Energy,Resources and Environmental Technology CO2Removal from Biogas by Water Washing System☆
2014-07-12YongXiaoHairongYuanYunzhiPangShulinChenBaoningZhuDexunZouJingweiMaLiangYuXiujinLi
Yong Xiao,Hairong Yuan,YunzhiPang,Shulin Chen,Baoning Zhu,Dexun Zou,JingweiMa, Liang Yu,Xiujin Li,*
Energy,Resources and Environmental Technology CO2Removal from Biogas by Water Washing System☆
Yong Xiao1,Hairong Yuan1,YunzhiPang1,Shulin Chen2,Baoning Zhu1,Dexun Zou1,JingweiMa2, Liang Yu2,Xiujin Li1,*
1Centre for Resource and Environmental Research,Beijing University of Chemical Technology,Beijing 100029,China
2Department of Biological Systems Engineering,Washington State University,Pullman,WA 99164,USA
A R T I C L E I N F o
Article history:
Received 8 November 2012
Received in revised form 17 June 2013
Accepted 16 August 2013
Available online 14 June 2014
Biogas puri fi cation
CO2removal
Water washing
Pilot system
CO2removal from biogas by water washing system was investigated with various parameters,including liquid/ gas ratio,pressure,temperature,and CO2content.The results indicate that CO2removalratio could reach 34.6%-94.2%as liquid/gas ratio increased from 0.14 to 0.50.Increasing pressure(from 0.8 to 1.2 MPa)could improve gas purification with a constant in flow rate of gas.Temperature played a key role in the process and lower temperature in absorption tower was beneficial for reducing CO2content.CO2removalratio could reach 24.4%-83.2% when CO2content in the simulated gas was 25%-45%.The lowest CO2content after absorption was 2.6%at 1.2 MPa with 400 L·h-1gas flow and 200 L·h-1water fl ow,which meets the requirement of CO2contentin natural gas for vehicle fuel.
©2014 The Chemical Industry and Engineering Society of China,and Chemical Industry Press.All rights reserved.
1.Introduction
With increasing concerns in globalenergy crisis and environmental pollution,development of clean and renewable energy has been a strategic issue for national security and environmental protection[1].As an important biomass energy,biogas from the anaerobic digestion can not only reduce the discharge of house refuses,city sludge,and other wastes,but also play a positive role in the remission of severe“greenhouse effect”[2].Although biogas technologies in China have been well developed,the use of biogas remains in fancy[3,4].Traditionally, the biogas is mainly used for living energy for ruralfamilies with low efficiency[5,6].It can be more widely used in value-added way if the raw gas is purified to natural gas quality level[7-9].
Raw biogas is generated from various biomass wastes,and mainly consists of CH4(55%-65%)and CO2(35%-45%).The calorific value of raw biogas is 22000-25000 kJ·m-3.After CO2is removed,the methane gas has a calorific value up to 39000 kJ·m-3[10].Purified biogas with methane content above 96%has similar property as naturalgas,which can replace fossilgas and has much wider and highervalue applications. Therefore,it is important to develop purification technology for biogas upgrading.
The methods for purifying biogas mainly include absorption with organic amine solution,membrane separation,and pressure swing adsorption[11,12].The absorption with organic amine solution is highly efficient,but the regeneration of a mine solution usually involves high energy consumption and potential high corrosion,which is not environ mentally friendly[13].For the membrane separation,the membrane it self is expensive,often suffers thermal shock and chemical corrosion, and is easily contaminated[14].Although the adsorption method does not involve equipment corrosion and environmental pollution,the high operation costs,frequent adsorption/desorption cycles,and complex equipment limit its applications[15].Water washing method is a physical absorption process.The change of CO2solubility in water depends on the pressure and temperature[16,17],so the absorption and desorption of CO2can be achieved in water.Compared to above methods,water washing method uses water as absorbent,with the advantages such as lower cost,higher stability and safety,and more environmentally friendly.
The key for purifying biogas to naturalgas quality is to remove a large amount of CO2in raw biogas.Water washing method is used in this study to investigate the effects of various parameters on CO2removalin a simulated gas,including liquid/gas ratio,pressure,temperature, and initial CO2content.The study could provide guidelines for biogas purification and engineering application.
2.Materials and Methods
2.1.Materials
Raw biogas is a mixture of a few components including CH4,CO2, NH3,and H2S,and it is difficult to use it for experiments.Thus simulated biogas was employed in this study,which consists of CO2and air.The
☆Supported by the National Technology Research and Development Program of China (2008AA062402),the China-US International Cooperation Project(2011DFA90800),and the Ministry of Science and Technology,China.
