Biological characteristics of[18F]-THK523 for tau imaging∗
2014-08-05KONGYanYan孔艳艳SIZhan司展ZHANGZhengWei张政伟GUANYiHui管一晖CAOGuoXian曹国宪XUEFangPing薛方平HUAFengChun华逢春WUPing吴平ZHAOJun赵军ZHUJianHua朱建华LICong李聪CHENJian陈键andQIANJun钱隽
KONG Yan-Yan(孔艳艳),SI Zhan(司展),ZHANG Zheng-Wei(张政伟),GUAN Yi-Hui(管一晖),,CAO Guo-Xian(曹国宪),XUE Fang-Ping(薛方平),HUA Feng-Chun(华逢春),WU Ping(吴平),ZHAO Jun(赵军),ZHU Jian-Hua(朱建华),LI Cong(李聪),CHEN Jian(陈键),and QIAN Jun(钱隽)
1PET Center,Huashan Hospital,Fudan University,Shanghai 200235,China
2Key Laboratory of Nuclear Medicine,Ministry of Health, Jiangsu Key Laboratory of Molecular Nuclear Medicine, Jiangsu Institute of Nuclear Medicine,Wuxi 214063,China
3Key Laboratory of Smart Drug Delivery,Ministry of Education&PLA, School of Pharmacy,Fudan University,Shanghai 200032,China
Biological characteristics of[18F]-THK523 for tau imaging∗
KONG Yan-Yan(孔艳艳),1SI Zhan(司展),1ZHANG Zheng-Wei(张政伟),1GUAN Yi-Hui(管一晖),1,†CAO Guo-Xian(曹国宪),2XUE Fang-Ping(薛方平),1HUA Feng-Chun(华逢春),1WU Ping(吴平),1ZHAO Jun(赵军),1ZHU Jian-Hua(朱建华),3LI Cong(李聪),3CHEN Jian(陈键),3and QIAN Jun(钱隽)3
1PET Center,Huashan Hospital,Fudan University,Shanghai 200235,China
2Key Laboratory of Nuclear Medicine,Ministry of Health, Jiangsu Key Laboratory of Molecular Nuclear Medicine, Jiangsu Institute of Nuclear Medicine,Wuxi 214063,China
3Key Laboratory of Smart Drug Delivery,Ministry of Education&PLA, School of Pharmacy,Fudan University,Shanghai 200032,China
Reliable and non-invasive diagnostic tools are highly valuable for successful therapeutic strategies for the treatment of Alzheimer’s disease(AD).The existence of neurofibrillary tangles(NFTs)consisting of tau protein are one kind of the pathological features of AD,and its level of severity is correlated with the stage of AD. However,no clinically approved positron emission tomography(PET)probe is currently available for selective imaging of neurofibrillary tangles on patients.In this paper,we report our studies on biological characteristics of[18F]-THK523 as a novel tau imaging probe.With low molecular weight,[18F]-THK523 is stable,electrically neutral,lipophilic and non-mass concentration-dependent.Preliminary biological studies have shown the excellent properties of[18F]-THK523 as brain imaging tracer for further research.
[18F]-THK523,Neurofibrillary tangles(NFTs),Alzheimer’s disease(AD),Tau-specific probe,Biological characteristics
I.INTRODUCTION
NationalInstituteonAging-Alzheimer’sAssociation (USA)has suggested that Alzheimer’s disease(AD)would be optimally treated before significant cognitive impairment, defined as a‘presymptomatic’or‘preclinical’stage[1].Diagnosis and treatment strategies for AD are based on sensitive and specific detection of the incipient neuropathological characteristics,combined with emerging treatments that counteract molecular processes in AD pathogenesis.The hyperphosphorylation of tau protein and formation of intraneuronal neurofibrillary tangles(NFTs)represent a characteristic neuropathological feature in AD brain.
Tau,localizing in the axons of neurons,is a microtubuleassociated protein(MAP)and maintains microtubules(MTs) stability,neurite outgrowth and chromosome stability[2–5]. NFTs are constituent of aggregated paired helical filaments (PHF)comprising of aberrantly phosphorylated tau.NFTs are formed in entorhinal cortex at the early stage of AD,and spread to the dentate gyrus,hippocampus,and cingulate cortex as the memory loss develops[6].NFTs,especially soluble hyperphosphorylated tau aggregations,interact with Aβmediated toxicity,oxidative stress,inflammation and abnormal mitochondrial function[7,8].Also,anti-tau treatmentcan reduce Aβ formation and the excitotoxicity levels[9,10].
