新型杂化材料[(2,2′-bipy)2Cu][((2,2′-bipy)2Cu)2PW12O40]·4H2O的水热合成及结构表征
2014-07-27马云飞孙乙庭王瑞库冯引弟刘双英马琢琪栾国有
张 科,马云飞,孙乙庭,王瑞库,冯引弟,刘双英,马琢琪,栾国有
(1.吉林省水利水电勘测设计研究院,吉林 长春 130012;2.现代天丰农业集团有限公司,吉林 长春 130000;3.吉林农业大学资源与环境学院,吉林 长春 130118)
新型杂化材料[(2,2′-bipy)2Cu][((2,2′-bipy)2Cu)2PW12O40]·4H2O的水热合成及结构表征
张 科1,马云飞1,孙乙庭1,王瑞库1,冯引弟2,刘双英1,马琢琪1,栾国有3
(1.吉林省水利水电勘测设计研究院,吉林 长春 130012;2.现代天丰农业集团有限公司,吉林 长春 130000;3.吉林农业大学资源与环境学院,吉林 长春 130118)
用水热方法合成了一种新型的无机-有机杂化材料[(2,2′-bipy)2Cu][((2,2′-bipy)2Cu)2PW12O40]·4H2O,并采用元素分析、形貌分析、红外光谱、紫外光谱、热重分析和X射线单晶结构分析对其进行了表征.结果表明:该化合物属于单斜晶系,P2(1)/n空间群;晶胞参数a=1.890 4 nm,b=2.047 8 nm,c=2.172 8 nm,α=90°,β=96.404°,γ=90°,V=8.358 9 nm3,Z=4,R1=0.050 2,wR2=0.117 1.
多金属氧酸盐;无机-有机杂化材料;晶体结构;水热合成
0 引言
1 实验部分
1.1 试剂与仪器
试剂:所用化学试剂均为分析纯;所用水为蒸馏水,使用时未进一步纯化.
仪器:元素分析采用美国Leeman labs Inc产PLASMASPE电感耦合等离子体发射光谱仪;样貌分析采用日本岛津公司产的SSX-550型扫描电子显微镜测定晶体的外观;红外光谱采用KBr压片,用美国Nicolet公司AV360型傅里叶红外光谱仪测定,测量范围400~4 000 cm-1;紫外光谱采用北京普析通用仪器有限责任公司TU-1901紫外可见分光光度计测量,测量范围190~900 nm;热重分析采用日本岛津公司产TA-60型差热分析仪,升温速度为10℃;晶体结构采用德国Bruker Smart CCD X单晶衍射仪测定.
1.2 化合物的合成
将H3PW12O40(0.22 g,0.076 mmol),Cu(CH3CO2)2·H2O (0.15 g,0.75 mmol),2,2′-bipy(0.10 g,0.64 mmol)溶解于10 mL H2O中,充分搅拌均匀,用1 mol/L的盐酸调节pH值至6.5,然后将溶液转移到25 mL的聚四氟乙烯反应釜中,填充度为60%,在160℃条件下晶化5 d,自然冷却至室温.过滤得到黑色块状晶体C60H56Cu3N12O44PW12.元素分析(单位为质量分数/%,括号内为计算值):C 17.66 (17.54),N 4.12 (4.21),H 1.37 (1.29).
1.3 晶体结构的测定
选取大小为0.29 mm×0.26 mm×0.22 mm的晶体用于单晶结构分析.晶体的X射线衍射数据在德国Bruker Smart CCD X单晶衍射仪上收取,在1.47<θ<26.05°范围内,采用直接法以SHELXTL-97程序解出,全矩阵最小二乘法修正[26].结果表明:晶体属于单斜晶系,P2(1)/n空间群;晶胞参数a=1.890 4 nm,b=2.047 8 nm,c=2.172 8 nm,α=90°,β=96.404°,γ=90°,V=8.358 9 nm3,Z=4,R1=0.050 2,wR2=0.117 1.