*Corresponding author.
E-mail address:xjli@mail.buct.edu.cn(X.Li).CO2gas(99.99%)was purchased from Beijing Orient Medical Gas Company.Both absorbent and cooling water were tap water without any purification.
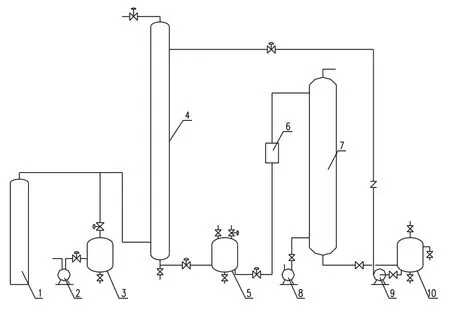
Fig.1.Experimental facility for CO2absorption.
2.2.Experimental facility
Experimental facility is a pilot device developed by our research group(Fig.1),with an air compressor,an absorption tower,a desorption tower,an air blower,a gas buffer tank,a lean solution tank,a rich solution tank,a heat exchanger,and pumps.The absorption tower is 50 mm in diameter with a total effective height of 0.9 m,packed with θ-type stainless steel rings(6 mm×6 mm).The desorption tower for regeneration is 0.1 m in diameter,with effective height of0.9 m and filled with polypropylene rings.
2.3.Experimental methods
The simulated biogas was pumped into the gas buffer tank and induced to the absorption tower.The in flow gas was controlled at different flow rates with a gas flow gauge.The tap water in the lean solution tank was pumped to and sprayed from the top of absorption tower.The CO2-rich water fl owed into the rich solution tank from the absorption tower and was further pumped into the desorption tower through a cooling process by a heat exchanger.The regenerated water was recycled from the desorption tower and fl owed into the lean solution tank.The purified simulated biogas was discharged from the top of the absorption tower.
According to the actual content of CO2in raw biogas,the volume fraction of CO2in the simulated gas was in the range of25%-45%.The pressure in the absorption tower was in the range of 0.8-1.2 MPa and the gas flow rate into the tower was 400-700 L·h-1.The flow rates of tap water and cooling water in the heat exchanger were controlled at 100-200 and 150 L·h-1,respectively.The gas flow of the air blower was kept at15 m3·h-1.
The experiments were conducted by varying the flow rates of gas and tap water,initial CO2content,temperature,and the pressure in the absorption tower.The system was kept running stably for 12 h every day under certain conditions.To determine the CO2removal, the gas at the exit of the absorption tower was collected and its CO2content was determined by a gas chromatograph(SP2100,Beifen RuiliCo., Beijing)every 0.5 h.The 24 values were averaged for the calculation of CO2removal.
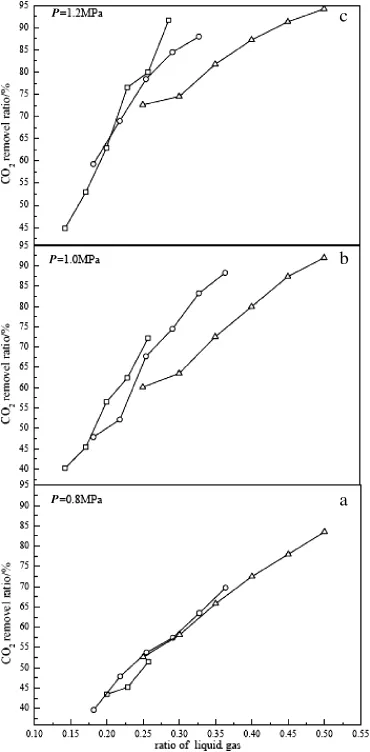
Fig.2.Effect of liquid/gas ratio and pressure on CO2removal.Inlet gas:CO245%,(□) 700 L·h-1,(○)550 L·h-1,(△)400 L·h-1;temperature:17.0-20.0°C.
3.Results and Discussion
3.1.Effect of liquid/gas ratio and pressure
The ratio of liquid/gas in the absorption could be controlled by adjusting the tap water flow with gas in flow kept constant.Different water flow rates were used in the experiments(100,120,140,160, 180,and 200 L·h-1).The gas flow rates into the absorption tower were 400,550 and 700 L·h-1.The pressure in the absorption tower was maintained at0.8,1.0 and 1.2 MPa.The volume fraction of CO2in the simulated biogas was 45%.