The utility of positron emission tomography(PET),with a radio-ligand for translocator protein as a biomarker for tau-triggered toxicity tau imaging and diagnostic assessment of tauopathies,with and without Aβ pathologies,shall be of technical importance for both clinical and basic research aimed at prodromal pathologies of AD.
There is no effective treatment target tau pathology used clinically.Nakamuraet al.[11]reported in 2012 that unlike trans p-tau,cis isomerization p-tau by proline-directed kinases appeared early in the brains of humans with mild cognitive impairment,accumulated exclusively in degenerated neurons,and localized to dystrophic neuritis during AD progression.Cs isomer cannot promote MTs assembly.It is more resistant to dephosphorylation and degradation,and more prone to aggregation.Conventional peptidyl-prolyl cistrans isomerases(PPIases)Pin1 can convertcistotransptau to prevent Alzheimer’s tau pathology.Tau-specific PET probe can effectively evaluate such new approach with accurate,reliable,and reproducible noninvasive monitoring of tau protein aggregates in the living brain.
There has been an increasing focus on developing PET imaging radiotracers for preclinical diagnosis of AD,especially[18F]-THK523,which may be a potential tau targeted probe.In vitrobinding studies demonstrated that[18F]-THK523 had higher af fi nity to a greater number of binding sites on recombinant tau(k18Δ280k)than β-amyloid1−42fi brils.[18F]-THK523 bound to tau pathology on autoradiographic and histo fl uorescence analysis of AD hippocampal serial sections.It had higher retention in tau transgenic mice brain than wild-type littermates mice,and that it bound to recombinant tau with much higher af fi nity than it did toβ-amyloid plaques[12].And it showed higher af fi nity totau fi brils than Aβfi brils by comparing the binding properties of[18F]-THK523 and other amyloid imaging agents,including PiB,BF-227 and FDDNP,to synthetic protein fi brils and human brain tissue[13].Zenget al.[14]demonstrated that[3H]-THK523 binds to NFTs and Aβ plaques in human AD brain sections.However,in transgenic mouse brain sections,[3H]-THK523 binds only to Aβ but fails to bind to NFTs.Okamuraet al.[15]reported that novel18F-labeled arylquinolinederivatives,18F-THK-5105and18F-THK-5117, had higher binding affinity for tau protein aggregates and tau-rich AD brain homogenates,and higher brain uptake and faster clearance in normal mice than[18F]-THK523.In this paper,wereportourcomplementarybiologicalcharacteristics studies to investigate whether[18F]-THK523 can meet ligand criteria for tau imaging tracer.
II.MATERIALS AND METHODS
A.Labeling procedure
The 2-((2-(4-(tert-butoxycarbonyl)amino)phenyl)quinolin-6-yl)oxy)ethyl 4-methylbenzenesulfonate(THK-7),as protected precursor,was synthesized at our lab[16].[18F]-THK523 was radio-synthetized with high yield from THK-7 by a fully automated module(PET Science&Technology Co.Ltd.,Beijing,China)[16].Aqueous18F–trapped on a quadrupole mass analyzer(QMA)cartridge was washedby1.5mLofK2CO3(2.73mg/mL)/KryptofixTM2.2.2 (11.82mg/mL),and the solvents were evaporated.After 2mg of THK dissolved in 1mL of acetonitrile(2mg/mL)was added,the nucleophilic substitution reaction was carried out at 120◦C.To hydrolyze the Boc protecting group,1N HCl (250µL)solution was added.The mixture was allowed to react at 105◦C for 5 minutes.The excess HCl was neutralized by 2N NaOH(125µL).Saturated 1N NaHCO3(125µL)was added to adjust the pH value to 7.4.The product was loaded on a Sep-Pak tC18 SPE cartridge,and washed with water to remove free18F–,polar byproducts,KryptofixR○TM2.2.2,etc. The cartridge was then washed with 2mL of ethanol.Crude product was collected after passing through a sterile filter, followed by further purification using semipreparative highperformance liquid chromatography(Waters XBridgeTMprep Shield RP18 10µm,250mm×10mm,part No.186003990, serial No.101/123041GG01,70%EtOH:30%H2O;Waters Corporation,Milford,Massachusetts,USA)equipped with Bioscan radioactivity detector at a flow rate of 4mL/min and stabilized with ascorbic acid(2mg,0.011mmol)before sterile filtration.Quality control of[18F]-THK523 was achieved by thin layer chromatography(TLC)and radio highperformance liquid chromatography(RHPLC).
