一种二维的四核铁取代夹心型锑钨酸盐NdNa3[Fe4(H2O)10][β-B-SbW9O33]2·36H2O
2014-09-01赵俊伟
曹 静, 张 静, 吉 帆, 赵俊伟
(河南大学 化学化工学院, 河南省多酸化学重点实验室, 河南 开封 475004)
一种二维的四核铁取代夹心型锑钨酸盐NdNa3[Fe4(H2O)10][β-B-SbW9O33]2·36H2O
曹 静, 张 静, 吉 帆, 赵俊伟*
(河南大学 化学化工学院, 河南省多酸化学重点实验室, 河南 开封 475004)
在常温水溶液条件下,合成了一种二维四核铁取代夹心型锑钨酸盐NdNa3[Fe4(H2O)10][β-B-SbW9O33]2·36H2O (1), 并通过IR光谱和单晶X射线衍射对其进行了表征. 化合物1属于三斜晶系,P-1空间群, 晶胞参数:a= 1.264 8(3) nm,b= 1.270 1(3) nm,c= 1.616 0(4) nm,α= 74.467(4) °,β= 77.592(4) °,γ= 83.658(4)°,V= 2.438 9(9) nm3,Z= 1,Dc= 4.000 g/cm3,GOOF= 1.047,R1= 0.065 6,wR2= 0.162 2 . X射线单晶结构分析表明, 化合物1的多阴离子由两个相同的三缺位Keggin[β-B-SbW9O33]9-单元通过四个八面体配位的铁离子连接而成. 四个FeO6八面体没有直接相连, 四个FeIII离子可以分为两组: 内部的FeIII离子带有两个水配体, 而外部的FeIII离子连有三个水配体. 邻近二聚多阴离子通过无序的钠/钕桥配离子构筑了二维层结构.
多金属氧酸盐; 锑钨酸盐; 夹心型化合物

1 Experimental
1.1 Physical measurements
The IR spectrum was recorded from a sample powder palletized with KBr on a Nicolet170 SXFT-IR spectrometer over the range of 4 000-400 cm-1. Na9[α-SbW9O33]·19.5H2O was synthesized according to the published procedure, and the purity was confirmed by IR spectra[21]. All other reagents were obtained from commercial resources and used without further purification.
1.2 Synthesis of 1
Na9[α-SbW9O33]·19.5H2O (0.220 g, 0.830 mmol), NdCl3(0.07 g, 0.279 mmol) and FeCl3·6H2O (0.032 g, 0.118 mmol) were successively dissolved in 15 mL water under stirring. The mixture was stirred for 2 h, heated at 80 ℃ for 2 h, and then filtered when it cooled to room temperature. Slow evaporation of the filtrate at room temperature led to green yellow crystals of1for several days. Yield: 25% (based on FeCl3·6H2O).
1.3 X-ray crystallography
A good single crystal for1was carefully selected under an optical microscope and glued at the tip of a thin glass fiber with cyanoacrylate adhesive. Intensity data were collected on Bruker APEX-II CCD detector at 296(2) K with Mo Kα radiation (λ= 0.071 073 nm). Intensity data were corrected for Lorentz and polarization effects as well as for empirical absorption. The structure was solved by direct methods and refined by the full-matrix least-squares method onF2using the SHELXTL-97 package[22]. The remaining atoms were found from successive full-matrix least-squares refinements onF2and Fourier syntheses. All the non-hydrogen atoms were refined anisotropically. Those hydrogen atoms attached to lattice water molecules were not located. In1, all the W centers split into two positions and Na1 and Na2 positions are simultaneously occupied by sodium(I) and neodymium(III) components with 75% sodium(I) and 25% neodymium(III) for each site. The crystallographic data and structural refinements for1are listed in Table 1.
