Synthesis and characterization of a dysprosium-organic framework incorporating 2-hydroxyl-6-methyl-isonicotinic acid and oxalate mixed ligands
2014-09-02TIANAiqinFENGXunWANGCailuLANGXuemingYANGSong
TIAN Aiqin, FENG Xun, WANG Cailu, LANG Xueming, YANG Song
(1. Basic Courses Education Department, Henan Forestry Vocational College, Luoyang 471002, Henan, China; 2. College of Chemistry and Chemical Engineering, Luoyang Normal University, Luoyang 471022, Henan, China; 3. School of Life Science and Technology, Nanyang Normal University, Nanyang 473601, Henan, China)
Synthesisandcharacterizationofadysprosium-organicframeworkincorporating2-hydroxyl-6-methyl-isonicotinicacidandoxalatemixedligands
TIAN Aiqin1,2, FENG Xun2*, WANG Cailu3, LANG Xueming2, YANG Song2
(1.BasicCoursesEducationDepartment,HenanForestryVocationalCollege,Luoyang471002,Henan,China; 2.CollegeofChemistryandChemicalEngineering,LuoyangNormalUniversity,Luoyang471022,Henan,China; 3.SchoolofLifeScienceandTechnology,NanyangNormalUniversity,Nanyang473601,Henan,China)
A new lanthanide-organic framework incorporating both substituted isonicotinic acid and oxalate coligand has been obtained through solvo-thermal reaction, namely, {[Dy(μ2-H2minca) (μ2-C2O4)·2H2O]·H2O}n, (H3minca = 2-hydroxyl-6-methyl-isonicotinic acid). The title complex exhibits two dimensional (2D) corrugated networks with 63topology, in which {DyO8} polyhedron are linked through carboxylate and oxalate into a wavelike sheet. The luminescence measurement shows that the title complex exhibits characteristic f-f transition emission in visible region. Thermogravimetric and differential thermal analysis measurement indicates that the title complex displays high thermal stability. Variable-temperature magnetic susceptibility measurements indicate that there may exist ferromagnetic magnetic interactions between adjacent Dy (III) centers.
X-ray single structure; dysprosium (III) complex; magnetic property; fluorescence property
In recent years, the fluorescent properties of lanthanide complexes have been the subject of numerous research activities, especially due to their potential applications in areas of science as diverse as electroluminescent devices, MRI imaging agents or bioprobes for immunoassays[1]. Numerous lanthanide complexes have been studied in detail through light absorption by other constituents of the lanthanide complex, with subsequent transfer of energy to the lanthanide ion[1-2]. Even so the judicious selection of ligands and metal ions has remained a challenging issue and has been at the forefront of inorganic chemistry research fields[3-4]. On the other hand, a great many multicarboxylate imidazoline/triazine/pyridine-based ligands have been extensively studied and are found to show various coordination modes along with a variety of structures[5-7]. However, among various strategies, most of the works have focused on the assembly of the d-block metal organic frameworks, and there are few reports on lanthanide transition metal MOFs or lanthanide clusters incorporating both substituted nitrogen heterocyclic carboxylate and small rigid ligands[8]. At the same time, it has also been demonstrated that oxalate, the smallest dicarboxylate, is rigid and coplanar, and it has a small stereo effect which is beneficial for constructing MOFs[9]. Oxalate has been proved to be a good candidate for pillar ligand due to its various bridging abilities to generate from 1D to 3D moderately robust frameworks, exhibiting tunable ferro- or antiferro-magnetic exchanges[10-11]. In addition, the oxalate within lanthanide multi-carboxylate system can reduce or eliminate water molecules from the coordination sphere of the central ions, increasing the luminescent intensity and lifetime[12]. Utilization of heavy lanthanide ions such as Dy(III) becomes more popular in the design and synthesis of single molecule magnets due to their larger angular momentum and strong magnetic anisotropy in the ground multiple states[13]. In order to better understand the role of oxalate in the self-assembly processes and the properties in this system, a new lanthanide-organic framework has been isolated.
