic interactions between[α-PW12O40]3-anion and monoprotonated[HTA]+cation of the target product belonging to rhombohedral space groupR-3as well as the beautiful trigonal arrangement of[α-PW12O40]3-anions whose interspaces are filled with free monoprotonated[HTA]+cations.
1 Experimental
1.1 Reagents and physical measurements
Na3[α-PW12O40]·6H2O was prepared according to reference[12].All other reagents used for synthesis were purchased from commercial resources and used without further purification.Elemental analyses were performed with a Perkin-Elmer 240Celemental analyzer.Infrared(IR)spectra(KBr pellets)were recorded with a Nicolet 170SXFT-IR spectrometer in the range of 4 000-400cm-1.
1.2 Synthesis of[HTA]3[PW12O40]·6H2O
Na3[α-PW12O40]·6H2O (0.791g,0.259mmol)was added to a solution of 10mL water containing CuCl2·2H2O (0.206g,1.205mmol),L-serine(0.064g,0.610mmol)and 1,2,4-triazole(0.058g,0.841 mmol).Then 5mL ethanol was slowly added into the solution.Resulting mixture was stirred for 2hand then heated in a water bath(80℃)for 2h.Upon completion of heating,as-obtained blue solution was cooled,filtered,and slowly evaporated at room temperature.Several days later,colorless transparent block crystals suitable for X-ray diffraction were collected.Anal calcd(%)for C6H24PW12N9O46:C,2.26;H,0.76;N,3.95.Found(%):C,2.38;H,0.88;N,3.86.Although CuCl2·2H2O andL-serine were used as reactants in the present research,they were not observed in the final product.The parallel experiments indicate that[HTA]3[PW12O40]·6H2O cannot be obtained in the absence of CuCl2·2H2O andL-serine,which suggests that they might play a synergistic role with other materials in the reaction.Similar phenomena have been encountered previously[13].
1.3 X-ray crystallography
A good single crystal of the title compound with the dimension of 0.37mm×0.35mm×0.34mm was selected under an optical microscope and glued at the tip of a thin glass fiber with cyanoacrylate adhesive.Intensity data were collected with Bruker APEX-II X-ray diffractometer(XRD)equipped with a graphitemonochromated Mo-Kαradiation(λ=0.071 073nm)at 296(2)K.A total of 13 168(3 722uique,Rint=0.072 7)reflections were measured(-22≤h≤23,-20≤k≤23,-32≤l≤26).The structure of the title compound was solved by direct methods and refined by the full-matrix least-squares method onF2using the SHELXTL 97program package[14].All of the non-hydrogen atoms were refined anisotropically.All hydrogen atoms attached to C and N atoms were placed in idealized positions and refined with a riding model using default SHELXL parameters.Those hydrogen atoms attached to lattice water molecules were not located.Crystal data and structural refinements of the title compound are given in Table 1.Selected bond lengths are listed in Table 2.