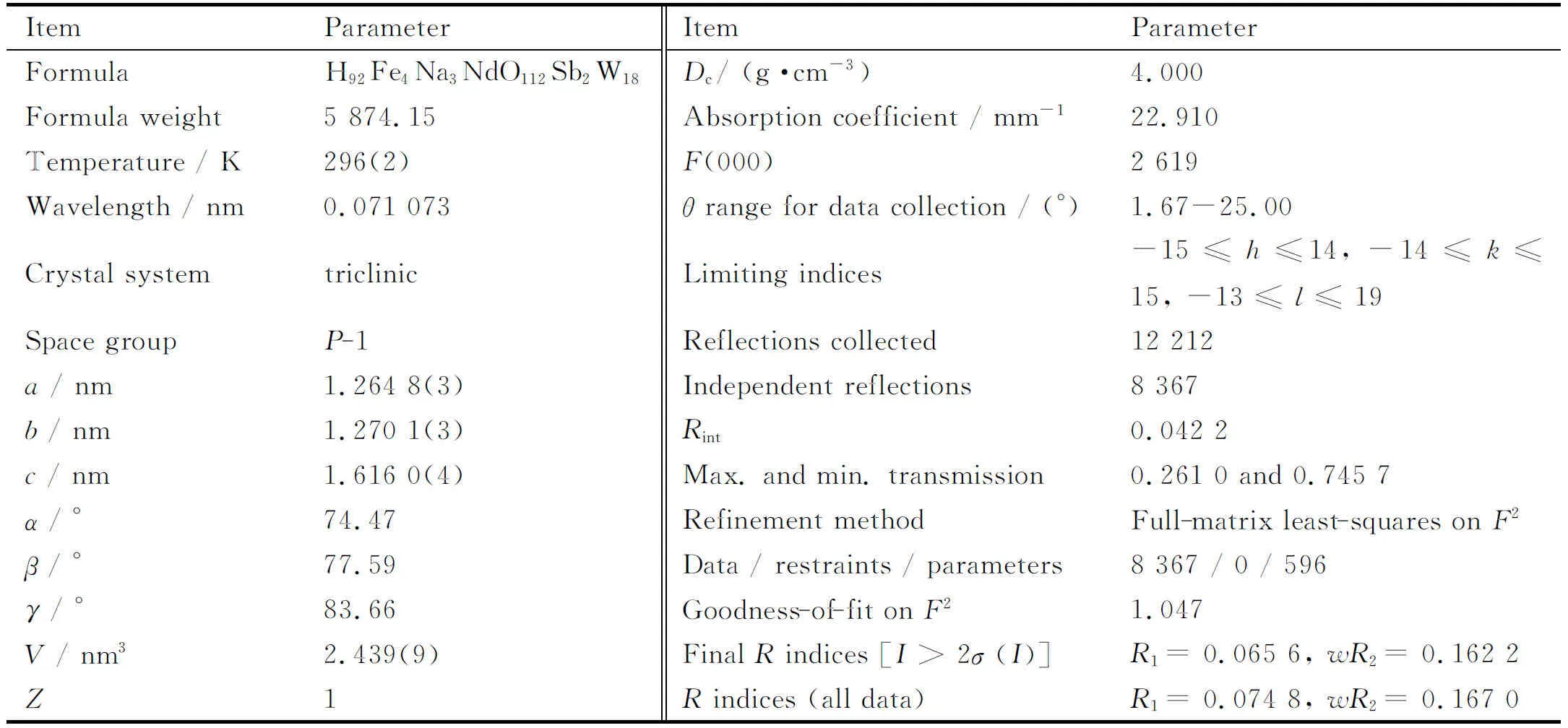
Table 1 Crystallographic data and structural refinements of 1
2 Results and discussion
2.1 Synthesis
With the profound research of POT chemistry, the synthesis and exploration of POTs with mixed TM/Ln cations has gradually been developed as an important research area. To the best of our knowledge, since the first POTs with mixed Ni/Ln components [Ln(H2O)5{Ni(H2O)}2As4W40O140]21(Ln = Y / Ce / Pr / Nd / Sm / Eu / Gd) were discovered by XUE et al in 2004[23], only a small number of such species have been obtained mainly because of the unavoidable competitive reactions among highly negative POM precursors, strongly oxyphilic Ln metal cations and less active TM cations in the same reaction system[24]. Recently, we have launched the study on the reaction of the [α-SbW9O33]9-precursor with iron and Ln metal cations under conventional aqueous solution method, by trial and error, a novel 2-D sheet tetra-FeIIIsandwiched AT1was prepared. Although the [B-α-SbW9O33]9-polyoxoanion was used as the starting material,1contains the [B-β-SbW9O33]9-fragments, indicates that the isomerization of [B-α-SbW9O33]9-→[B-β-SbW9O33]9-must have taken place during the course of the reaction. Similar phenomenon has been previously encountered by us[25].