1 Experimental
1.1 Materials and physical measurements
All reagents used in the syntheses were of analytical grade and used as received. Elemental analyses for carbon, hydrogen and nitrogen atoms were performed on a Vario EL III elemental analyzer. The infrared spectra (4 000-400 cm1) were recorded by using KBr pellet on an Avatar TM 360 E. S. P. IR spectrometer. Thermogravimetric analyses (TGA) were performed under atmosphere with a heating rate of 10 ℃/min using TGA/SDTA851e. The powder X-ray diffraction (PXRD) patterns were measured using a Bruker D8 Advance powder diffractometer at 40 kV, 40 mA for MoKαradiation (λ= 0.071 073 nm), with a scan speed of 0.2 s/step and a step size of 0.02° (2θ). Luminescence spectra of the complexes were carried out on a Cary Eclipse fluorescence spectrophotometer. Variable-temperature magnetic susceptibilities were measured using a MPMS-7 SQUID magnetometer under a 0.2 T applied magnetic field and over the range of 2-300 K.
1.2 Synthesis of complex {[Dy(μ2-H2minca)](μ2-C2O4)·2H2O]·H2O}n
Ligand H3minca (0.039g, 0.2 mmol) and sodium oxalate dihydrate (0.029 g, 0.2 mmol) in a solution of water/DMF (V/V=2.0, 10 mL) were mixed with an aqueous solution (10 mL) of 0.1 mmol Dy(NO3)3·5H2O (0.043 g). After stirring for 20 min in air, the pH value was adjusted to 5.5 with nitric acid, and the mixture was placed into 25 mL Teflon-lined autoclave under autogenous pressure being heated at 150 ℃ for 72 h, then the autoclave was cooled over a period of 24 h at a rate 5 ℃/h. After filtration, the product was washed with distilled water and dried, light red crystals were obtained suitable for X-ray diffraction analysis. Yield (based on Dy element): 0.021 7 g (46%). Elemental analysis (%): Calc. for C9H12DyNO10: C 23.67, H 2.65, N 3.07; Found: C 23.26, H 2.23, N 2.98. IR (KBr pellet, cm-1): 3 423 br, 3 341 s, 2 934 s, 1 609 s, 1 537 s, 1 413 m, 1 374 s, 1 087 s, 929 vs, 747 m, 668 s, 467 m.
1.3 X-ray structure determination
A single crystal of the title complex was mounted on a Bruker SMART APEX II CCD diffractometer equipped with a graphite-monochromatized Moαradiation (λ= 0.071 073 nm) by using aφ/ωscan mode at room temperature in the range of 2.04° ≤θ≤ 27.50°. Corrections forLpfactors were applied and all non-hydrogen atoms were refined with anisotropic thermal parameters. The structure was