The effect of liquid/gas ratio on CO2absorption is illustrated in Fig.2. The CO2removalratio was enhanced from 34.6%to 94.2%as the liquid/ gas ratio varied from 0.14 to 0.50 at different pressures.At the pressureof1.2 MPa with in flow gas of400 L·h-1and water flow of200 L·h-1,the CO2removalratio reached the highest value of94.2%.This value corresponds to 2.6%CO2content in exitgas,which is lower than the requirement of3%CO2in naturalgas for vehicle utilization.When the pressure in the absorption tower was at relatively lowerlevel,0.8 MPa,increasing gas flow rate did notaffect CO2removal significantly(Fig.2a).Athigher pressures of1.0 and 1.2 MPa(Fig.2b and c),CO2removal could be improved as the gas flow rate increased at liquid/gas ratios higher than 0.25.The turbulence of liquid and mass transfer between CO2and water are strengthened in the absorption tower with higher gas flow rate[18].When the liquid/gas ratio is low(e.g.,less than 0.25),the turbulence may not appear lacking of enough energy input,leading to weak mass transfer and slight improvement on CO2removal.Therefore, better CO2removalcan be achieved by controlling both pressure and liquid/gas ratio.It is recommended to apply relatively higher pressure and liquid/gas ratio.Here,the theoretical minimum liquid/gas ratio is 0.12 and the operating ratio is 0.14-0.50,and their ratio is 1.2-4.2, which is in a reasonable range.It should be noted that high ratio may cause liquid fl ooding.
3.2.Effect of temperature
Fig.3 shows the effect of temperature on the CO2removal in the absorption tower in the range of7-40,which corresponds to the operation temperature in winter and summer seasons in China.The CO2removalratio decreases from 85.3%to 52.2%as the temperature increases from 7°C to 40°C.At higher temperatures,such as 35-40°C,the CO2removal changes little.The CO2absorption in water is a physical process,in which a large amount of dissolution heat is released.Atcertain pressure,the dissolvability of CO2is temperature-dependent,which decreases with the increase of temperature[19].Thus temperature is a decisive factor in this process.Lower temperature in the absorption tower is beneficial for the improvement of CO2removal.At temperatures higher than 35°C,dissolved CO2in the water reaches saturation equilibrium at the pressure,without the CO2removal.Hence,the temperature control in water washing system for biogas separation should be given great attentions,especially when the facility is operated in summer season.A cooling system should be used when temperature is high.
3.3.Effect of initial CO2content
Fig.4 shows the effect of initial CO2content in the inlet gas on CO2removal and content at exit.The CO2removal ratio increases from 24.4%to 83.2%as CO2content in the simulated gas increases from 25% to 45%at different water in flow rates(Fig.4a).CO2content at the exit of the absorption tower also increases(Fig.4b).This may be caused by enhanced gas-liquid mass transfer area at higher initial CO2content.It is of benefit to the CO2removal.The CO2removal is improved by increasing water in flow rate.CO2absorption in water is the liquid film controlling process[20].The liquid-film resistance decreases as the water in flow rate increases.This result again proves that high liquid/ gas ratio is beneficial for CO2absorption.

Fig.3.Effect oftemperatures on CO2removal.Pressure:1.0 MPa;gas in fl ow:550 L·h-1; water fl ow:160 L·h-1;inlet gas:CO245%.

Fig.4.Effect of initial CO2content on CO2removal.Water flow rate(L·h-1):(□)100,(○) 120,(△)140,(▽)160,()180;pressure:1.0 MPa;in flow gas:550 L·h-1.
4.Conclusions
The liquid/gas ratio,pressure,temperature,and CO2content are important parameters for CO2removal in biogas.CO2removalratio was increased from 34.6%to 94.2%as liquid/gas ratio increased from 0.14 to 0.50.Relatively higher pressure improves CO2removal.Temperature plays a key role in CO2removal.Lower temperature is beneficial for CO2removal.CO2removalratio could reach 24.4%-83.2%with CO2content of 25%-45%.The lowest CO2content after absorption could reach 2.6%at 1.2 MPa with 400 L·h-1gas flow and 200 L·h-1water fl ow, which meets the requirement of CO2content in natural gas for vehicle fuel.
[1]S.Chu,Carbon capture and sequestration,Science 325(2009)1599.
[2]Y.Z.Pang,X.J.Li,Future development of biogas industrialization and key technologies in China,Trans.CSAE 22(Supp.1)(2006)53-57.