Radiochemical yield of[18F]-THK523 was evaluated by TLC using silica gel G60 with fluorescence(F254)plates (cut into 10cm×0.4cm strips)as stationary phase while ethyl acetate:n-hexane:triethylamine=4:1:0.005(V/V/V) as mobile phase.The reaction product was spotted with a capillary and developed by mobile phase.After development,the strips were dried at room temperature,cut into 1cm×0.4cm pieces and counted by Wizard 1470 automatic gamma counter(Perkin Elmer Company,USA)equipped with a multi-channel analyzer.Retention factor(Rf)and labeling yield were determined from TLC chromatogram data. Two TLCs ran for each tested reaction condition and the data were averaged as the labeled rate.
The radiochemical purity(RCP)of[18F]-THK523 was determined by analytical RHPLC.The sample was passed through a Millipore fi lter carefully and then injected into the HPLC column(PurospherR○STAR LPRP-18e endcapped (5µm),250×4.6mm,sorbent Lot No.TA1752311,column No.210072)at room temperature.The absorbance was measured at 350nm and the fl ow rate was adjusted to 0.6mL/min. An injection volume of 20µL tracer was used with a mobile phase at volume(acetonitrile)/volume(containing triethylamine 0.05%water)ratio of 80%/20%.Retention time (Rt)was measured and checked with the standard product.
B.Electrophoresis
The charge of[18F]-THK523 was determined by paper electrophoresis using kalium phosphate buffer solution:alcohol:distilled water,1:1:1(V/V/V)with pH 7.4 as electrolyte and Xinhua No.1 papers strips as a support.The sample was run at a constant voltage of 110V for 2.5h of standing time. The strip was scanned by gamma-counter.For comparison,a sample of18F–was run under the identical condition.
C.Determination of lipid–water partition coef fi cient of [18F]-THK523
Lipid–water partition coef fi cient of[18F]-THK523 was measured in two steps.Step 1:1mL of phosphate-buffered saline(PBS)(pH=7.4)saturated by n-octyl alcohol and 1mL of n-octyl alcohol saturated by PBS(pH=7.4)were added to centrifuge tube containing 100µL of sample.Step 2:The tube was capped and vortexed for 10min at room temperature,and then stood for 5 min.After reaching equilibrium,the tube was centrifuged at 2000r/min(r=6.0cm)for 10min.100µL of the organic phase and water phase were pipetted out respectively and each phase was counted by the gammacounter.Theorganicphaseof500µLwaspipettedout into another centrifuge tube and then followed by the addition of 500µL of n-octyl alcohol saturated by PBS(pH=7.4)and 1mL of PBS(pH=7.4)saturated byn-octyl alcohol.Step 2 was repeated for six times.The partition coef fi cient was calculated as(cpm in organic phase)/(cpm in water phase).
D.Measurement of plasma protein binding rate
Heparin anticoagulant fresh blood plasma of 10 volunteers was provided by Nuclear Medicine Department,Huashan Hospital af fi liated to Fudan University.Trichloroacetic acid with volume fraction of 10%and 25%was prepared,respectively.The experiment was divided into the high,middle,mid-low and low dose groups,each having four parallel samples.Each tube contained 0.2mL blood plasma and 0.1mL of[18F]-THK523 in activity of 22.20,2.22,0.22 or 0.02MBq for the high,middle,mid-low and low dose groups,respectively.Being incubated for 2 hours at 37◦C,each tube was added with 1mL of 25%trichloroacetic acid.They were vortex blended,and centrifuged at 2000r/min(r=6.0cm)for 10min.Then,the supernatant was collected.Afterwards, 1mL of 10%trichloroacetic acid was added to the precipitate. This step was repeated twice.According to the radioactivity counts of precipitation and supernatant,plasma protein binding rate was calculated as following:plasma protein binding rate=[(precipitationradioactivecounts)/(precipitation+supernatant radioactive counts)]×100%.