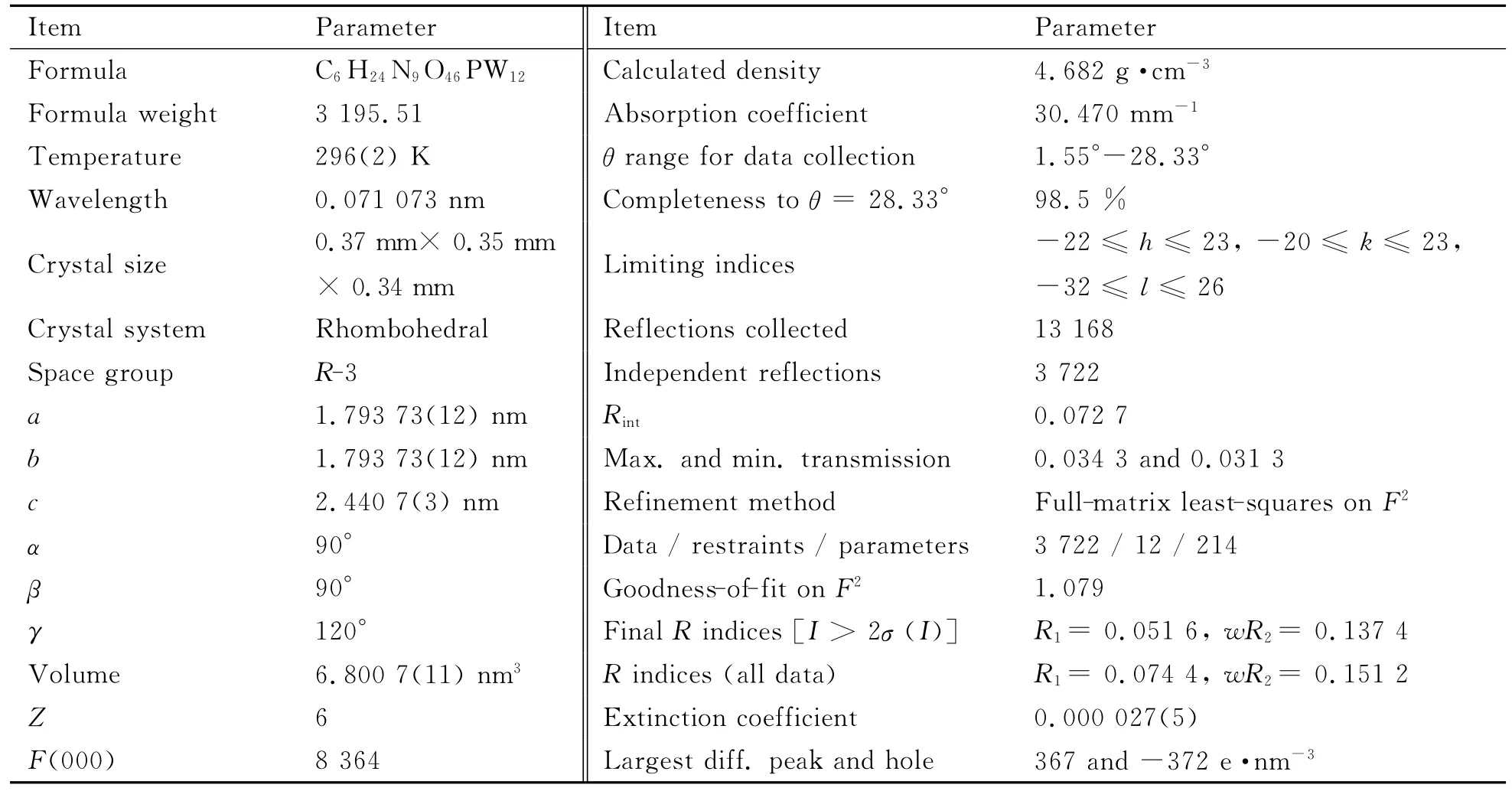
Table 1 Crystallographic data and structural refinements of the title compound

Table 2 Selected bond lengths of the title compound
2 Results and discussion
2.1 IR spectrum
The IR spectrum of the title compound(Fig.1)has been recorded in the wavenumber range of 4 000-400cm-1,which is very useful for the identification of characteristic vibration bands of the saturated Keggin polyoxoanion and organic components.The characteristic vibration patterns derived fromα-Keggintype polyoxoanion framework are observed in 1 100-700cm-1.The obvious characteristic bands at 980,893,and 799cm-1are attributed to theν(W-Od),ν(W-Ob),andν(W-Oc)stretching vibration,respectively[15].The vibration resonances at 1 088cm-1and 1 081cm-1are assigned to theν(P-Oa)stretching vibration.In addition,theν(C-H)stretching band emerges at about 3 142cm-1,and theν(C-N)stretching band appears at 1 406cm-1;whereas the characteristic peaks centered at 1 617and 1 579cm-1are attributed to the bending vibration ofν(O-H)andν(C-H),respectively.The vibration band at 3 548cm-1is assigned to theν(O-H)stretching vibration of water molecules.The appearance of these characteristic signals confirms the presence of[HTA]+cation and lattice water molecules,being in good agreement with relevant single-crystal structural analysis results.
