Recovery and Recycling of Ti Supported Bimodal Mesoporous Catalysts Prepared via Ship-in-a-bottle Method in the Epoxidation of Cyclohexene☆
2014-07-12ShiyangBaiXintaoHuJihongSunBoRenJinpengWang
Shiyang Bai,Xintao Hu,Jihong Sun*,Bo Ren,Jinpeng Wang
Department of Chemistry and chemical engineering,College of Environmental&Energy Engineering,Beijing University of Technology,Beijing 100124,China
Recovery and Recycling of Ti Supported Bimodal Mesoporous Catalysts Prepared via Ship-in-a-bottle Method in the Epoxidation of Cyclohexene☆
Shiyang Bai,Xintao Hu,Jihong Sun*,Bo Ren,Jinpeng Wang
Department of Chemistry and chemical engineering,College of Environmental&Energy Engineering,Beijing University of Technology,Beijing 100124,China
A R T I C L E I N F o
Article history:
Received 3 January 2014
Received in revised form 16 January 2014
Accepted 11 February 2014
Available online 18 June 2014
Ti/BMMs
Ship-in-a-bottle method
Recovery
Recycling
Epoxidation of cyclohexene
Ti/BMMs(Tisupported bimodalmesoporous silica)catalysts have been prepared via self-assembly route combined with ship-in-a-bottle method.The recovery and recycling performances of Ti/BMMs were investigated in the epoxidation of cyclohexene.In order to the evaluate the regeneration methods and to examine the deactivation behaviors,the deactivated Ti/BMMs catalysts were washed in chloroform or calcinated at 450°C for 6 h and then activity of the recovery catalysts were examined.Meanwhile,the structure features and surface properties of the regenerated catalysts were characterized by X-ray diffraction,N2-sorption analysis,scanning electron microscopy,transmission electron microscopy,Fourier transform infrared spectroscopy,thermogravimetric analysis,UV visible spectroscopy and X-ray photoelectron spectroscopy.The results showed that the typical bimodal mesoporous structure of recycled Ti/BMMs catalysts was stillmaintained,and the phenomenon of Tileaching during the catalytic process and recovery was negligible.In particular,spectroscopic observations indicated that the effects of the regeneration methods on the tetrahedrally-coordinated Tispecies and catalytic deactivation were remarkable.The main reasons were related to the polarities of used solvents during recovery tests,the environment medium of adsorbed water inside mesopore channels and the deposition of bulky molecules of by-products on the mesoporous surface.
©2014 The Chemical Industry and Engineering Society of China,and Chemical Industry Press.Allrights reserved.
1.Introduction
Catalytic epoxidation processes play an important role in the manufacture of fine chemicals[1,2].Ti-containing molecular sieves have attracted much attention in the past decades,since Ti-silicalite[3],Tiβ-zeolite[4],and Ti-MWW[5,6]have shown excellent performances in various oxidation reactions under mild conditions.However,their small pores hinder the accessibility of bulky substrates as well as large organic oxidants,leading to the low catalytic activities.Presently, several Ti-containing mesoporous materials,such as Ti-MCM-41[7-9], Ti-HMS[10],Ti-MCM-48[11-14],and Ti-SBA-15[15-21]have been systematically investigated by incorporation of tetrahedrally coordinated Ti species into the framework via one-pot routes using co-condensation of silicon and Tiprecursors or post-synthesis methods through functional modification.Recently,investigators described that the rapid deactivation of mesoporous catalysts originated from the serious leaching of Tispecies.Some researchers[22,23]reported Ti-SBA-15 catalysts in which the stability of the tetrahedrally-coordinated Tispecies was improved by using acetylacetone(acac)as coordinating ligands,and showed that the presence of acac is essential to prevent the formation of anatase TiO2on the mesoporous surface and therefore to exhibit remarkably slow catalytic deactivation during the recycling tests.Capel-Sanchez et al.[24]prepared Ti/silica catalysts by grafting method using Ti-isopropoxide as precursor and triethanolaminate as complexing agent,which is in favor of anchoring the isolated Tispecies even after calcination.
In our previous work[25],we grafted Tispecies onto the bimodal mesoporous surface of the bimodal mesoporous silica(BMM)by a ship-in-a-bottle technique.The post-synthesis procedures were investigated,involving the self-assembly of ionic liquid[spmim][Cl-]and titanocene dichloride as Tiresource,as well as triethanolamine(TEA)as coordinating ligand,respectively.The results showed that the obtained Ti/ BMMs catalysts presented high catalytic activities in epoxidation of cyclohexene,due to the high dispersion of tetrahedrally-coordinated Ti species on the mesoporous surface.