E.Stability studies
Stability assessment of the complex was carried out by measuring its radio chemical purity at 25◦C.The radiochemical purity of[18F]-THK523 was determined by TLC and the radioactivity of[18F]-THK523 was counted by gamma counter at 0.5,1,1.5,2,3,4,5,6 and 7h after preparation.
F.Blood kinetic studies
Blood clearance studies were performed in C57 mice (n=5,(21±1)g).For each animal,5.18MBq/140µCi of the[18F]-THK523(0.1mL)was administered intravenously through the tail vein.Blood samples(10µL)were collected from the tail vein and radioactivity was measured by the gamma counter at different time intervals(2,5,10,15,20, 30,45 and 60min)after intravenous injection.The data was expressed as percentage of the administered dose at each time point.The weight of each blood sample was determined by weighing the microcentrifuge tube before and after blood collection.The concentrations of radioactivity in the blood were calculated as%ID/g.The blood clearance patterns of [18F]-THK523 were simulated using Pharmacokinetics Local Model(PLM)software developed by Caoet al.[17].
G.Micro PET Imaging
Normal C57 mice((20±2)g)were acquired with a SiemensInveonPET/CTsystem(SiemensMedicalSolutions, Knoxville,USA).After induction of anesthesia and placement of the catheter systems,the animals were placed with their bodies in the center of the fi eld of view and were fi xed in the scanner in prone feet fi rst position(FFP).At the beginning of the PET scanning procedure,a CT scan(Inveon)was performed for all animals.[18F]-THK523 was given via the catheter system intravenously in a slow bolus.The total applied volume was(0.18±0.02)mL.The amount of injected activity was(0.15±0.03)mCi.Radioactivity in the syringe and catheter was measured immediately before and after injection.Dynamic data acquisition was performed by Inveon Acquisition Workplace(IAW,Siemens)for 60min starting immediately after injection(p.i.)of the tracer.A PET image was reconstructed from 600 million coincidental 511keV photon counts.A reconstruction of sinograms yielded a 3D mapping of positron signal using Fourier rebinning and a 2D fi ltered back-projection algorithm with a ramp fi lter.And the voxel size was set as 0.80mm×0.86mm×0.86mm.CT images were reconstructed using a modi fi ed Feldkamp cone beam reconstruction algorithm(COBRA)from 360 projections with isotropic pixel size of 110µm.The emission data were normalized and corrected for decay and dead time.The resulting sinograms were reconstructed with FBP( fi ltered back-projection)into 8 frames(1@120;1@180;3@300; 1@600;2@900)of equal length used for motion correction, ratio measurements and image production for time-activity curve(TAC)generation.
For each micro PET scan,three-dimensional regions-ofinterest(ROIs)were drawn over the major organs by using vendor software(Inveon Research Workshop;IRW)on decay-corrected whole-body images.All PET and CT image datasets were scaled to calibrated kBq/cc and saved in fl oat format.Orientation of planes was con fi rmed to radiological human brain standard such that theZ-axis was perpendicular to horizontal sections.
To retrieve reliable small-animal PET results,accurate and standardized co-registration of PET to CT is essential.A twostep matching process of PET data was used.The initial automatic rigid matching was performed fi rst,and manual adjustment was applied if necessary.High-resolution CT scan was used as the basis for VOI de fi nition.To quantify the dynamic data,TACs with high initial time resolution were used.
H.Biodistribution studies in mice
Ex vivobiodistribution studies were carried out to confi rm that the quantitative tracer uptake values based on non-invasive micro PET imaging truly represented the actual tracer distribution in normal mice.Fifty C57 mice ((20±2)g)from Shanghai Slac Laboratory Animal CO.Ltd. were used in animal experiments(25 female,25 male).They were divided into ten groups randomly according to sacri fi ce time points.[18F]-THK523(0.1mL)in activity of 5.18MBq/140µCi was injected into the tail vein of each mouse and the animals were sacri fi ced at 2,5,10,15,30, 45,60,120,180 and 240min after injection.Samples of the major organs/tissues of interest,including liver,spleen,pancreas,stomach,intestine,femur,muscle,gonad,lung,kidney, heart,brain and blood,were collected and wet-weighed.Speci fi c radioactivity of the tissue samples was measured using a gamma-counter.The percent dose per organ was calculated byacomparisonofthetissuecountstothecountsofasuitably diluted aliquot of the injected material.The concentrations of radioactivity in the blood were also calculated as%ID/g.
