苯丙氨酸锂催化醛和胺以及三甲基硅腈三组分的Strecker反应
2014-09-01刘梦杰王幸伟王心义邓冬生
刘梦杰,郭 辉,王幸伟,王心义,邓冬生*
(1.河南理工大学 资源环境学院, 河南 焦作 454150; 2.洛阳师范学院 化学化工学院, 河南 洛阳 471022)
苯丙氨酸锂催化醛和胺以及三甲基硅腈三组分的Strecker反应
刘梦杰1,2,郭 辉2,王幸伟1,2,王心义1*,邓冬生1,2*
(1.河南理工大学 资源环境学院, 河南 焦作 454150; 2.洛阳师范学院 化学化工学院, 河南 洛阳 471022)
以苯丙氨基酸锂为催化剂,成功催化了醛、胺和三甲基硅腈三组分的Strecker反应,并结合一系列氨基酸盐催化剂的筛选以及各种溶剂的选择优化了反应条件. 结果表明,利用所述反应可以得到较高收率的α-氨基腈;该三组份的Strecker反应具有反应条件温和、反应收率较高、操作简便、无需繁琐的分离步骤、催化剂便宜且环境友好等优点.
苯丙氨基酸锂;醛;胺;三甲基硅腈;三组分Strecker反应
Modified Strecker reaction has been proven to be one of the preeminent multicomponent reactions useful for the synthesis ofα-amino acids via the intermediacy ofα-amino nitriles[1-2]. Generally, the reaction has to be carried out in two steps: i) the preparation of imines; and ii) cyanide addition. Thus, the method has to suffer from some disadvantages such as longer reaction time, intermediate waste disposal, and fussy operation. Meanwhile, there are not many examples[3]for efficient and clean three-component Strecker reaction. For developing an efficient synthetic process, reduction of reaction steps is commonly attempted by employing a one-pot synthesis. In this regard, several catalysts for modified Strecker synthesis have recently been identified, including NHC-amidate palladium(ii) complex[1], gallium(III) triflate[2], and Nd-MOFs[3]. Since the catalytic use of amino acid alkali metal salts was first reported by YAMAGUCHI's group in 1991[4], amino acid salts have effectively catalyzed many organic transformation reactions[5-7]. In this paper, we describe a new catalytic system for Strecker reaction which utilizes readily available amino acid lithium as an efficient catalyst for the three-component reaction of aldehyde, amine and trimethylsilyl cyanide.
1 Experimental
1.1 Materials and instrumentation
All starting materials and solvents were purchased commercially and used as received. Nuclear magnetic resonance (1H NMR) spectra were recorded with a Varian UNITY/NOVA 400 MHz spectrometer (CDCl3solvent) at room temperature; and chemical shifts were given inδrelative to tetramethylsilane.
1.2 Typical experimental procedure for Strecker reactions
Aromatic aldehyde (10.3 μL, 0.1 mmol), aromatic amine (12.7 mg, 0.1 mmol) andL-phenylalanine lithium salt (1.7 mg, 0.01 mmol) were mixed in toluene (4.5 mL) for 2 h at room temperature. Trimethylsilyl cyanide (20 mg, 0.2 mmol) andn-BuOH (0.01 mmol) were added into the mixture and stirred for 12 h under monitoring by thin layer chromatograph (TLC). The reaction mixture was then quenched with saturated NaHCO3solution and extracted into ethyl acetate (3×10 mL). The resulting solution was washed with brine and dried over anhydrous MgSO4to afford the crude product after the solvent was removed in vacuum. Purification by 100-200 mesh silica gel chromatograph ZCX II (typical eluant:Vhexane∶Vethyl acetate= 12∶1) gave the desired aminonitriles. The spectroscopic characteristics of the known products3a-3fare in agreement with the published data.
1.3 Spectral data of the resulting compounds
2-(p-tolylamino)-2-phenylacetonitrile (3a). White solid, 20 mg, 90% yield.1H NMR (400 MHz, CDCl3)δ: 7.60 (d,J= 4 Hz, 2H, ArH), 7.45 (d,J= 4 Hz, 3H, ArH), 7.09 (d,J= 8 Hz, 2H, tolylH), 6.71 (d,J= 8 Hz, 2H, tolylH), 5.40 (d,J= 8 Hz, 1H, -CH), 3.92 (d,J= 8 Hz, 1H, -NH), 2.28 (s, 3H, -CH3).
2-phenyl-2-(phenylamino)acetonitrile (3b). White solid, 18 mg, 87% yield.1H NMR (400 MHz, CDCl3)δ: 7.61-7.45 (m, 5H, ArH), 7.30-7.21 (m, 2H, ArH), 6.93-6.77 (m, 3H, ArH), 5.45 (d,J= 12 Hz, 1H, -CH), 4.04 (d,J= 8 Hz, 1H, -NH).