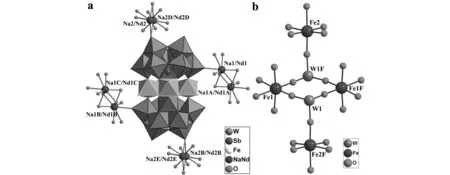
Fig.1 (a) Ball-and-stick/polyhedral representation of the molecular unit of 1 with the selected labeling scheme. Lattice water molecules are omitted for clarity. The atoms with the suffix A, B, C, D, E, F are generated by the symmetry operation: A: -x, 1-y, 1-z; B: 1- x, -y, 1-z; C: 1+x, -1+y, z; D: 2-x, 1-y, -z; E: -1+x, -1+y, 1+z; F: 1-x, 1-y, 1-z. (b) The tetra-FeIII cations sandwiched by two trivacant Keggin-type [B-β-SbW9O33]9- fragments in 1
2.2 Description of crystal structure
Single-crystal structural analysis indicates that1crystallizes in the triclinic space groupP-1 and its molecular unit consists of two [B-β-SbW9O33]9-moieties linked by four Fe3+ions (Fig.1a). Formally, the well-known trivacant [B-β-SbW9O33]9-unit can be envisioned as a derivative from the parentα-Keggin structure by removing three edge-sharing {WO6} octahedra and consists of three corner-sharing W3O13fragments with a Sb3+center linked by triply bridging oxygen atoms and a 60° rotation of one W3O13subunit. Four Fe3+ions consist of two inequivalent pairs, namely, two inner Fe3+ions (Fe1, Fe1F) and two outer Fe3+ions (Fe2, Fe2F). Moreover, their coordination environments are somewhat different. The octahedral Fe1 ion is defined by two O atoms from one [B-β-SbW9O33]9-fragment [Fe-O: 0.196 33(132)-0.196 38(167) nm], two O atoms from the same [B-β-SbW9O33]9-fragment [Fe-O: 0.193 17(184)-0.196 33(132) nm], and other O atoms from two terminal H2O ligands [Fe-O: 0.207 42(203)-0.208 29(184) nm], whereas the octahedral Fe2 ion is built by two O atoms from one [B-β-SbW9]9-fragment [Fe-O: 0.192 68(167)-0.192 74(177) nm], one O atoms from the same [B-β-SbW9]9-fragment [Fe-O: 0.185(15) nm], the other three O atoms from two terminal H2O ligands [Fe-O: 0.202 59(201)-0.210 10(178) nm]. The Fe1 and Fe1F ions are crystallographiclaly equivalent whereas the Fe2 and Fe2F ions are also crystallographiclaly equivalent. Interestingly, four iron centers are separated by four Fe-O-W-O-Fe bonds (Fig. 1b), and such connection mode is somewhat distinct from those in the tetra-FeIII-sandwiched units [Fe4(en)(α-GeW9O34)2]8-and [Fe4(en)2(α-GeW9O34)2]8-reported by our group recently[26], in which four iron centers are separated by two Fe-O-Fe bonds.