2 结果与讨论
2.1 晶体结构
标题化合物是由一个双支撑Keggin型[((2,2′-bipy)2Cu)2PW12O40]-阴离子、一个分离的[Cu(2,2′-bipy)2]+阳离子和4个溶剂水组合而成,[((2,2′-bipy)2Cu)2PW12O40]-通过过渡金属Cu将阴离子簇[PW12O40]3-与2个2,2′-bipy组装到一起,如图1所示.杂多阴离子[((2,2′-bipy)2Cu)2PW12O40]-是由典型的Keggin型[PW12O40]3-阴离子和2个复杂的[(2,2′-bipy)2Cu]+基团组合而成.在Keggin型[PW12O40]3-阴离子模型中包含一个稍微扭曲的PO4四面体,围绕着四面体周围的是12个WO6八面体.P—O键长范围为0.153 0(9)~0.153 5(9) nm,∠O—P—O范围为109.0(5)°~109.5(5)°.W—O键长范围为0.169 7(9)~0.249 2(8) nm,∠O—W—O范围为71.3(3)°~173.0(5)°.键价计算(BVS)表明[27],阴离子[PW12O40]3-中的P和W的价态分别是+5和+6价,阳离子[Cu(2,2′-bipy)2]+中Cu的价态是+1价,并且3个Cu的价态都一样.考虑元素分析、电荷平衡和配位体系,该有机-无机杂化多金属氧酸盐晶体化合物的化学分子式确定为[(2,2′-bipy)2Cu][((2,2′-bipy)2Cu)2PW12O40]·4H2O.磷钨多氧阴离子作为二齿配体,通过桥氧原子与[Cu(2,2′-bipy)2]+阳离子进行配位,Cu—O键长分别为0.210 5(9)和0.224 3(9) nm,Cu(1)与Cu(2)分别与来自2个2,2′-bipy的4个N原子和1个[PW12O40]3-阴离子的氧原子形成5配位环境,Cu—N键长范围为0.197(3)~0.212(3) nm.在被分离的阳离子[Cu(2,2′-bipy)2]+中,Cu(3)的化合价被确定为+1价,Cu(3)与来自2个2,2′-bipy的4个N原子相连构成四配位体系,Cu—N键长范围为0.201(3)~0.206(3) nm,Cu(3)与4个N原子构成扭曲的四面体结构.化合物的主要键长和键角见表1.

图1 标题化合物的晶体结构

化学键键长/nm化学键键角/(°)W(1)—O(36)0.172(2)O(36)—W(1)—O(10)102.2(9)W(1)—O(19)0.1914(19)O(36)—W(1)—O(19)101.5(10)W(1)—O(18)0.194(2)O(10)—W(1)—O(19)86.6(9)W(2)—O(7)0.1722(19)O(36)—W(1)—O(3)102.0(9)W(2)—O(29)0.1896(17)O(10)—W(1)—O(3)155.8(8)W(2)—O(30)0.1887(19)O(19)—W(1)—O(3)88.3(8)W(3)—O(33)0.1697(19)O(3)—W(1)—O(18)87.7(8)W(3)—O(4)0.1899(18)O(7)—W(2)—O(29)101.0(9)W(3)—O(12)0.1905(19)O(30)—W(2)—O(20)89.0(8)
在晶体结构中,存在着分子内氢键和分子间氢键(见表2).沿着bc面看,分子内氢键有2种形式构成:一是晶格水分子提供H原子与相邻杂多阴离子的桥氧(O(23))和(O(34))之间形成的O(3w)—H(3A)…O(23)和O(1w)—H(1B)…O(34)氢键;二是2个结晶水中的2个氧(OW)形成的O(3w)—H(3B)…O(4w)氢键.分子间的氢键是水分子与杂氧阴离子的氧形成的O(1w)—H(1B)…O(34)和O(1w)—H(1A)…O(40)氢键.2个相邻的杂多阴离子[((2,2′-bipy)2Cu)2PW12O40]-上的2,2′-bipy基团之间存在π-π堆积作用,苯环之间的质心距离为0.339 nm,化合物通过氢键作用和π-π堆积作用最终形成超分子网络结构.

表2 标题化合物的氢键参数
对称操作:#1为-x+1,-y+1,-z;#2为-x+1/2,y-1/2,-z+1/2;#3为-x+1,-y+1,-z+1.
2.2 形貌分析
将化合物粉晶放在双面导电胶上,进行喷金处理后,用扫描电子显微镜对其不同表面形貌进行分析,形貌见图2.从图2b看出,晶体中有大量孔隙,这种孔隙应该具有吸附作用,作为催化剂时可以增大表面积,提高催化效率.
2.3 红外光谱分析


图3 标题化合物的红外光谱

图4 标题化合物的紫外光谱
2.4 紫外-可见光谱分析

2.5 热重分析
标题化合物的热重分析是在室温条件下,空气中测定的.化合物在25℃~600℃之间存在2步失重,总失重为24.92%,与理论失重24.72%一致.第1步失重约为1.83%,对应着化合物中水的失去 (1.77%);第2步在375℃~600℃失重为23.09%,归属于配体2,2′-bipy的失去[24-25,31].
[1] WANG Z L,LU Y,LI Y G,et al. Visible-light photocatalytic H2evolution over a series of transitionmetal substituted Keggin-structure heteropoly blues[J]. Chin Sci Bull,2012,57:2265-2268.