[3]X.J.Li,B.Zhou,H.R.Yuan,Y.Z.Pang,Y.Meng,China biogas industry—challenges and future development,Trans.CSAE 27(Suppl.2)(2011)352-355.
[4]X.Y.Jiang,S.G.Sommer,K.V.Christensen,A review of the biogas industry in China, Sustain.Biofuels 39(10)(2011)6073-6081.
[5]S.R.Fang,The handicap and strategy of biogas industrialization in rural area of China,J.Agric.Mechanization Res.2(2010)216-219.
[6]H.Catwalk,A.K.Bohara,Biogas:a promising renewable technology and its impact on rural households in Nepal,Renew.Sust.Energ.Rev.13(9)(2009)2668-2674.
[7]J.L.Walsh,C.C.Ross,M.S.Smith,S.R.Harper,Utilization of biogas,Biomass 20(3,4) (1989)277-290.
[8]Y.L.Zhang,The current situation and development countermeasures of ruralbiogas in China,Renew.Energy 116(4)(2004)5-8.
[9]L.S.Chang,J.Zhao,X.F.Yin,J.Wu,Z.B.Jia,L.X.Wang,Comprehensive utilizations of biogas in Inner Mongolia,China,Renew.Sust.Energ.Rev.15(3)(2011)1442-1553.
[10]B.Yin,L.M.Chen,Q.P.Kong,Research on puri fi cation technology for vehicle biogas, Modern Chem.Ind.29(11)(2009)28-31.
[11]S.S.Kepi,V.K.Vijay,S.K.Rajesh,R.Prasad,Biogas scrubbing,compression and storage:perspective and prospectus in India context,Renew.Energy 30(8)(2005) 1195-1202.
[12]E.Ryckebosch,M.Drouillon,H.Vervaeren,Techniques for transformation of biogas to biomethane,Biomass Bioenergy 35(5)(2011)1633-1645.
[13]H.Bay,A.C.Yeh,Removalof CO2greenhouse gas by ammonia scrubbing,Ind.Eng. Chem.Res.36(1997)2490-2493.
[14]S.P.Yan,M.X.Fang,W.F.Zhang,Experimental study on the separation of CO2from flue gas using hollow fi ber membrane contactors without wetting,Fuel Process. Technol.88(5)(2007)501-511.
[15]L.Tina,Z.Deng,Z.Xia,Y.Hu,Y.Zhang,The application of variable pressure adsorption in biogas purification,Environ.Eng.28(2010)78-82.
[16]P.Chiquet,J.L.Daridon,D.Broseta,S.Thibeau,CO2/water inter tensions under pressure and temperature conditions of CO2geological storage,Energy Convers.Manag. 48(3)(2007)736-744.
[17]G.K.Anderson,Solubility of carbon dioxide in water under incipient clathrate formation conditions,J.Chem.Eng.Data 47(2)(2002)219-222.
[18]S.Y.Zhu,The Environmental and Industrial Gas Puri fi cation Technology,Chemical Industry Press,Beijing,2001.176-178.
[19]W.S.Dodds,L.F.Stutzman,B.J.Sollami,Carbon dioxide solubility in water,Ind.Eng. Chem.1(1)(1956)92-95.
[20]S.Khaisri,D.deMontigny,P.Tontiwachwuthikul,R.Jiraratananon,Comparing membrane resistance and absorption performance of three different membranes in a gas absorption membrane contactor,Sep.Purif.Technol.65(3) (2009)290-297.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Promoting Xylene Production in Benzene Methylation using Hierarchically Porous ZSM-5 Derived from a Modified Dry-gel Route☆
- Preparation,Structure Characterization and Hydrodesulfurization Performance of B-Ni2P/SBA-15/Cordierite Monolithic Catalysts☆
- Enhanced Photocatalytic Properties of Silver Oxide Loaded Bismuth Vanadate☆
- Recovery and Recycling of Ti Supported Bimodal Mesoporous Catalysts Prepared via Ship-in-a-bottle Method in the Epoxidation of Cyclohexene☆
- Biotechnology and Bioengineering The Effect of pH Controlon Acetone-Butanol-Ethanol Fermentation by Clostridium acetobutylicum ATCC 824 with Xylose and D-Glucose and D-Xylose Mixture☆
- Energy,Resources and Environmental Technology Experimental and Modeling Study on de-NOxCharacteristics of Selective Non-catalytic Reduction in O2/CO2Atmosphere☆