solved by direct methods with SHELXS-97[14]. The hydrogen atoms were assigned with common isotropic displacement factors and included in the final refinement by use of geometrical restrains. A full-matrix least-squares refinement onF2was carried out using SHELXL-97[15]. Crystallographic and experimental details are summarized in Table 1. The selected bond lengths and bond angles are listed in Table 2.

Table 1 Crystallographic data and experimental details for title complex

Table 2 Selected bond lengths and angles for the title complex
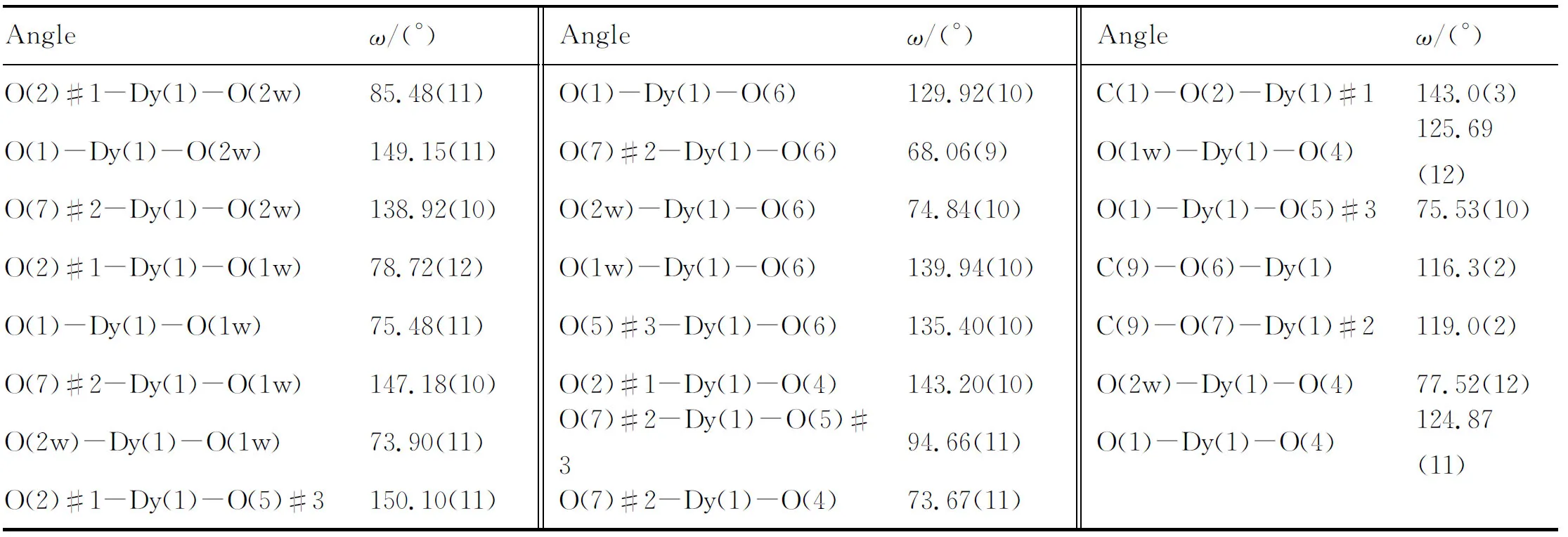
Continued to Table 2
2 Results and discussion
2.1 Crystal structure description
Single X-ray diffraction analysis reveals that the title complex is lanthanide-organic polymers based on discrete binuclear lanthanide carboxylate aggregates and one free water molecule. As illustrated in Fig. 1, the asymmetric unit of the title complex is composed of one Dy(III) cation, two H3minca ligands, two oxalate and two coordinated water molecules, as well as one lattice water molecule. The coordination environment around the Dy(III) cation can be described as a distorted dodecahedron. Each Dy(III) cation is eight-coordinated by two O atoms of H2minca ligands, four O atoms of oxalate and two lattice water O atoms. Interestingly, the pyridine-N and hydroxyl-O do not coordinate to the central ion. In addition, in the complex H2minca ligand acts as a bidentate ligand bridge connecting two Dy cations, which results in one eight-membered ring and two five-membered rings around the Dy(III) cation, as displayed in Fig.1.
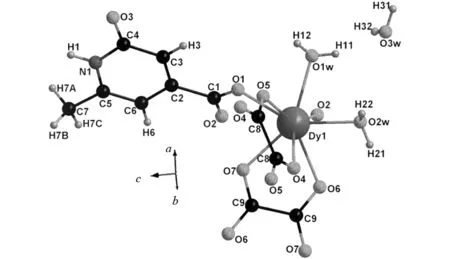

Fig.1 The asymmetry unit (a) and illustration of coordination environments of Dy(III) ion in the title complex(b)
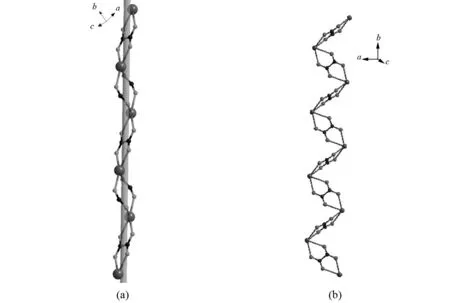
Fig.2 Ball and stick diagram of the 1D chain of Dy(III) cations alternate linked by the H2minca and oxalate ligands (a) and Dy-oxalate zigzag chain (b)

Fig.3 Diamond illustration of the 1D ribbon chain being connected by oxalate along b axis. The H atoms and free water molecules are not included for clarity
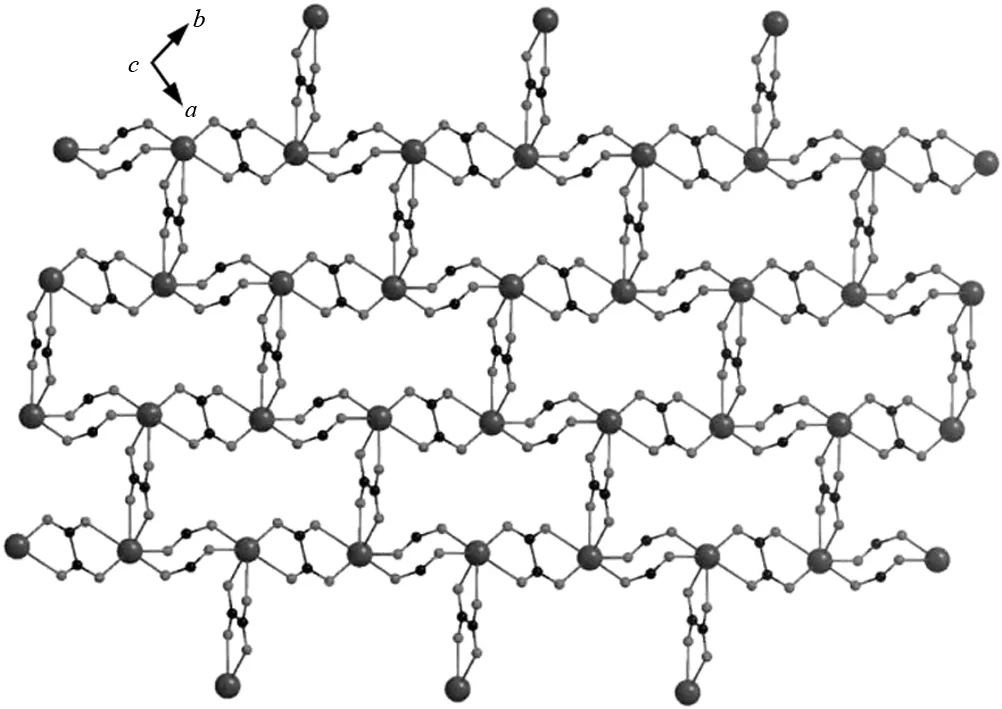
Fig.4 Projective view of 2D corrugated layer consisting of 1D zigzag chain along ab plane