[2] SHA J Q,PENG J,LI Y G,et al. Synthesis and characterization of the highest connected 3D Wells-Dawson POM/TMC hybrid[J]. Inorg Chem Commun,2008,11:907-910.
[3] ZHANG Z M,LIU J,LI Y G,et al. Polyoxometalate-based eight-connected self-catenated network and five fold interpenetrating framework[J]. J Solid State Chem,2010,183:228-233.
[4] KATO C N,NAGAMI M,UKAI N. Strong influence of structures around aluminum centers constructed in polyoxotungstates for catalytic oxidation of alcohols with dioxygen in water[J]. Appl Catal A Gen,2013,452:69-74.
[5] MA B C,ZHANG Z X,SONG W F,et al. Solvent-free selective oxidation of C—H bonds of toluene and substituted toluene to aldehydes by vanadium-substituted polyoxometalate catalyst[J]. J Mol Catal A Chem,2013,368/369:152-158.
[6] SOUSA P M P D,GRAZINA R,BARBOSAB A D S,et al. Insights into the electrochemical behaviour of composite materials monovacant polyoxometalates @ porous metal-organic framework[J]. Electrochim Acta,2013,87:853-859.
[7] MENG X,WANG H N,WANG X L,et al. Construction and property investigation of inorganic-organic hybrid materials based on metal-salens and Keggin polyoxometalates[J]. Inorg Chim Acta,2012,390:135-142.
[8] HUANG J,HAN Z G,ZHANG H,et al. Bi-antimony capped Keggin polyoxometalate modified with Cu-ligand fragment[J]. J Solid State Chem,2012,194:65-70.
[9] HUSSAIN F,SANDRIESSER S,SPELDRICH M,et al. A new series of lanthanoid containing Keggin-type germanotungstates with acetate chelators:[{Ln(CH3COO)Ge W11O39(H2O)}2]12-{Ln=EuⅢ,GdⅢ,TbⅢ,DyⅢ,HoⅢ,ErⅢ,TmⅢ,and YbⅢ}[J]. J Solid State Chem,2011,184:214-219.
[10] CLEMENTE-JUAN J M,CORONADO E,FORMENT-ALIAGA A,et al. A new heptanuclear cobalt(Ⅱ) cluster encapsulated in a novel heteropolyoxometalate topology:synthesis,structure,and magnetic properties of [Co7(H2O)2(OH)2P2W25O94]16-[J]. Inorg Chem,2004,43:2689-2694.
[11] YAO S,ZHANG Z M,LI Y G,et al. A {Cu6}-containing inorganic-metal-organic sandwich-type tungstoantimonite and its 3D supramolecular framework[J]. Inorg Chem Commun,2009,12:937-940.
[12] YAN B B,HONDSDON S A,LI Y F,et al. Synthesis,structures and properties of new hybrid solids containing ruthenium complexes and polyoxometalates[J]. J Solid State Chem,2011,184:3179-3184.
[13] ZHANG L Z,GU W,DONG Z L,et al. Syntheses,structures and properties of a series of photochromic hybrids based on Keggin tungstophosphates[J]. J Solid State Chem,2009,182:1040-1044.
[14] ZHANG T Z,LU Y,CHEN W L,et al. Two new organic-inorganic hybrid materials based on the polyoxotungstoaluminates[J]. Inorg Chim Acta,2011,365:377-383.
[15] NIU J Y,WANG Z L,WANG J P. Hydrothermal synthesis and structure characterization of a Keggin tungstocobaltate [Co(2,2′-bpy)3]2H2[CoW12O40]·9.5H2O[J]. Polyhedron,2004,23:773-777.
[16] 方光荣,祝媛媛,杜春芳,等. (4,4′-bpyH)3[PW12O40] (4,4′-bpy)·7H2O的水热合成、晶体结构与热性质[J]. 分子科学学报,2006,22(6):367-371.
[17] WANG Y,XIAO D R,FAN L L,et al. Syntheses and characterizations of two novel networks formed by Keggin clusters and copper-organonitrogen complexes[J]. J Mol Struct,2007,843:87-94.
[18] JIN H,QIN C,LI Y G,et al. [Cu5Cl(4,4′-bpy)5] [SiW12O40]·1.5H2O a novel three-dimensional framework constructed from polyoxometalate clusters and trinuclear Cu(I) complex[J]. Inorg Chem Commun,2006,9:482-485.