Those dimeric sandwich-type anions are also known with two tungsten atoms and two TM cations linking two B-β-XW9O33moieties (X = AsIII, SbIII, BiIII, SeIV, TeIV). For example, in 1997, KREBS and co-workers reported the 22-tungsto-2-antimonate(III) [Sb2W22O74(OH)2]12-comprising two trilacunary Keggin-type [B-β-SbW9O33]9-units linkedviaa belt of two internal [WO2]2+and two external [WO2(OH)]+groups[21]. Subsequently, they also reported a Bi(III) species with the same structure[27]. The group of KREBS revealed that the two external WVIcenters in such structures can be easily substituted by various first-row TM ions, such as [(WO2)2M2(H2O)6(B-β- XW9O33)2](14-2n)-(X = SbIII, BiIII; Mn+= Mn2+, Fe3+, Co2+, Ni2+,VO2+; X = BiIII, Mn+= Fe3+, Co2+, Ni2+, Cu2+, Zn2+)[28-29]and [(WO2)2(WO2(OH))0.5Sn1.5(B-β-XW9O33)2]10.5-(X = SbIII, BiIII)[30]. In 2006, KORTZ et al obtained an organoruthenium derivative [Sb2W20O70(RuC6H6)2]10-under conventional aqueous solution conditions, in which two [B-β-SbW9O33]9-fragments are held together by a belt of two Ru(II) and two W centers[31]. KREBS′ and KORTZS′ groups showed that derivatives of this structural type can be prepared with all internal and external tungsten ions replaced by one kind of TM ions, resulting in [M4(H2O)10(B-β-XW9O33)2]n-(X = AsIII, SbIII, SeIV, TeIV; M = Fe3+, Mn2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Hg2+, In3+, Al3+)[11-12,21,32].
More interestingly, each dimeric {[Fe4(H2O)10[B-β-SbW9O33]2}6-building unit is combined with adjacent four same ones alternatively by the O-Na/Nd-O-Na/Nd-O and O-Na/Nd-O bonds resulting in the unique 2-D sheet architecture (Fig.2). Such 2-D tetra-FeIIIsubstituted sandwich-type AT with mixed TM/Ln components is observed for the first time.

Fig.2 2-D sheet architecture established by distinctive dimeric {[Fe4(H2O)10][B-β-SbW9O33]2}6- building units via Na+/Nd3+ bridges
It is noteworthy that the tetra-FeIII-sandwiched unit {[Fe4(H2O)10[B-β-SbW9O33]2}6-in1is the same to that in Na6[Fe4(H2O)10(β-SbW9O33)2]·32H2O (2)[11]. The common feature of1and2is that they can be described as two trivacant Keggin-type [B-β-SbW9O33]9-subunits connected by four octahedral Fe3+ions. Moreover, both were prepared under conventional aqueous solution conditions. However, two obvious differences can be found among them: (a) the synthetic processes and strategies are different:1was prepared by making use of Na9[α-SbW9O33]·19.5H2O, NdCl3and FeCl3·6H2O at 80 ℃, while2was obtained by using Na9[α-SbW9O33]·19.5H2O and FeCl3·6H2O at 90 ℃; (b) their structure types are distinct:2utilizes the discrete (0-D) structure whereas1demonstrates a beautiful 2-D network through the disordered Na+/Nd3+linkers.
2.3 IR spectra
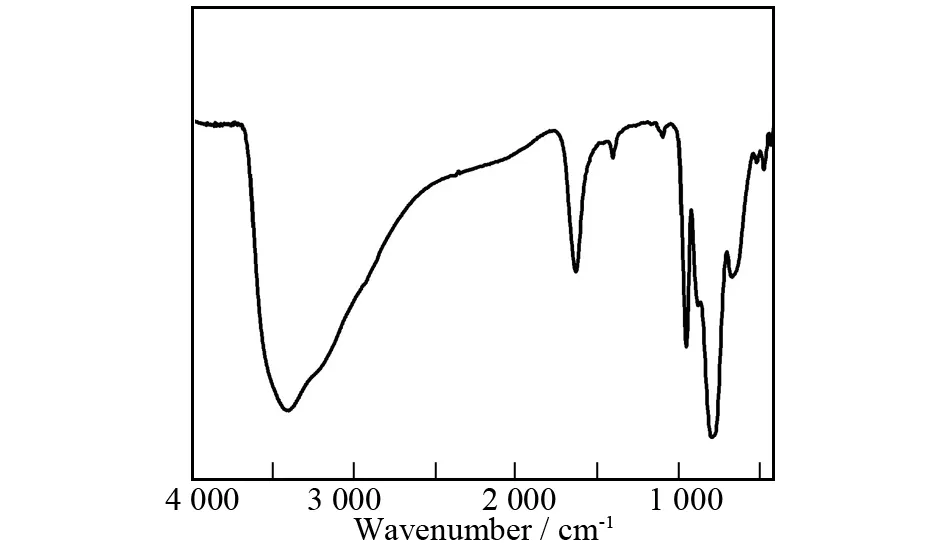
Fig.3 The IR spectrum of 1