The Dy-O bond lengths involving the central ion are in the range of 0.228 6-0.246 5 nm, and a slightly distorted dodecahedral geometry is also confirmed by the bond angles of O(6)-Dy(1)-O(7) and O(2)-Dy(1)-O(2w), which are found to be 68.07° and 85.49°, respectively. They are closely similar to those observed in several related lanthanide species[16]. The selected bond lengths and angles for the title complex are listed in Table 2 for details. The two carboxylate connected two Dy(III) cations, resulting in an eight-membered chair-like ring with the Dy…Dy separation of 0.516 6 nm, followed by the oxalate connecting two neighboring Dy(III) using a bis-bidentate bridging mode to give rise to an infinite 1D helix chain along the crystallographicbdirection, as displayed in Fig. 2(a). There is another unique bridging oxalate ligand chelating the two adjacent Dy(III) cations in binuclear unitviaits oxygen atoms with the Dy…Dy separation of 0.632 4 nm, connecting these Dy2dimers, giving rise to a 1D ribbon zigzag chain array along the crystallographiczaxis, as described in Fig. 2(b) and Fig. 3. Moreover, the oxalate ligand acts as a linker, connecting alternately 1D chains into an interesting corrugated 2D lamellar lattice sheet alongabplane, as displayed in Fig.4. In this sheet, six Dy(III) cations were alternately connected by four oxalate and two carboxylates from H3minca to form a six-membered ring. However, when the binuclear unit doubly connected through carboxylic group is regarded as a three connected node, the whole structure can be described as a 3-connected corrugated network, with 3, 6hcbtopology[17], and the point symbol is 63, as shown in Fig. 5. Close inspection reveals that the hydrogen bonding interactions (O(1w)-H(12)…O(5)#4:0.281 4(4) nm) between carboxylic group and oxygen atoms of water molecules further interlink the adjacent 2D lamellar sheet into 3D supramolecular network structure.

Fig.5 Illustration of 3-connected hcb topological network
2.2 Powder X-ray diffraction patterns and thermogravimetric analysis
PXRD patterns were performed on crystalline powder of the title complex. The powder XRD patterns of the title complex shows strong peaks at 8.8°, 13.18°, 15.08°, 16.41°, 17.65°, 23.51°, 27.98°, respectively, as illustrated in Fig. 6, which are in consistent with the simulation ones based on single-crystal data analysis, suggesting that the bulk sample of the title complex obtained is in a pure phase.