Based on the observations mentioned above,the aim of this study is focused mainly on the recovery and recycling performances of Ti/BMMs mesoporous catalysts in the epoxidation of cyclohexene.In order to evaluate the regeneration methods and to investigate the deactivation feature,the deactivated Ti/BMMs mesoporous catalysts were either washed in chloroformorcalcined at450°C.Meanwhile,the regeneratedcatalysts were characterized by X-ray diffraction(XRD),Brunauer-Emmett-Teller(BET)method,scanning electron microscopy(SEM), transmission electron microscopy(TEM),Fourier transform infrared spectroscope(FT-IR)spectra,thermogravimetric analysis(TGA),UV visible spectroscopy(UV-vis)and X-ray photoelectron spectroscopy (XPS).
2.Experimental
2.1.Chemicals
The chemicals used in this work including cetyltrimthylammonium bromide(CTAB),ethyl silicate(TEOS),ammonium hydroxide(25-27%,by mass),triethanolamine,triethylamine,ether,dichloromethane and chloroform were obtained from Beijing Chemicals Works.Besides, 3-chloropropyl-trimethoxysilane(97%),1-methylimidazole(99%), titanocene dichloride(Cp2TiCl2),cyclohexene were supplied by Alfa Aesar Co.,USA.Tert-butyl hydroperoxide(TBHP,65%in water,by mass),mesitylene,anhydrous magnesium sulfate was obtained from Sinopharm Chemical Reagent Co.,Beijing.All the chemicals were all A.R.grade.
2.2.Synthesis of catalyst
2.2.1.Synthesis ofionic liquid[spmim][Cl−]
8 ml of 3-chloropropyltrimethoxysilane and 4 ml of 1-methylimidazole were added into a glass fl ask,and then mixture was continuously stirred and re fl uxed at 95°C under N2atmosphere.After reacting for 24 h,the white mixture gradually changed into a brown viscous liquid.It was subsequently washed with ether,and dried under vacuum at 60°C for 3 h.Finally,the ionic liquid[spmim][Cl-] was obtained and denoted as IL.
2.2.2.Grafting IL onto the surface of BMM
The synthetic procedure of BMMwas similar to the method reported by Sun etal.[26].In a typicalsynthesis procedure,CTAB(2.6115 g)was stirred with 104 mlofdoubly distilled water at room temperature until it fully dissolved.8 mlofTEOS was then added to the solution while stirring,followed immediately by 4 mlofammonium hydroxide.The mixture was stirred continuously to get a white gel until precipitates resulted,which were filtered,washed,and dried at120°C for3 h.To remove surfactant,the solid was calcined at 550°C for 6 h,with a heating rate of5°C min-1from room temperature to 550°C.
Prior to the grafting process,BMMwas degassed under vacuum at 150°C for 3 h.In a round-bottom fl ask with a flux condenser,0.5 g of IL were dissolved in 10 mlofchloroform,and treated with 1 g of previously prepared BMM.Then,the mixture was keptstirring and refluxing at 60°C under N2atmosphere.After reacting for 24 h,the obtained mixture was filtered,washed with dichloromethane,dried under high vacuum at 60°C for 3 h.Thus,the IL grafted BMM was obtained and denoted as ILBMM.
2.2.3.Preparation of Ti/BMM mesoporous catalysts
0.03 g of Cp2TiCl2were dried under vacuum at 120°C for 1 h,and then cooled down under N2atmosphere.The dried Cp2TiCl2were dissolved in 5 ml of chloroform and then 0.65 g of ILBMMand 1.7 mlof triethanolamine were added.After stirring at room temperature under a dry N2atmosphere for 8 h,the obtained mixture was filtered,washed with dichloromethane,dried at 60°C under high vacuum for 3 h.To remove IL,the solid was calcined at 450°C for 6 h and the sample was termed as Ti/BMM.
2.3.Catalytic tests
The epoxidation of cyclohexene with TBHP was carried outin a three necked fl ask equipped with a condenser under vigorous magnetic stirring which was heated with an oil bath.TBHP was dried using anhydrous MgSO4before use.Typically,100 mg of catalyst,10 ml of chloroform as solvent,10 mmol of cyclohexene,11 mmol of TBHP, and 5 mmolmesitylene(internal standard)were mixed in the fl ask and heated to 60°C under a dry nitrogen atmosphere.The reaction medium was withdrawn at different sampling times and analyzed by gas chromatography(GC-7890II,TianmeiCo.,China)equipped with a fl ame ionization detector,using a HP-5MS capillary column.After the reaction for 6 h,the powder was removed from the reaction mixture by filtration and washed with chloroform.For the regeneration by calcinations,the used catalyst was calcined at 450°C under air for 6 h.