The IR spectrum of1has been collected from a solid sample palletized with KBr in the range of 400-4 000 cm-1, which exhibits the characteristic vibration patterns derived from the Keggin AT framework in the low wavenumber region (Fig.3). Four characteristic vibration absorption bands attributable to terminalν(W-Ot),ν(Sb-Oa), corner-sharingν(W-Ob) and edge-sharingν(W-Oc) are observed at 954, 796, 875 and 668 cm-1, respectively[21,33]. In comparison with the IR spectrum of Na10[α-SbW9O34]·19.5H2O [920, 767, 890 and 715 cm-1forν(W-Ot),ν(Sb-Oa),ν(W-Ob) andν(W-Oc)][21], theν(W-Ot,b,c) and (Sb-Oa) vibration peaks of1have different shifts, which may be related to incorporation of FeIIIcations into the vacant sites of [B-β-SbW9O33]9-fragments, coordination of Na+/NdIIIcations to the terminal oxygen atoms of [B-β-SbW9O33]9-fragments and isomerization of [B-α-SbW9O33]9-→[B-β-SbW9O33]9-. The vibration band centered at 3 415 cm-1is indicative of the presence of water molecules. In a word, the result of IR spectrum is in good agreement with that of single-crystal structural analysis.
3 Conclusion
In summary, a 2-D tetra-FeIIIsubstituted sandwich-type AT1was synthesized and structurally characterized.1displays a pure inorganic 2-D sheet architecture built by the dimeric {[Fe4(H2O)10][SbW9O33]2}6-moieties through the disordered Na+/Nd3+linkers, representing the first 2-D tetra-FeIIIsubstituted sandwich-type AT with mixed TM/Ln components. These results reveal the extreme structural versatility of ATs and it is expected that a large class of ATs with mixed TM/Ln components can be obtained in this way by varying TM and Ln cations. In the following time, we will exploit this domain.
[1]XU L, BORING E, HILL C L. Polyoxometalate-modified fabrics: new catalytic materials for low-temperature aerobic oxidation [J]. J Catal, 2000, 195: 394-405.
[2]VASYLYEV M V, NEUMANN R. New heterogeneous polyoxometalate based mesoporous catalysts for hydrogen peroxide mediated oxidation reactions [J]. J Am Chem Soc, 2004, 126: 884-890.
[3]KORTZ U, MÜLLER A, SLAGEREN J VAN, et al. Polyoxometalates: fascinating structures, unique magnetic properties [J]. Coord Chem Rev, 2009, 253: 2315-2327.
[4]COMPAIN J D, MIALANE P, DOLBECQ A, et al. Iron polyoxometalate single-molecule magnets [J]. Angew Chem Int Ed, 2009, 48: 3077-3081.
[5]HOWELL R C, PEREZ F G, JAIN S, et al. A new type of heteropolyoxometalates formed from lacunary polyoxotungstate ions and europium or yttrium cations [J]. Angew Chem Int Ed, 2001, 40: 4031-4034.
[6]MAL S S, KORTZ U. The wheel-shaped Cu20tungstophosphate [Cu20Cl(OH)24(H2O)12(P8W48O184)]25-ion [J]. Angew Chem Int Ed, 2005, 44: 3777-3780.
[7]ZHENG S T, ZHANG J, CLEMENTE-JUAN J M, et al. Poly(polyoxotungstate)s with 20 nickel centers: from nanoclusters to one-dimensional chains [J]. Angew Chem Int Ed, 2009, 121: 7312-7315.