To examine the thermal stability of this Ln-MOF, TGA were measured on crystalline sample of title complexunder nitrogen atmosphere from room temperature (RT) to 900 ℃. The thermal behavior is reported in Fig. 7. For the title complex, the first weight loss of 13.5 % is observed from 65 to 200 ℃, corresponding to the release of the free water molecule and coordination water molecules. The second weight loss begins above 300 ℃, which is attributed to departure of the oxalate groups with weight loss of 17.3 %. Further weight loss is ascribed to the decomposition of the organic compound and the residual weight is 24.65% at 800 ℃. This indicates that the compound has high thermal stability.

Fig.6 Comparison of simulated and experimental PXRD patterns for the title complex
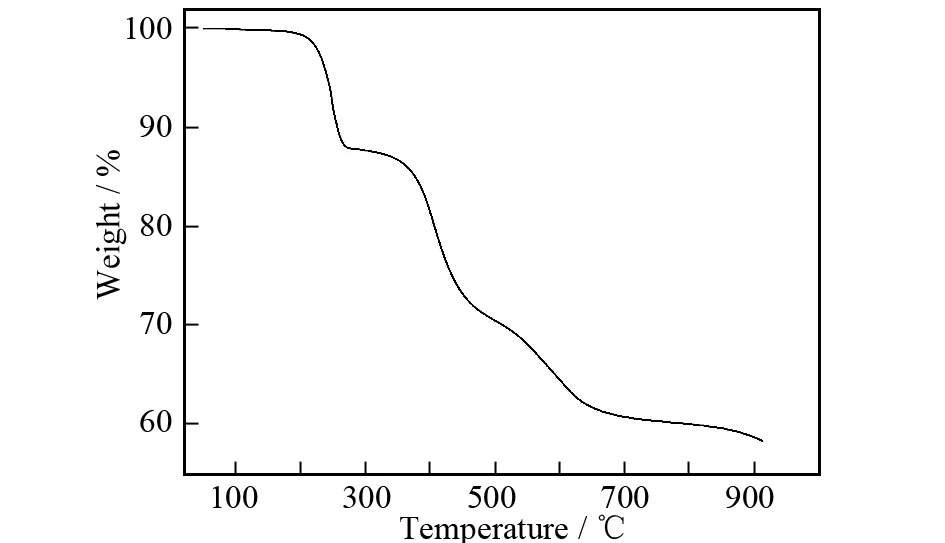
Fig.7 TG curve of the title complex
2.3 Photoluminescence property
The solid state luminescent properties of free H3minca ligand and title compound were investigated at room temperature. As shown in Fig.8(a), the emission spectrum of free H2minca ligand gives the medium strong emission peak at 453 nm at the excited wavelength of 287 nm, which is attributed to the π*→π transition. As illustrated in Fig. 8(b), under the same excitation, the title complex displays intense luminescence with bands peaking at 396, 485, 548 and 577 nm. The two strong emission peaks in visible region are originated from the characteristic transitions of Dy(III) ion, which belong to the transitions of4F9/2→6H15/2(485 nm) and4F9/2→6H13/2(577 nm) of Dy III)[18-19]. The intensity is strong, which indicates that the absorption energy of the ground state of the ligand can be effectively transferred to the excited state of the Dy(III) ion[20].

(a)

(b)
2.4 Magnetic property
As illustrated in Fig.9, the correctedχMTvalue of per formula unit is 13.24 cm3·K·mol-1at 300 K, corresponding well to the expected value 13.27 cm3·K·mol-1, for magnetically insulated Dy(III) ions with a6H15/2ground state withJ= 15/2,g= 4/3[21]. When the temperature is lowered,χMTincreases very slowly to 13.98 cm3·K·mol-1atca. 65 K. This is indicative of possible presence of very weak ferromagnetic coupled interactions between the adjacent two Dy(III) ions bridged by carboxylate at higher temperature. Then it slightly decreases down to 2 K, and reaches a minimum of 11.44 cm3·K·mol-1at the lowest temperature, indicating possible presence of weak antiferromagnetic interactions at low temperature or the depopulation of Stark sublevels together with crystal-affection on consideration of strong spin-orbital coupling in Ln(III)[22].