The experiments were carried out in compliance with national laws for the conduct of animal experimentation and were approved by the local committee for animal research.
III.RESULTS
A.Electrophoresis
Charge of the complex was con fi rmed by paper electrophoresis.Table 1 shows that 95.8%of[18F]-THK523 stay still under the condition of current,indicating that it was electrically neutral;while the18F-species moved to anode,indicating that the compound exhibited anionic behavior.

TABLE 1.Electrophoresis of[18F]-THK523
B.Determination of lipid–water partition coef fi cient of [18F]-THK523
The lipophilicity of[18F]-THK523 is determined by lipid–water partition coef fi cient(lgP),and the results are listed in Table 2(lgP=0.99±0.06,n=7),indicating[18F]-THK523 is lipophilic,which is consistent with Ref.[12,13].
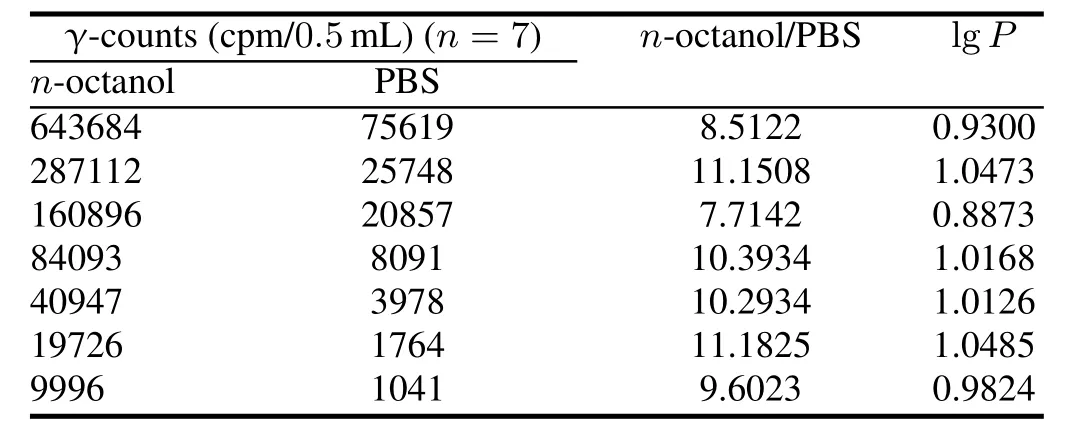
TABLE 2.Lipid-water partition coef fi cient of[18F]-THK523
C.Measurement of plasma protein binding rate
Plasmaproteinbindingratewas(8.68±0.45)%, (7.86±0.32)%,(8.13±0.35)%and(8.11±0.53)%for the high,middle,mid-low and low concentration groups, respectively.They do not differ signi fi cantly,indicating that the protein binding rate of[18F]-THK523 is not of the mass concentration-dependent nature.
D.Stability studies
Stability of the radiolabeled compound over time was investigated.According to our test results,radiochemical purity of[18F]-THK523 was stable,remaining at the level of about 90%for up to 5h.It was decreased to 87.6%and 86% at 6h and 7h respectively(Fig.1).Considering the radioactive decomposition and decay of18F,it is better to applied [18F]-THK523 within 5h after preparation.

Fig.1.Stability of[18F]-THK523 at room temperature.
E.Blood kinetic studies
The following dual-exponential equation was adopted to model the pharmacokinetic of[18F]-THK523 in mice in mice:Y=2.23e−0.014t+1.89−0.0007t,whereYis%ID g−1in blood;tis time in minute.The distribution and elimination were coincident with the results given by compartment modeling.Thepharmacokineticsparametersof[18F]-THK523are listed in Table 3.