2.4.Characterization
The amounts of Tiwere determined by inductively coupled plasma emission spectrometry(ICP,Optima 2000DV,PerkinElmer Co.,US). The powder XRD measurements were recorded using a Beijing PERSEE XD-3 X-ray diffactometer using CuKαradiation(λ=0.154056 nm) source for 2θranging from 0.6°to 10.0°with a scanning speed of 0.02(°)·s-1at 36 kV and 20 mA.FT-IR spectra were observed on a Bruker TENSOR-27 analyzer.The SEMimages were captured on a Hitachi field-emission scanning electron microscope(S-4300)operated at an accelerating voltage of 15 kV.The TEM analysis was performed on an electron microscope(JEM-2010F,JEOLCo.,Japan)under 200 kV accelerating voltage.N2adsorption and desorption isotherms at-196°C were obtained using a Micromeritics Tristar II.Before nitrogen adsorption,the samples were pretreated for 5 h under helium at 100°C.The isotherm data were analyzed with BET(Brunauer-Emmett-Teller)and the plots of the corresponding pore size distribution were obtained from the desorption branches of the isotherms by using BJH(Barrett-Joyner-Halenda)model.The TGA were illustrated between 25 and 800°C using a Perkin-Elmer Pyris 1 TG analyzer under 20 ml·min-1N2flow with a heating rate of10°C·min-1.UV-vis spectra were collected on a UV-2450 spectrophotometer(Shimadzu Co.,Japan).XPS analysis was carried out with a Thermo Fisher Scientific ESCALAB 250 system using monochromated Al Kα200 W(spot size=650μm, pass energy=200 eV for survey;30 eV for high resolution scan), Analyzer mode was CAE.The Ticontentis tested by PerkinElmer Optima 2000DV ICP spectrometer(ICP).
3.Results and Discussion
3.1.XRD analysis
The XRD patterns of the Ti/BMMregenerated via washing or calcination at different times are shown in Fig.1.As can be seen in Fig.1(a)-I, the fresh sample displayed the characteristic(100)diffraction peak of the mesoporous structure at2θ=2.32°,in agreement with the literature report[27,28].However,as illustrated in Fig.1(a)II-VI,all of regenerated samples via washing method showed that the(100)peak intensity was decreased with the recycle times,suggesting the decreasing of the mesoporous structuralorder degree.Meanwhile,the characteristic(100)diffraction peak of the Ti/BMM after 3 recycle times shifted toward high angles,implying the transformation of mesoporous structures.In addition,Fig.1(b)presented the XRD patterns of Ti/BMM samples regenerated by calcination,showing the similar phenomena to that of obtained by washing in Fig.1(a).These observations suggested that the ordered mesoporous framework was still preserved after regeneration by washing or calcination as expected,and the intensity of the(100)diffraction peak decreased after regeneration along with increasing recycle times,meaning the disordered mesostructure.
3.2.SEMand TEM micrograph
The morphology and microstructure of the Ti/BMMsamples before and after regeneration by washing with chloroform and calcination at450°C after5 recycles of reaction are clearly shown by the SEMand TEM images.As can be seen in Fig.2,the SEMimage of allsamples,including the fresh[Fig.2(a)]and regenerated catalysts[Fig.3(b)and(c)],revealed nanosized spherical particles of around or less than 50 nm, which was almost the same as that of typical BMMs[26,27].Meanwhile, as illustrated with TEMimages in Fig.3,allsamples possessed a large number of mesopores with a mean pore size of about 3 nm.These photomicrographs provided further evidences that the morphologies and the mesoporous structural integrities were maintained even after 5 recycles of regeneration by washing or calcination,which is in good agreement with the conclusion from the XRD patterns in Fig.1.