[8]SHI D Y, CHEN L J, ZHAO J W, et al. Two novel 2D organic inorganic hybrid lacunary Keggin phosphotungstate 3d-4f heterometallic derivatives: [Cu(en)2]2H6[Ce(α-PW11O39)2]·8H2O and [Cu(dap)2(H2O)][Cu(dap)2]4.5[Dy(α-PW11O39)2]·4H2O [J]. Inorg Chem Commun, 2011, 14: 324-329.
[9]NIU J Y, ZHANG S W, CHEN H N, et al. 1-D, 2-D and 3-D organic-inorganic hybrids assembled from Keggin-type polyoxometalates and 3d-4f heterometals [J]. Cryst Growth Des, 2011, 11: 3769-3777.
[10]BI L H, WANG E B, HUANG R D, et al. Room temperature synthesis and structural characterization of (NH4)15Co0.5(NH3)3[Co(NH3)4NaSb9W21O86]·15H2O—a novel chain-like inorganic cryptate [J]. J Mol Struct, 2000, 553: 167-174.
[11]KORTZ U, SAVELIEFF M G, BASSIL B S, et al. Synthesis and characterization of iron(III)-substituted, dimeric polyoxotungstates [Fe4(H2O)10(β-XW9O33)2]n-(n= 6, X = AsIII, SbIII;n= 4, X = SeIV, TeIV) [J]. Inorg Chem, 2002, 41: 783-789.

[14]VOLKMER D, BREDENKÖTTER B, TELLENBRÖKER J, et al. Structure and properties of the dendron-encapsulated polyoxometalate (C52H60NO12)12[(Mn(H2O))3(SbW9O33)2], a first generation dendrizyme [J]. J Am Chem Soc, 2002, 124: 10489-10496.
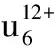
[16]DOLBECQ A, COMPAIN J D, MIALANE P, et al. Water substitution on iron centers: from 0D to 1D sandwich type polyoxotungstates [J]. Inorg Chem, 2008, 47: 3371-3378.
[17]MCGLONE T, VIL-NADAL L, MIRAS H N, et al. Assembly of titanium embedded polyoxometalates with unprecedented structural features [J]. Dalton Trans, 2010, 39: 11599-11604.
[18]BOLAND K S, CONRADSON S D, COSTELLO A L, et al. Stabilising pentavalent actinides visible near infrared and X-ray absorption spectroscopic studies of the utility of the [(Np3W4O15)(H2O)3(MW9O33)3]18-(M = Sb, Bi) structural type [J]. Dalton Trans, 2012, 41: 2003-2010.
[19]HAN Z G, ZHANG Q X, GAO Y Z, et al. Novel hexagonal {V=O}6-containing sandwich-type cluster accompanied by in situ carbon-carbon bond formation of organic cations [J]. Dalton Trans, 2012, 41: 1332-1337.
[20]PILETTE M A, FLOQUET S, MARROT J, et al. Synthesis, structure, and tungsten NMR characterization of {SbW9O33}9-{Mo2O2S2}2+sandwich-like polyoxothiometalates [J]. Eur J Inorg Chem, 2013:1726-1730.
[21]BÖSING M, LOOSE I, POHLMANN H, et al. New strategies for the generation of large heteropolymetalate clusters: theβ-B-SbW9fragment as a multifunctional unit [J]. Chem Eur J, 1997, 3: 1232-1237.
[22]SHELDRICK G M. SHELXTL-97, Program for crystal structure solution [CP]. University of Göttingen, Germany, 1997.
[23]XUE G L, LIU B, HU H M, et al. Large heteropolymetalate complexes formed from lanthanide (Y, Ce, Pr, Nd, Sm, Eu, Gd), nickel cations and cryptate [As4W40O140]28-: synthesis and structure characterization [J]. J Mol Struct, 2004, 690: 95-103.
[24]CHEN W L, LI Y G, WANG Y H, et al. A new polyoxometalate-based 3d-4f heterometallic aggregate: a model for the design and synthesis of new heterometallic [J]. Dalton Trans, 2008: 865-867.