TABLE 3.Pharmacokinetic parameters of[18F]-THK523 in mice (131.35MBq/mL,n=5)
F.Micro PET imaging and Biodistribution studies in mice
The biodistribution of[18F]-THK523 was determinedex vivoin healthy mice at 2,5,10,15,30,45,60,120,180 and 240min after intravenous injection.The uptakes in liver((7.96±0.97)%ID/g),kidney((4.32±0.33)%ID/g) and heart((4.18±0.28)%ID/g)were the highest initially at 2min,followed by fast clearance(Fig.2(a)).Preclinical study showed that the highest[18F]-THK523 uptake occurred in gall bladder,followed by liver,kidney,heart and intestine,whereas femur and gonads showed the lowest uptake(Fig.2(b)).Within less than 15min,[18F]-THK523 essentially cleared from blood and plasma.[18F]-THK523was mainly metabolized by liver and excreted through biliary,thus leading to substantial rise in the uptake in intestine at 15min and then slow decline started approximately at 120min.Micro PET imaging demonstrated such changes vividlyin vivo,agreeing with the biodistribution analysisexvivo(Figs.2(a)and 2(c)).The bone uptake rose and then decreased slowly because of radioactive decomposition,which could be improved with the presence of ascorbic acid(2mg, 0.011mmol).
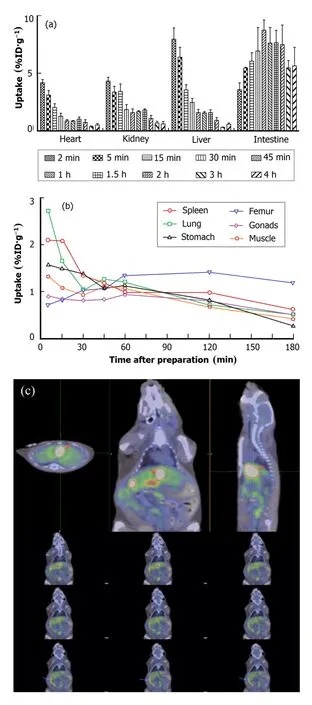
Fig.2.(Color online)Biodistribution of[18F]-THK523 in main metabolic organs(a)and other organs(b)in normal mice,and smallanimal PET images of a mouse at 60min after[18F]-THK523 injection.The orange arrows indicate gall bladder,and the blue arrows indicate intestine.The data are expressed as means±SD(n=5).

Fig.3.(Color online)Biodistribution of[18F]-THK523 in main regions(a)and cortex of in normal mice brain(expressed as means,n=5).
The brain uptake was(2.62±0.39)%ID/g at 2min after injection.Mouse brains were dissected into following regions:cortex(front cortex,parietal cortex, temporal cortex,occipital cortex),striatum,hippocampus,thalamus,cerebellum,pons and medulla oblongata. The uptakes in occipital cortex((4.91±0.94)%ID/g), temporal cortex((3.33±0.72)%ID/g)and hippocampus ((3.07±0.35)%ID/g)were the highest initially at 2min,followed by fast clearance(Figs.3(a)and 3(b)).The tracer uptake for the occipital cortex and temporal cortex were higher than other cortex(Fig.3(b)).The brain uptake trend of[18F]-THK523 was similar betweenex vivoandin vivofrom injec-tion to 60min.The highest brain uptake was at 2min after injection,followed by quick clearance during 90min postinjection.The clearance was relatively slow from 90min to 240min after injection(Fig.4).