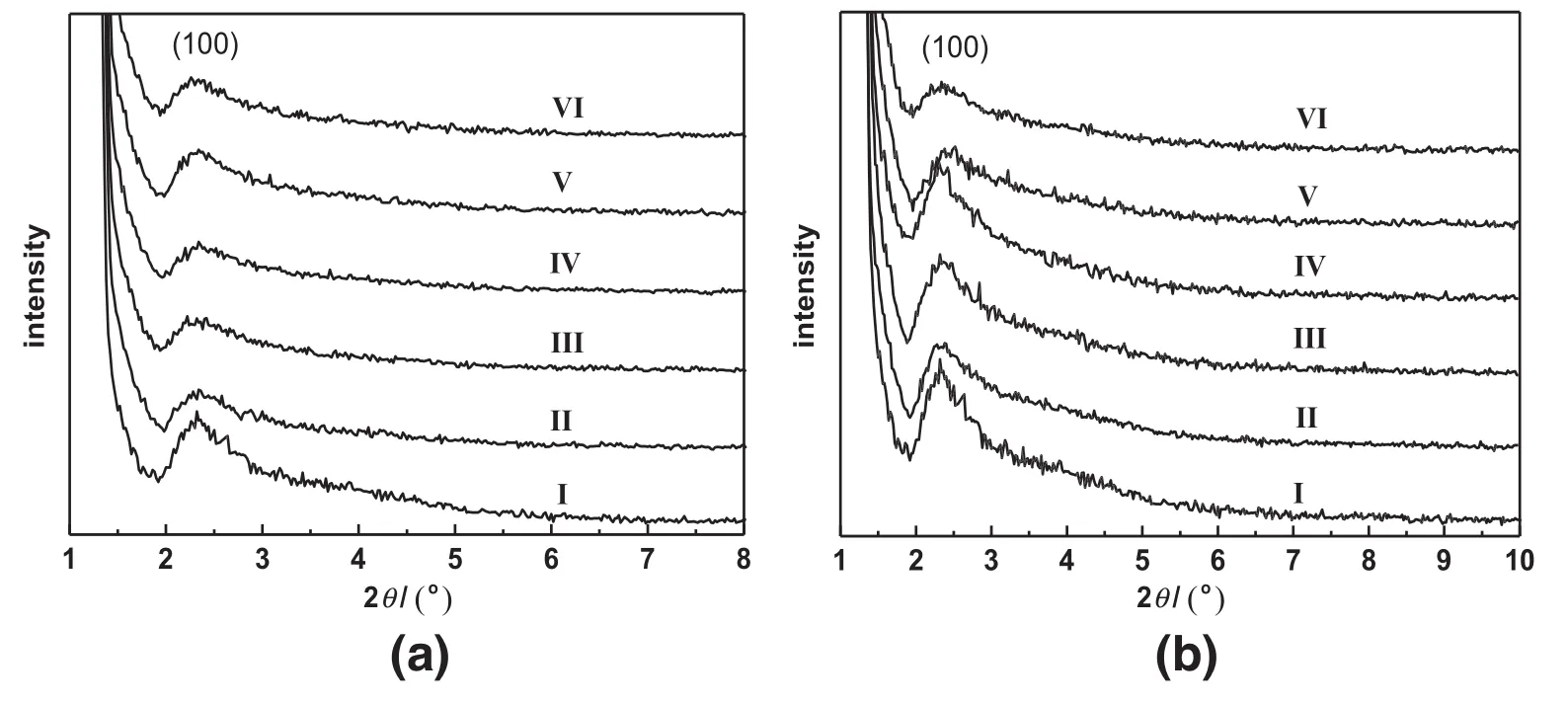
Fig.1.XRD patterns of the Ti/BMMcatalysts regenerated by washing with chloroform(a)and by calcination(b)with different recycle times:fresh(I),after 1 recycle(II),after 2 recycles (III),after 3 recycles(IV),after 4 recycles(V),and after 5 recycles(VI).
3.3.N2-sorption analysis
Fig.4 exhibits the N2adsorption/desorption isotherms of all samples and the corresponding pore size distributions(inset).Evidently,their isotherms were classified as type IV characteristic of the mesoporous materials with two hysteresis loops[26-30],whereas the isotherm of the fresh sample showed that the first inflection occurs atrelative pressure P/P0of0.30-0.40,which was stemmed from the capillary condensation,and corresponding to small pore size distribution with a mean pore size of around 2.7 nm in Fig.4a inset.Comparably,the second one at relative pressure scale of 0.75-0.99 was steeper than the first one,corresponding to the large mesopore distributions with the pore size centered at 21.0 nm,which was originated from inter-particles, suggesting the presences of the bimodalmesoporous structures[26,27]. Besides,the fresh sample exhibited the BET surface area of865 m2·g-1and pore volume of1.4 cm3·g-1,respectively.

Fig.2.SEMimages of Ti/BMMsamples:the fresh sample(a),regenerated sample by washing with chloroform(b)and by calcinations(c)after 5 recycles.