2-(p-tolylamino)-2-p-tolylacetonitrile (3c). White solid, 21 mg, 88% yield.1H NMR (400 MHz, CDCl3)δ: 7.48 (d,J= 8 Hz, 2H, tolylH), 7.26 (d,J= 8 Hz, 2H, tolylH), 7.09 (d,J= 8 Hz, 2H, tolylH), 6.71 (d,J= 8 Hz, 2H, tolylH), 5.36 (d,J= 8 Hz, 1H, -CH), 3.86 (d,J= 8 Hz, 1H, -NH), 2.39 (s, 3H, -CH3), 2.28 (s, 3H, -CH3).
2-(p-tolylamino)-2-(4-methoxyphenyl)acetonitrile (3d). White solid, 21 mg, 85% yield.1H NMR (400 MHz, CDCl3)δ: 7.50 (d,J= 8 Hz, 2H, tolylH), 7.08 (d,J= 8 Hz, 2H, tolylH), 6.96 (d,J= 8 Hz, 2H, anisolylH), 6.69 (d,J= 8 Hz, 2H, anisolylH), 5.32 (s, 1H, -CH), 3.86 (s, 1H, -NH), 3.83 (s, 3H, -OCH3), 2.27 (s, 3H, -CH3).
2-(phenylamino)-2-p-tolylacetonitrile (3e). White solid, 19 mg, 86% yield.1H NMR (400 MHz, CDCl3)δ: 7.48 (d,J= 8 Hz, 2H, tolylH), 7.29-7.26 (m, 4H, tolylH+ArH), 6.91 (d,J= 8 Hz, 1H, ArH), 6.78 (d,J= 8 Hz, 2H, ArH), 5.39 (d,J= 8 Hz, 1H, -CH), 4.00 (d,J= 8 Hz, 1H, -NH), 2.39 (s, 3H, -CH3).
2-(4-methoxyphenylamino)-2-phenylacetonitrile (3f). White solid, 19 mg, 83% yield.1H NMR (400 MHz, CDCl3)δ: 7.61 (d,J= 8 Hz, 2H, ArH), 7.46 (d,J= 8 Hz, 2H, ArH), 6.86 (d,J= 8 Hz, 2H, anisolylH), 6.77 (d,J= 8 Hz, 2H, anisolylH), 5.36 (d,J= 8 Hz, 1H, -CH), 3.81 (d,J= 8 Hz, 1H, -NH), 3.77 (s, 3H, -OCH3).
2 Results and discussion


Table 1 Catalyst screen for the modified Strecker reactiona
Secondly, other readily obtainable amino acid lithium salts were evaluated for the Strecker reaction (Table 1, entries 7-13). However, other lithium salt showed lower catalytic activity in comparison with phenylalanine lithium.
Next, we examined a solvent screen with Phe OLi (Table 2). The modified Strecker reaction in a high-polarity solvent, such as dimethyl sulphoxide (DMSO), dimethylformamide (DMF) or CH3CN, afforded corresponding adduct3ain low yield (Table 2, entries 1-3). While low-polarity solvents including CHCl3, CH2Cl2, and toluene were used, better product yield was observed than that with high-polarity solvents (Table 2, entries 4-6). Among these low-polarity solvents, toluene gave relatively good results (Table 2, entry 6). Thus, toluene was chosen as a solvent for further investigations.

Table 2 Solvent screen for the modified Strecker reaction
Then, we examined further optimization of the reaction conditions for the modified Strecker reaction with various alcohols as additives. Three additives includingn-BuOH, EtOH andi-PrOH have been screened for their co-catalytic results andn-BuOH as additives gives excellent conversions. Therefore, 10%(amount of substance fraction) Phe-OLi withn-BuOH additive in toluene was chosen as the optimization condition for further experiments.
Finally, the scope of the catalytic Strecker reaction of aldehyde, amine and trimethylsilyl cyanide was evaluated under the optimized cooperative catalysis conditions. The reaction was applicable to a range of aromatic aldehydes and aromatic amines. It is noted that, these three-component Strecker reactions can be smoothly performed under the typical conditions with Phe-OLi catalyst system to produce the amino nitriles with moderate good yields, as summarized in Table 3.