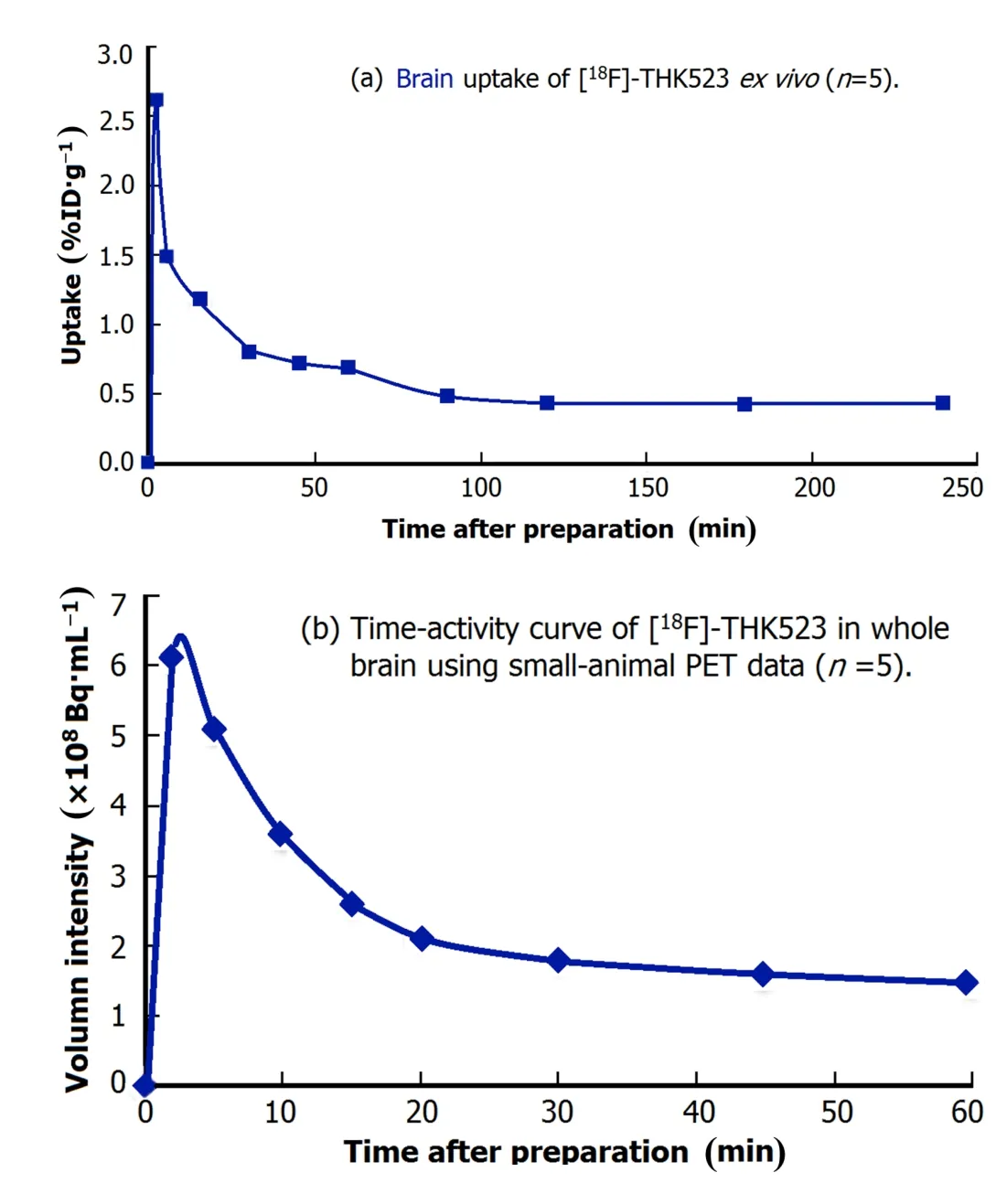
Fig.4.(Color online)The brain uptake trend of[18F]-THK523 over time in normal miceex vivo(a)andin vivo(b).
IV.DISCUSSION
NFTs are one kind of the pathological hallmarks found in AD brains and are closely associated with the severity of dementia,indicating the contribution of NFTs to neuronal dysfunction.NFTs are therapeutic target of AD for disease modifying therapy.A PET tracer for imaging NFTs in the brain shall be valuable in developing new therapies for AD.
Considering the factors related to brain uptake,a radioactive tracer can hardly cross the blood-brain barrier(BBB)if it is not electrically neutral or non-lipophilic.For brain tracer, lowplasmaproteinbindingrateisalsocriticaltobrainuptake. If plasma protein binding rate is too high,the tracer would be unable to get access to target region.The analytical data and favorable lgPsuggest that[18F]-THK523 should cross the BBB and enter the central nervous system(CNS).Also, with the presence of ascorbic acid,[18F]-THK523 is stable at room temperature up to 5 h.All these biological characteristics demonstrate that[18F]-THK523 satis fi es the basic requirements of brain tracer.Our blood kinetic studies show that[18F]-THK523 distributes quickly from blood to other organs(t1/2α=47.9min),with relatively favorable retention time in target organs(t1/2β=965.1min).
Metabolism and biodistribution patterns should be evaluated for any radiopharmaceutical candidate being considered for clinical translation.Ourex vivostudies reveal a high brain uptake at 2min after injection,especially in the hippocampus,followed by rapid clearance in healthy mice.In vitroandin vivostudies have con fi rmed that[18F]-THK523 is of high af fi nity and selectivity for tau pathology[12,13],and our biological characteristics results shall help ful fi lling its brain ligand criteria for further imaging trials.