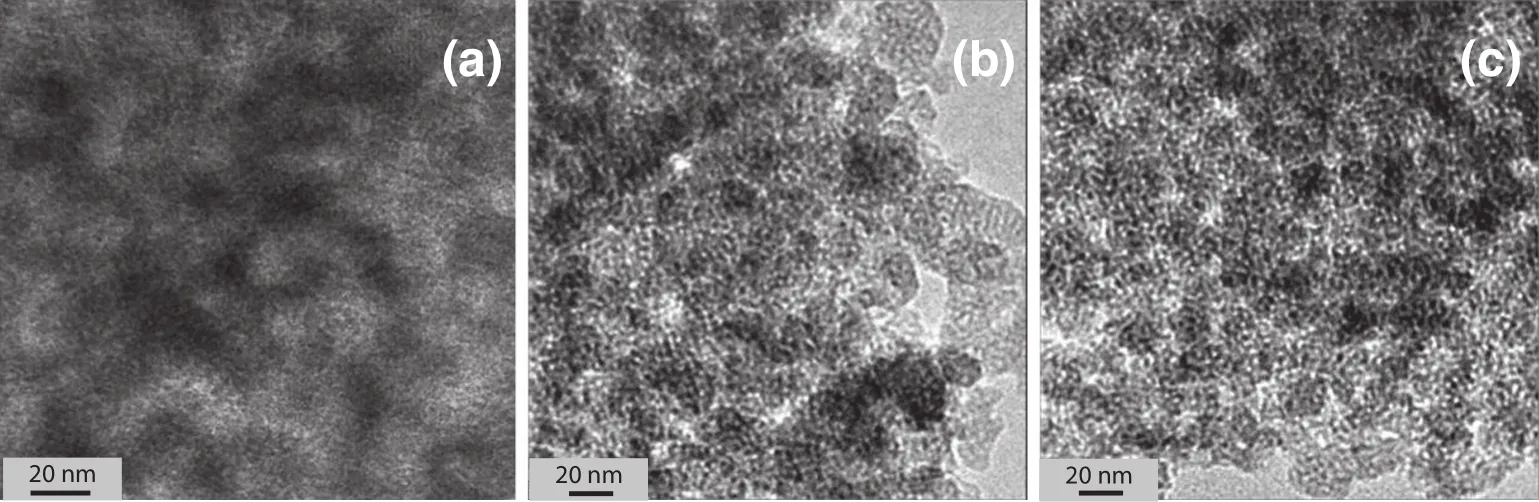
Fig.3.TEMphotomicrograph of Ti/BMMsamples,the fresh sample(a),regenerated sample after 5 recycles by washing with chloroform(b)and by calcinations(c).
As compared,Fig.4b shows that N2isotherms of reused sample regenerated by washing with chloroform after 5 recycles,in which both capillary condensation steps shifted to lower relative pressure, and correspondingly,BET surface area and pore volume showed the lower values of around 556 m2·g-1and 1.05 cm3·g-1,implying the presences of organic molecules existing inside the mesoporous surfaceof catalyst[31,32].This conclusion was also supported by the pore size distributions,whereas both of the mean pore size decreased to 2.3 nm and 18.8 nm,respectively.Apparently,as depicted in Fig.4c,the N2adsorption isotherms of other Ti/BMMsamples regenerated by calcinations showed a similar pro fi le to that obtained by washing with BET surface area of632 m2·g-1and pore volume of1.2 cm3·g-1,meanwhile the mean pore size decreased to 2.5 nm and 19.2 nm,respectively.
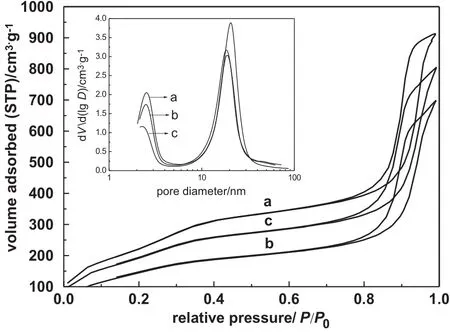
Fig.4.N2adsorption/desorption isotherms of Ti/BMM samples and the corresponding pore size distributions(inset):fresh(a),regenerated by washing with chloroform after 5 cycles(b),and by calcinations after 5 cycles(c).
3.4.FT-IR analysis
The FT-IRspectra of Ti/BMMs before and after regeneration by washing with chloroform are displayed in Fig.5(a).As can be seen,the main absorption bands were observed at 1230 cm-1,1085 cm-1,960 cm-1, 815 cm-1,respectively,which were in accordance with that of Ticontaining molecular sieves reported in the literature[33-35].Additionally,the fresh catalyst in Fig.5(a)-I had three peaks centered at 815 cm-1,960 cm-1and 1085 cm-1,which were assigned to symmetric stretch of Si-O,stretching vibration of Si-OH,and asymmetric stretch of Si-O-Si,respectively.Recently,some reports elucidated that the band at 960 cm-1was assigned to the stretching vibrations of the SiO4tetrahedral bond with Tiatoms,and therefore considered as a proof of Ti species in Ti-containing nanoporous silica[33].However, the 960 cm-1band also presented in the spectra of pure mesoporous silica.Thus,this band can be interpreted in terms of the overlapping of both Si-OH group and Ti-O-Si bond vibrations.Comparably, for other samples regenerated by washing with different recycle times in Fig.5(a)-(II-VI),some of stretching bands at 2951 cm-1and 2870 cm-1appeared,which can be ascribed to asymmetric vibrations of saturated C-H,implying that some substances with saturated C-H existed on the mesoporous surface of regenerated catalysts[32], which could easily resemble tetrahedrally-coordinated Tispecies with TBHP[36].In addition,Fig.5(b)displayed the FT-IR spectra of reused samples regenerated by calcination with different recycle times,which showed almost the same as that of the Ti/BMMcatalysts regenerated by washing in Fig.5(a),except that the bands centered at 2951 cm-1and 2870 cm-1completely disappeared after calcination.