[19] JIN H,WANG X L,QI Y F,et al. Hybrid organic-inorganic assemblies built up from saturated hteropolyoxoanions and copper coordination polymers with mixed 4,4′-bpyridine and 2,2′-bpyridine ligands[J]. Inorg Chim Acta,2007,360:3374-3353.
[20] DAI L M,YOU W,WANG E B,et al. 2D Rhombus-grid networks constructed from vanadium-substituted Keggin-type polyoxomolybdophosphates and Cd/Zn complex fragments[J]. Inorg Chim Acta,2009,362:4967-4971.
[21] FU H,CHEN W L,WANG E B,et al. Three new multidimensional organic-inorganic hybrids based on polyoxometalates and copper coordination polymers with 4,4′-bipyridine ligands[J]. Inorg Chim Acta,2009,362:1412-1420.
[22] WANG J P,MA P T,NIU J Y. The first polyoxometalate based on Keggin dodecatungstoselenate framework supported copper(Ⅰ) coordination group:[Cu2(2,2′-bpy)4Cl][Cu(2,2′-bpy)2SeW12O40]·2H2O[J]. Inorg Chem Commun,2006,9:1049-1052.
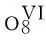
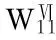
[25] SHA J Q,XIN L,ZHOU B B,et al. A novel Keggin tungstocobaltate framework supported by copperbipyridyl complexes [Cu(Ⅰ)(2,2′-bipy)2]{[Cu(Ⅱ)(2,2′-bipy)2]2[HCoW12O40]}·4H2O[J]. Z Naturforsch,2010,65B:1-6.
[26] 李翠莉,孙萍,刘术侠.一维链状化合物[Cu(C10N2H8)2(H2O)]2{[Cu(C10N2H8)][Cr(OH)6Mo6O18]2}·10H2O的合成与晶体结构[J]. 东北师大学报:自然科学版,2010,42(1):83-88.
[27] BROWN I D,ALTERMATT D. Automatic searching for chemical bonds[J]. Acta Crystallogr,1985,41B:244-247.
[28] 马翔,马鹏涛,张超,等. 三维超分子结构多金属氧酸盐(H2en)3[(NiO6)Mo6O18(As3O3)2]Cl2·6H2O的合成与结构表征[J]. 科学通报,2011,56:928-933.
[29] XIAO L N,WANG Y,CHEN Y,et al. A novel polyoxometalate tri-supported mixed-valent transition metal coordination complex[J]. Inorg Chem Commun,2010,13:1217-1220.
[30] MA P T,YU C F,ZHAO J W,et al. Hydrothermal syntheses and structural characterization of two organic-inorganic hybrid compounds based on Lindqvist polyanions[J]. J Coord Chem,2009,62:3117-3125.
[31] ZHU M,PENG J,PANG H J,et al. A new 3D transition-metal complex-templated framework based on Wells-Dawson polyoxometalate and copper(Ⅰ)-pyrazine moieties[J]. Inorg Chim Acta,2011,370:260-264.
Abstract:A novel inorganic-organic hybrid material [(2,2′-bipy)2Cu][((2,2′-bipy)2Cu)2PW12O40]·4H2O has been hydrothermally synthesized and structurally characterized by elemental analysis,scanning electron microscope,IR spectrum,UV-Vis,TGA-DSC and single-crystal X-ray diffraction. Crystal data:monoclinic,space groupP2(1)/n,a=1.890 4 nm,b=2.047 8 nm,c=2.172 8 nm,α=90°,β=96.404°,γ=90°,V=8.358 9 nm3,Z=4,R1=0.050 2,wR2=0.117 1.
Keywords:polyoxometalate;inorganic-organic hybrid material;crystal structure;hydrothermal synthesis
(责任编辑:石绍庆)
Hydrothermal synthesis and structure characterization of a novel hybrid material [(2,2′-bipy)2Cu][((2,2′-bipy)2Cu)2PW12O40]·4H2O
ZHANG Ke1,MA Yun-fei1,SUN Yi-ting1,WANG Rui-ku1,FENG Yin-di2,
LIU Shuang-ying1,MA Zhuo-qi1,LUAN Guo-you3
(1.Jilin Province Water Resource and Hydropower Consultaive Company,Changchun 130012,China;2.Modern Tianfeng Agriculture Grouplimtied Company,Changchun 130000,China;3.College of Resource and Environment Science,Jilin Agriculture University,Changchun 130118,China)
1000-1832(2014)03-0099-06
10.11672/dbsdzk2014-03-019
2013-09-13
全球环境基金资助项目(4632);吉林农业大学科研启动基金资助项目(201218).
张科(1982—),男,硕士研究生;通讯作者:栾国有(1968—),男,教授,主要从事多酸化学研究.
O 611.4 [学科代码] 150·10
A