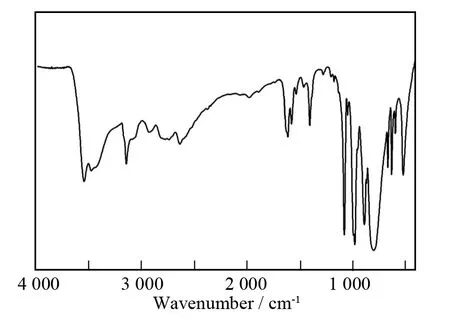
Fig.1 IR spectrum of the title compound
2.2 Crystal structure
X-ray single-crystal diffraction indicates that the title complex consists of one saturated Keggin-type phosphotungstate[α-PW12O40]3-anion,three free protonated[HTA]+cations and six lattice water molecules(Fig.2).Three free protonated[HTA]+cations are distributed around the saturated Keggin phosphotungstate [α-PW12O40]3-anion with an inter-[HTA]+cation angle of 120°to compensate the surface negative charge of Keggin-type polyanion.Besides,there exist strong hydrogen-bonding and electrostatic interactions between[α-PW12O40]3-anions and protonated[HTA]+cations;and the heteropolyanion in the title compound still retains well-known Keggin structure with theTdpoint symmetry.P atom in the PO4tetrahedron is located in the center of the[α-PW12O40]3-polyanion;and twelve W atoms can be divided into four groups of W3O13trimers.Four W3O13trimers are connected together by corner-sharing and edge-sharing mode with the aid of the central PO4tetrahedron.In each W3O13trimer,three WO6octahedra are combined together in the form of corner-sharing fashion.The P-O distances are in the range of 0.151 6(2)-0.153 7(11)nm,and they are consistent with the results of previous study[16].The W-O distances vary from 0.167 1(13)to 0.245 1(11)nm.These results indicate that the PO4tetrahedron and WO6octahedra are highly distorted.
Because the title compound crystallizes in the rhombohedral space groupR-3,a beautiful trigonal packing of[α-PW12O40]3-anions are formed,in which free monoprotonated[HTA]+cations occupy the gaps surrounded by[α-PW12O40]3-anions.In addition,from the viewpoint of supramelocular chemistry,supramolecular interactions also exist in the title compound.Namely,there exist hydrogen-bond interactions between the nitrogen atoms of[HTA]+ligands and the surface oxygen atoms of Keggin-type phosphotungstate[α-PW12O40]3-anion(the distance of N(1)-H(1B)…O(13)is 0.310(2)nm),which helps to enhance the chemical stability of the title compound(Fig.3).
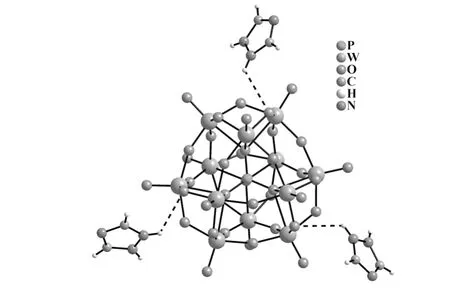
Fig.2 View of the molecular structure of the title compound.All lattice water molecules are omitted for clarity
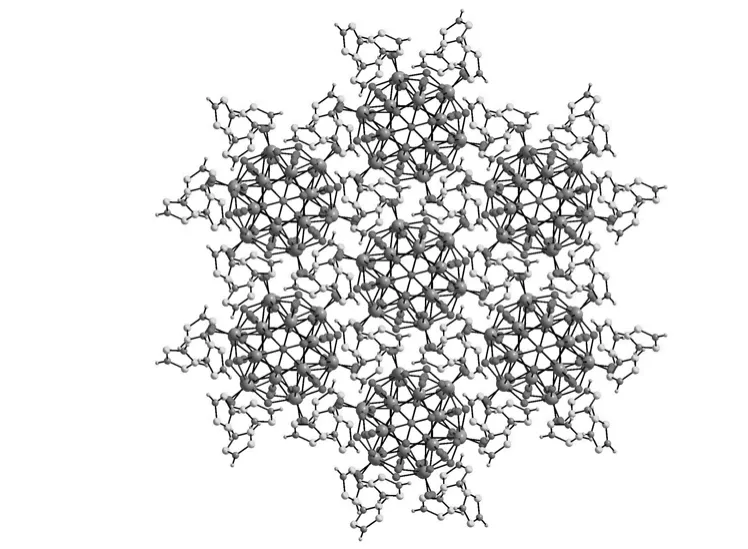
Fig.3 Three dimensional supramolecular architecture of the title compound
[1]POPE M T.Heteropoly and isopoly oxometalates[M].Berlin:Springer,1983.