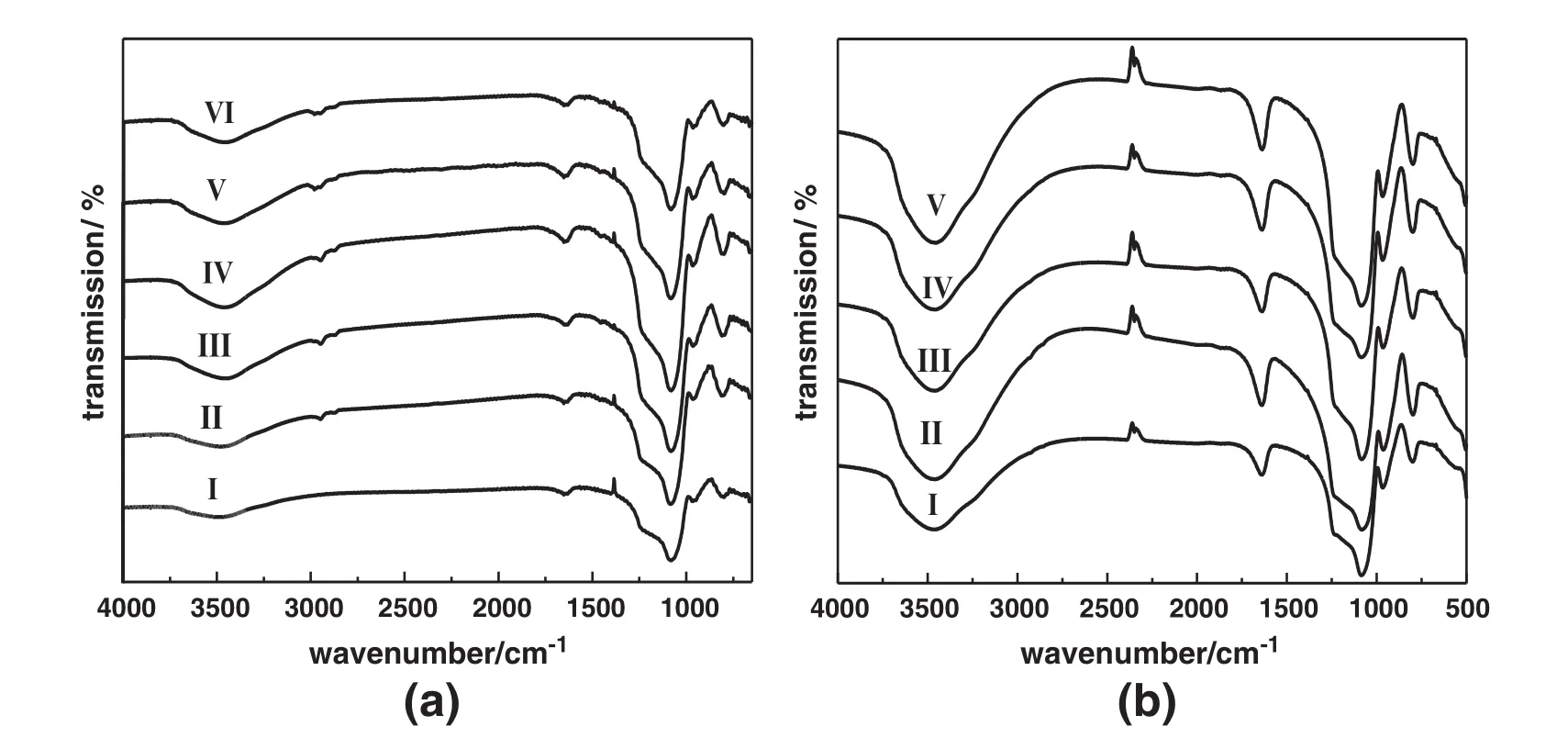
Fig.5.FT-IRspectra ofTi/BMMs before and after regeneration by washing with chloroform(a)and by calcinations(b)atdifferentrecycle times:fresh(I),after 1 recycle(II),after2 recycles (III),after 3 recycles(IV),after 4 recycles(V),after 5 recycles(VI).