Table3SubstratescopeforthemodifiedStreckerreaction
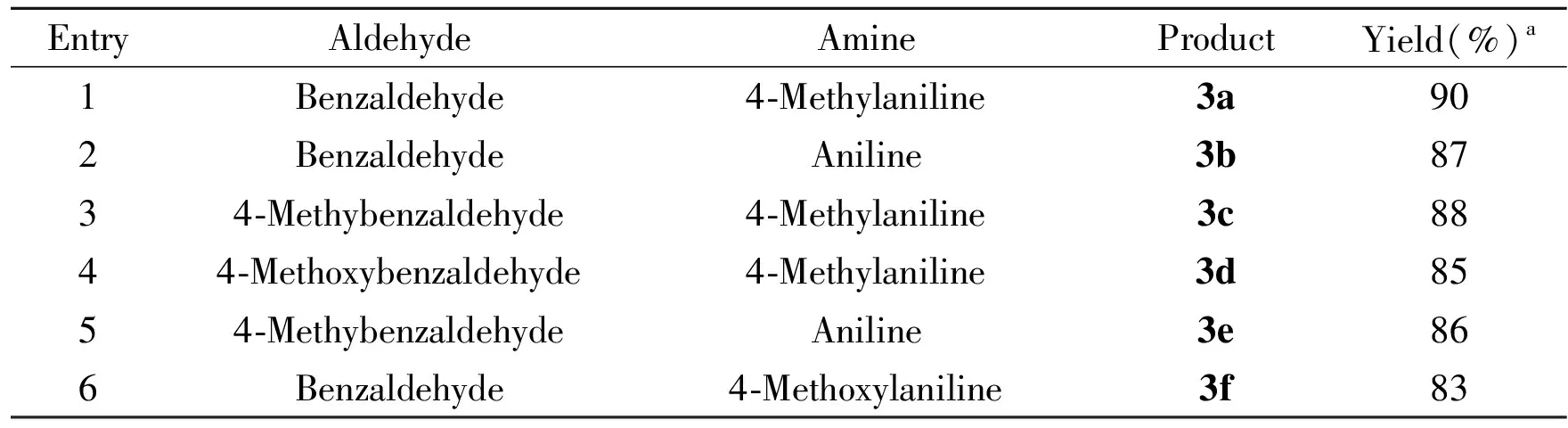
EntryAldehydeAmineProductYield(%)a1Benzaldehyde4⁃Methylaniline3a902BenzaldehydeAniline3b8734⁃Methybenzaldehyde4⁃Methylaniline3c8844⁃Methoxybenzaldehyde4⁃Methylaniline3d8554⁃MethybenzaldehydeAniline3e866Benzaldehyde4⁃Methoxylaniline3f83
aIsolated yield based on amine.
3 Conclusions
In summary, we present here the systematic investigation into the three-component Strecker reactions catalyzed by phenylalanine lithium in toluene. The reaction proves to be effective to produceα-amino nitriles in moderate to high yields under mild conditions with good substrate scopes.
[1]JARUSIEWICZ J, CHOE Y, YOO K S, et al. Efficient three-component Strecker reaction of aldehydes/ketonesviaNHC-amidate palladium(II) complex catalysis [J]. J Org Chem, 2009, 74 (7): 2873-2876.
[2]SURYA PRAKASH G K, MATHEW T, OLAH G A. Gallium(III) triflate: an efficient and a sustainable Lewis acid catalyst for organic synthetic transformations [J]. Acc Chem Res, 2012, 45 (4): 565-577.
[3]LIU Yan, MO Yan, CUI Yong. Porous and robust lanthanide metal-organoboron frameworks as water tolerant Lewis acid catalysts [J]. Inorg Chem, 2013, 52 (18): 10286-10291.
[4]YAMAGUCHI M, YOKOTA N, MINAMI T. The michael addition of dimethyl malonate toα,β-unsaturated aldehydes catalyzed by proline lithium salt [J]. J Chem Soc, Chem Commun, 1991 (16): 1088-1089.
[5]YAMAGUCHI M, SHIRAISHI T, HIRAMA M. Asymmetric michael addition of malonate anions to prochiral acceptors catalyzed byL-proline rubidium salt [J]. J Org Chem, 1996, 61 (10): 3520-3530.
[6]YOSHIDA M, SATO A, HARA S. Asymmetric michael addition of aldehydes to nitroalkenes using a primary amino acid lithium salt [J]. Org Biomol Chem, 2010, 8: 3031-3036.
[7]YOSHIDA M, NARITA M, HARA S. Asymmetric michael addition of malonates to enones catalyzed by a primaryβ-amino acid and its lithium salt [J]. J Org Chem, 2011, 76: 8513-8517.
date:2013-10-21.
National Natural Science Foundation of China (21272109).
Biography:LIU Mengjie(1988-), female, master, research field: asymmetric catalysis.*
, E-mail: wangxy@hpu.edu.cn;dengdongsheng168@sina.com.