V.CONCLUSION
[18F]-THK523 was radiosynthesized on automated module anditsbiologicalcharacteristicswereevaluated.Invitrostudies demonstrated that[18F]-THK523 was electrically neutral, lipophilic(lgP=0.99±0.06,n=7)and quite stable with its radiochemical purity of more than 90%maintained for up to 7h at room temperature.Due to its low molecular weight,[18F]-THK523 can easily cross blood brain barrier(BBB).With relatively low plasma protein binding rate, [18F]-THK523 is not of mass concentration-dependent nature.From calculations of pharmacokinetics parameters of the blood,and the blood kinetic study,the dual-exponential equation wasY=2.23e−0.014t+1.89−0.0007twitht1/2α= 47.9min−1,t1/2β=965.1min−1,K12=0.0067min−1,K21=0.0070min−1,Ke=0.0015min−1,plasma clearance=0.036%ID/g/min,area under concentration-time curve=2785.1ID%/g/min.[18F]-THK523 is of high brain uptake,and our study on its biodistribution in healthy C57 mice shows that[18F]-THK523 is mainly metabolized by liver and excreted through biliary.[18F]-THK523 may well be a promising candidate for molecular imaging of tau pathology.
ACKNOWLEDGEMENTS
We are grateful to GAO Xi-Hui,HUANG Cui-Yun, ZHENG Shu-Yan and WANG Feng for helpful brain anatomy and counseling in all aspects of animal handling and experimenting.
[1]Mori T,Maeda J,Shimada H,et al.Psychogeriatrics,2012,12: 106–114.
[2]Wang J Z and Liu F.Prog Neurobiol,2008,85:148–175.
[3]Rossi G,Dalpr`a L,Crosti F,et al.Cell Cycle,2008,7:1788–1894.
[4]Witman G B,Cleveland D W,Weingarten M D,et al.Proc Natl Acad Sci USA,1976,73:4070–4074.
[5]Guo J L,Covell D J,Daniels J P,et al.Cell,2013,154:103–117.
[6]de Calignon A,Polydoro M,Suarez-Calvet M,et al.Neuron, 2012,73:685–697.
[7]Revett T J,Baker G B,Jhamandas J,et al.J Psychiatr Neurosci, 2013,38:6–23.
[8]Ittner L M,Ke Y D,Delerue F,et al.Cell,2010,142:387–397.
[9]RobersonED,Scearce-LevieK,PalopJJ,etal.Science,2007,316:750–754.
[10]Nussbaum J M,Schilling S,Cynis H,et al.Nature,2012,485: 651–655.
[11]Nakamura K,Greenwood A,Binder L,et al.Cell,2012,149: 232–244.
[12]Fodero-Tavoletti M T,Okamura N,Furumoto S,et al.Brain, 2011,134:1089–1100.
[13]Harada R,Okamura N,Furumoto S,et al.Eur J Nucl Med Mol, 2013,40:125–132.
[14]Zeng Z,Chen T B,Miller P,et al.Alzheimers Dement,2012,8:P66.
[15]Okamura N,Furumoto S,Harada R,et al.J Nucl Med,2013,54:1420–1427.
[16]Kong Y Y,Cao G X,Zhang Z W,et al.Nucl Sci Tech,2014,25:040302.
[17]Cao G,Zhou X,Kong Y,et al.Nucl Tech,2013,36:60–65.(in Chinese)
10.13538/j.1001-8042/nst.25.050302
(Received January 7,2014;accepted in revised form February 21,2014;published online October 4,2014)
∗Supported by National Natural Science Foundation of China(Nos. 81271516 and 81371625),Program of Shanghai Science and Technology Commission(Nos.13JC1401503 and 14DZ1930402),Shanghai MunicipalHealthandFamilyPlanningCommission(No.2013313)andExchange Programme Foundation of Doctoral Student under the Office for Graduate Medical Education,Fudan University
†Corresponding author,guanyihui@hotmail.com
猜你喜欢
杂志排行
Nuclear Science and Techniques的其它文章
- Dose rate distribution of photoneutrons in an ID beamline of SSRF:simulations and measurements
- Calibration method for electrode gains in an axially symmetric stripline BPM∗
- A study of quasi-absolute method in photon activation analysis∗
- High-resolution boosted reconstruction of γ-ray spectra∗
- A fractionation model based on three lognormal particle size distributions
- Model-predictive control of power supply for particle accelerators∗