[2]SADAKANE M,STECKHAN E.Electrochemical properties of polyoxometalates as electrocatalysts[J].Chem Rev,1998,98:219-238.
[3]KORTZ U,JEANNIN Y P,TÉZÉA,et al.A novel dimeric Ni-substitutedβ-Keggin silicotungstate:structure and magnetic properties of K12[{β-SiNi2W10O36(OH)2(H2O)}2]·20H2O [J].Inorg Chem,1999,38:3670-3675.
[4]赵俊伟,陈利娟,王敬平.[4,4′-bpyH2]5H2[α-P2W18O62]2·4H2O的合成与结构表征[J].化学研究,2008,19(4):14-17.
[5]BANERJEE A,BASSIL B S,RÖSCHENTHALER G V,et al.Diphosphates and diphosphonates in polyoxometalate chemistry[J].Chem Soc Rev,2012,41:7590-7604.
[6]PRADEEP C P,MISDRAHI M F,LI F Y,et al.Synthesis of modular“inorganic-organic-inorganic”polyoxometalates and their assembly into vesicles[J].Angew Chem Int Ed,2009,48:8309-8313.
[7]YANG D H,Li S Z,Ma P T,et al.Carboxylate-functionalized phosphomolybdates:ligand-directed conformations[J].Inorg Chem,2013,52:8987-8992.
[8]NIU J Y,ZHAO J W,WANG J P,et al.Synthesis,property and crystal structure of a novel two-dimensional network organic-inorganic hybrid compound based on the neodymiumIIIcenter and Keggin-type heteropolyanion of[α-BW12O40]5-[J].J Mol Struct,2004,699:85-92.
[9]SHA J Q,PENG J,CHEN J,et al.Two novel Keggin tungstocobaltates grafted by cobaltIIcomplex group(s):K[Co(phen)2(H2O)]2[HCoW12O40]·2H2O and[Co(2,2′-bipy)3]1.5{[Co(2,2′-bipy)2(H2O)][HCoW12O40]}·0.5H2O [J].Solid State Sci,2007,11(9):1012-1019.
[10]WANG Q S,WANG S M,LIN S.A novel supramolecular compound 2,2′-bipyridyl-phosphotungstic acid:synthesis and catalysis[J].Carbohydr Res,2009,344:679-682.
[11]KAKHKI S,SHAMS E,BARSAN M M.Fabrication of carbon paste electrode containing a new inorganic organic hybrid based on[SiW12O40]4-polyoxoanion and Nile blue and its electrocatalytic activity toward nitrite reduction[J].J Electroanal Chem,2013,704:80-85.
[12]ROCCHICCIOLI-DELTCHEFF C,FOURNIER M,FRANK R,et al.Vibrational investigations of polyoxometalates.2.Evidence for anion-anion interactions in molybdenum(VI)and tungsten(VI)compounds related to the Keggin structure[J].Inorg Chem,1983,22:207-216.
[13]陈利娟,元 静,罗 婕,等.硅钨酸盐[H2bpy][H2bbpy][α-SiW12O40]·2H2O的合成与结构表征[J].化学研究,2013,24(4):365-369.
[14]SHELDRICK G M.SHELXL 97,Program for crystal structure refinement[CP].University of Göttingen,Germany,1997.
[15]马鹏涛,庆伟霞,张少伟.一种含稀土阳离子的多金属氧酸盐二聚体的合成及晶体结构 [J].化学研究,2013,24(4):370-374.
[16]NIU J Y,WANG J P.Introduction of heteropoly compound[M].Henan University Press,Kaifeng,2000:169.