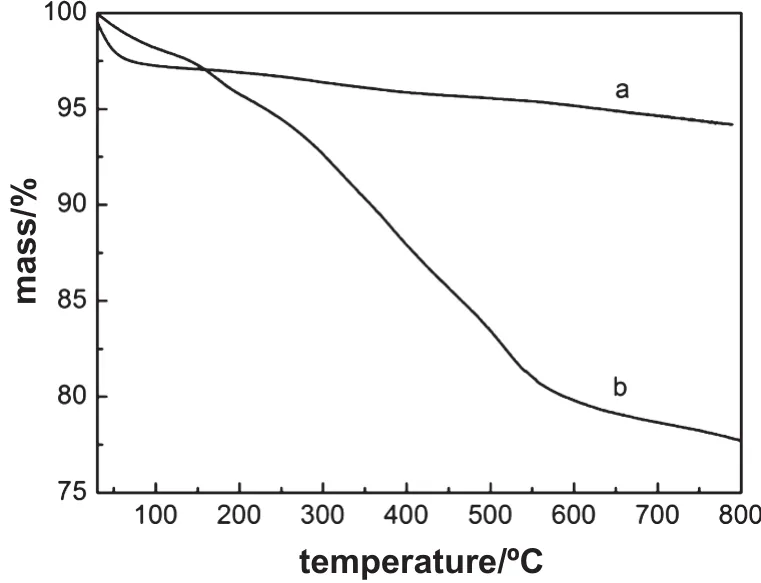
Fig.6.TG curves of before and after Ti/BMMsamples:fresh(a),regenerated by washing with chloroform after 5 recycles(b).
3.5.TG analysis
The TGA plots of the related samples under N2atmosphere were shown in Fig.6,indicating that the fresh catalyst(Fig.6a)had a slight mass loss of around 1%during the temperature rise from 100 to 800°C.Comparably,TG curve of the reused sample regeneratedby washing with chloroform after fi ve recycles(Fig.6b)revealed three distinct stages of mass loss in the temperature range of 100-800°C, and a totalmass loss was about 22.4%,which was derived from solvent evaporation,waterdesorption,and decomposition ofby-products deposited on the mesoporous surfaces of Ti/BMMcatalysts,respectively.

Fig.7.UV-vis spectra ofbefore and after Ti/BMMs regenerated by washing with chloroform(a)and by calcinations(b)with different recycle times:fresh(I),after 1 recycle(II),after 2 recycles(III),after 3 recycles(IV),after 4 recycles(V),after 5 recycles(VI).
3.6.UV-vis analysis
UV-vis diffuse re fl ectance spectroscopy of before and after Ti/BMMs regenerated by washing with chloroform are shown in Fig.7(a).It can be seen that the fresh sample[Fig.7(a)-I]exhibited a maximumabsorption centered at 220 nm,which was attributed to tetrahedrallycoordinated Tispecies in the mesoporous structure,in good agreement with that of Ti-containing mesoporous materials[37,38].As compared, for the Ti/BMM samples regenerated with different recycle times [Fig.7(a)-(II-VI)],the maximum absorptions were also centered at 220 nm,suggesting the presence of tetrahedral-Ti environments. However,a shoulder peak appeared at 276 nm indicating that the localmicrostructures of the Tispecies were some what changed during the epoxidation reaction,which was related to the bulky by-products deposited in the mesoporous channels[32,39,40].Meanwhile,a band at 330 nm was assigned to the presence of TiO2-like microclusters in the samples[40].In addition,as depicted in Fig.7(b),the UV-visible spectra of Ti/BMM samples regenerated by calcination showed almost similar to that obtained by washing[Fig.7(a)],except for the absence ofa band at330 nm.