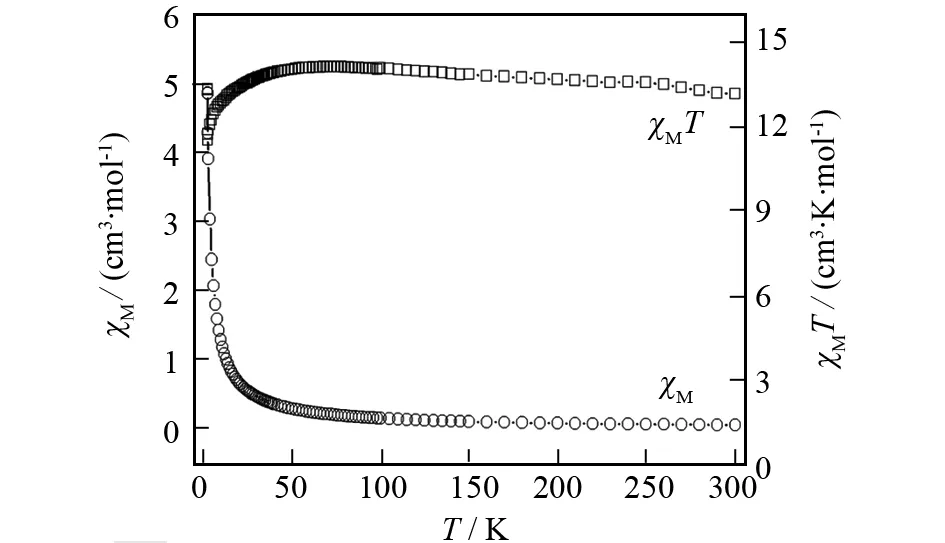
Fig.9 Temperature dependence of χMT and χM for the title complex under an applied field of 0.2 T
[1] BINNEMANS K, LENAERTS P, DRIESEN K, et al. A luminescent tris(2-thenoyltrifluoroacetonato) europium(III) to a 1,10-phenanthroline-functionalised sol-gel glass [J]. J Mater Chem, 2004, 14: 191-195.
[3] GUSTAFSSON M, BARTOSZEWICZ A, MARTIN-MATUTE B, et al. A family of highly stable lanthanide metal-organic frameworks: structural evolution and catalytic activity [J]. Chem Mater, 2010, 22: 3316-3322.
[4] CHANDLER B D, YU J O, CRAMB D T, et al. Series of lanthanide-alkali metal-organic frameworks exhibiting luminescence and permanent microporosity [J]. Chem Mater, 2007, 19: 4467-4473.
[5] CAO R, SUN D F, LIANG Y C, et al, Syntheses and characterizations of three-dimensional channel-like polymeric lanthanide complexes constructed by 1,2,4,5-benzenetetracarboxylic acid [J]. Inorg Chem, 2002, 41: 2087-2094.
[6] FENG X, LING X L, LIU L, et al. A series of 3D lanthanide frameworks constructed from aromatic multi-carboxylate ligand: Structural diversity, luminescence and magnetic properties [J]. Dalton Trans, 2013, 42: 10292-10303.
[7] FENG X, WANG J G, LIU B, et al. From two-dimensional double decker architecture to three-dimensional pcu framework with one-dimensional tube: syntheses, structures, luminescence, and magnetic studies [J]. Cryst Growth Des, 2012, 2: 927-938.
[8] ZHANG Z H, SONG Y, OKAMURA T, et al. Syntheses, structures, near-infrared and visible luminescence, and magnetic properties of lanthanide-organic frameworks with an imidazole-containing flexible ligand [J]. Inorg Chem, 2006, 45: 2896-2902.
[9] LIU M S, YU Q Y, CAI Q Y, et al. One-, two-, and three-dimensional lanthanide complexes constructed from pyridine-2,6-dicarboxylic acid and oxalic acid ligands [J]. Cryst Growth Des, 2008, 8: 4083-4091.
[10] XU G F, WANG Q L, GAMEZ P, et al. A promising new route towards single-molecule magnets based on theoxalate ligand [J]. Chem Commun, 2010, 46: 1506-1508.
[11] HE Z, GAO E Q, WANG Z M, et al. Coordination polymers based on inorganic lanthanide(III) sulfate skeletons and an organic isonicotinateo-oxide connector: segregation into three structural types by the lanthanide contraction effect [J]. Inorg Chem, 2005, 44: 862-869.