[25]HUSSAI F, REICKE M, KORTZ U. The hybrid organic inorganic 2-D material (CsNa2[{Sn(CH3)2}3(H2O)4(β-XW9O33)]·7H2O)∞(X = AsIII, SbIII) and its solution properties [J]. Eur J Inorg Chem, 2004: 2733-2738.
[27]LOOSE I, DROSTE E, BÖSING M, et al. Heteropolymetalate clusters of the subvalent main group elements BiIIIand SbIII[J]. Inorg Chem, 1999, 38: 2688-2694.
[28]BÖSING M, NÖH A, LOOSE I, et al. Highly efficient catalysts in directed oxygen-transfer processes: synthesis, structures of novel manganese-containing heteropolyanions and applications in regioselective epoxidation of dienes with hydrogen peroxide [J]. J Am Chem Soc, 1998, 120: 7252-7259.
[29]DREWES D, LIMANSKI E M, PIEPENBRINK M, et al. Neue heteropolyanionen des wolframs mit vanadium (IV) als heteroatom [J]. Z Anorg Allg Chem, 2004, 630: 58-62.
[30]KREBS B, DROSTE E, PIEPENBRINK M, et al. Novel Keggin stannato(II)tungstates with lone-pair assembling atoms: syntheses, crystal structure and spectroscopic studies [J]. C R Acad Sci Ser IIc Chim, 2000, 3: 205-210.
[31]BI L H, AL-KADAMANY G, CHUBAROVA E V, et al. Organo-ruthenium supported heteropolytungstates: synthesis, structure, electrochemistry, and oxidation catalysis [J]. Inorg Chem, 2009, 48: 10068-10077.
[32]PRINZ M, TAKACS A, SCHNACK J, BALASZ I, et al. Magnetic and electronic properties of the iron-containing polyoxotungstate [Fe4(H2O)10(β-SbW9O33)2]6[J]. J Appl Phys, 2006, 99: 08J505 1-3.
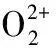
date:2013-11-08.
Supported by the Natural Science Foundation of China (21101055, 21301049, U1304208), the Natural Science Foundation of Henan Province (122300410106), the Foundation of Education Department of Henan Province (2010B150006) and the Students Innovative Pilot Plan of Henan University (2012, 2013).
Synthesisandcrystalstructureofanorganic-inorganiccomposite
phosphotungstate[HTA]3[α-PW12O40]·6H2O
QI Xiuli1,2, ZHAO Haozhe1, ZHANG Jingli1, CHEN Lijuan1,2*
(1.CollegeofChemistryandChemicalEngineering,HenanUniversity,Kaifeng475004,Henan,China;
2.BasicExperimentalTeachingCenter,HenanUniversity,Kaifeng475004,Henan,China)
An organic-inorganic composite Keggin-type phosphotungstate [HTA]3[α-PW12O40]·6H2O (TA = 1,2,4-triazole) was synthesizedviathe reaction of CuCl2·2H2O, Na3[α-PW12O40]·6H2O, andL-serine with 1,2,4-triazole in aqueous solution of ethanol. The structure of as-synthesized product was characterized by infrared spectrometry and single-crystal X-ray diffraction, and its composition was determined by elemental analysis. It was found that the title compound crystallizes in the rhombohedral space groupR-3 witha= 1.793 73(12) nm,b= 1.793 73(12) nm,c= 2.440 7(3) nm,α=β= 90°,γ= 120°,Z= 6,V= 6.800 7(11) nm3,Dc= 4.682 g·cm-3,GOOF= 1.079,R1= 0.051 6 andwR2= 0.137 4. In terms of the molecular structure, the title compound consists of one saturated Keggin phosphotungstate [α-PW12O40]3-anion, three free monoprotonated [HTA]+cations and six lattice water molecules.
polyoxometalate; phosphotungstate; Keggin structure; synthesis; crystal structure
O 614.3DocumentcodeAArticlcleID1008-1011(2014)01-0008-05
Biography:CAO Jing (1988-), female, master, majoring in polyoxometalate-based functional materials.*
. E-mail: zhaojunwei@henu.edu.cn.