[12] KE H S, GAMEZ P, ZHAO L, et al. Magnetic properties of dysprosium cubanes dictated by the M-O-M Angles of the [Dy4(μ3-OH)4] core [J]. Inorg Chem, 2010, 49: 7549-7557.
[13] ZHAO X Q, ZHAO B, SHI W, et al. Synthesis, structures and luminescent and magnetic properties of Ln-Ag heterometal-organic frameworks [J]. Inorg Chem, 2009, 48: 11048-11057.
[14] SHELDRICK G M. SHELXS-97, Program for the solution of crystal structure [M]. University of Göttingen: Germany, 1997.
[15] SHELDRICK G M. SHELXL97, Program for the crystal structure refinement [M]. University of Göttingen: Germany, 1997.
[16] FENG X, LI S H, WANG L Y, et al. A series of lanthanide-organic polymers incorporating ethyl-4,5-imidazole-dicarboxylato and formate coligand: structures, luminescent and magnetic properties [J]. CrystEngComm, 2012, 14: 3684-3693.
[17] FENG X, CHEN J L, WANG LY, et al. A series of homonuclear lanthanide complexes incorporating isonicotinic based carboxylate tectonic and oxalate coligand: structures, luminescent and magnetic properties [J]. CrystEngComm, 2014, 16: 1334-1343.
[18] FENG X, FENG Y Q, LIU L, et al. A series of Zn-4f heterometallic coordination polymers and a zinc complex containing a flexible mixed donor dicarboxylate ligand [J]. Dalton Trans, 2013, 41: 7741-7754.
[19] ALLENDORF M D, BAUER C A, BHAKTA R K, et al. Luminescent metal-organic frameworks [J]. Chem Soc Rev, 2009, 38: 1330-1352.
[20] ELISEEVA S V, BÜNZLI J C G. Lanthanide luminescence for functional materials and bio-sciences [J]. Chem Soc Rev, 2010, 39: 189-227.
[21] GUO X D, ZHU G S, LI Z Y, et al. A lanthanide metal-organic framework with high thermal stability and available Lewis-acid metal sites [J]. Chem Commun, 2006,100: 3172-3174.
[22] ZHOU T H, YI F Y, LI P X, et al. Synthesis, crystal structures and luminescent properties of two series of new lanthanide (III) amino-carboxylate-phosphonates [J]. Inorg Chem, 2010, 49: 905-915.
[责任编辑:吴文鹏]
一个含有2-羟基-6-甲基-异烟酸和草酸混合配体镝的有机框架化合物的合成与表征
田爱琴1,2, 冯 勋2*, 汪彩露3, 郎雪明2, 杨 松2
(1. 河南林业职业学院 基础教育部,河南 洛阳 471002; 2. 洛阳师范学院 化学化工学院, 河南 洛阳 471022;
3. 南阳师范学院 生命科学与技术学院, 河南 南阳 473601)
通过溶剂热合成了一个新的由2-羟基-6-甲基-异烟酸和草酸混合配体构筑的镝有机框架化合物, 化学式为{[Dy(μ2-H2minca) (μ2-C2O4)·2H2O]·H2O}n, (其中, H3minca 代表2-羟基-6-甲基-异烟酸). 标题化合物呈现复杂的二维(2D)瓦楞状网络63拓扑结构, 其中{DyO8}多面体是通过苯羧酸上的羧基和草酸联成片状结构. 光致发光研究表明, 标题化合物在可见光区域发射出Dy的特征f-f跃迁荧光光谱. 热重和差热分析表明, 该化合物具有较高的热稳定性. 变温磁化率测量表明, 相邻镝(III)中心之间有可能存在弱的铁磁相互作用.
X-ray单晶结构; 镝(III)化合物; 磁学性质; 荧光性质
date:2014-08-15.
National Natural Science Foundation of China (21273101), Foundation Program for Science & Technology Innovation Talents in Universities of Henan Province (2014HASTIT014), Tackle Key Problem of Science and Technology Project of Henan Province (142102310483), Program for Backbone Teachers in Universities of Henan Province ( 2012GGJS158).
Biography:TIAN Aiqin (1961-), male, assistant professor, majoring in coordination chemistry.*
, E-mail: hnlyfx@163.com.
O73DocumentcodeAArticleID1008-1011(2014)05-0466-08
10.14002/j.hxya.2014.05.007