3.7.XPS analysis
The XPS technology is one of the most usefultools for studying the chemical state,coordination,and relative dispersion of Tispecies on the mesoporous surface of silica[24].The XP spectra of Ti/BMMs regenerated by calcination after 1 and 5 recycle times and by washing with chloroform after 5 recycle times are shown in Fig.8.It can be found that the high-resolution XPS analysis of the Ti2p region on the surface was observed at binding energies near 458.5 eV and 465.5 eV,corresponding to the Ti 2p3/2and Ti 2p1/2states,respectively.Meanwhile, the Ti 2p3/2peak was split into two contributions:one centered at 459.8±0.2 eV was ascribed to typically tetrahedral coordination of Ti species,and another at lower binding energies(458.1±0.2 eV)was usually assigned to the octahedral-Ti coordination and/or Ti(IV) interacting with adsorbed water or penta-coordinated species[41]. In addition,the Ti/BMMs regenerated by calcination after 1 recycle [Fig.8(a)]displayed intense Ti2p3/2peak centered at460.1 eV,evidently indicating the tetrahedrally-coordinated Tispecies existing on the mesoporous surface,As compared,the sample regenerated by calcination after 5 recycles[Fig.8(b)]revealed a broadened peak centered at 459.4 eV with a very low intensity,which was mainly attributed to the existence of tetrahedral-Ticoordination[24,42],and therefore further proved a good dispersion of the Ticomplexes at internalmesoporous surfaces. However,with an increase of the recycle times regenerated by calcination,the Ti2p3/2peak shifted to a lower binding energy,meaning the presence ofan intermediate(penta-or hexa-Ti)coordinated state[22]. On the other hand,as shown in Fig.8(c),the XPS spectra of Ti/BMMsregenerated by washing with chloroform after 5 recycles illustrated that the Ti2p3/2peak wasalso separated into two peaks centered at460.3 and 459.3 eV,respectively,drawing similar observations to that of sample regenerated by calcination after 5 recycles.
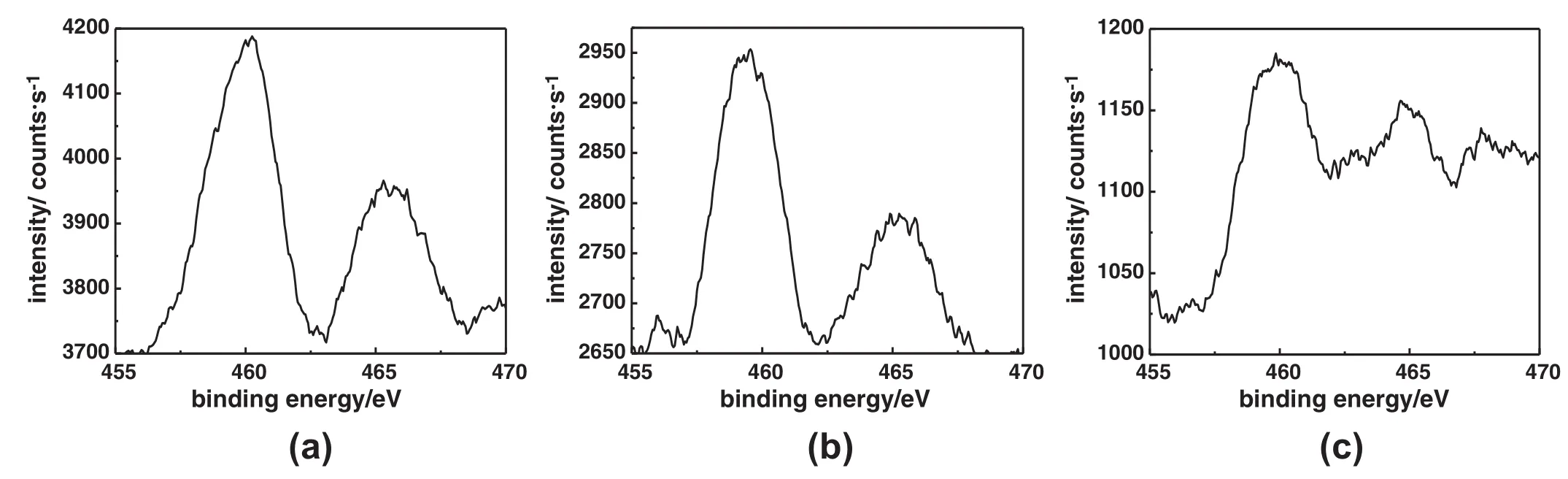
Fig.8.XPS spectra of before and after Ti/BMMs regenerated by calcinations after 1 recycle(a)and after 5 recycles(b),by washing with chloroform after 5 recycles(c).
On the basis of the mentioned above with the help of XRD,SEM/ TEM,FT-IR,TGA,and XPS characterizations,it can be easily concluded that the effects of solvent and reactive molecules existing on the mesoporous surfaces of regenerated Ti/BMM catalysts on the coordination environments of Tispecies were remarkable[32,43].

Table 1 Catalytic performances of the Ti/BMMsamples regenerated by different methods during oxidation of cyclohexene with TBHP①
3.8.The catalytic performance of regenerated Ti/BMMs catalysts
The catalytic performances of regenerated catalysts for the epoxidation of cyclohexene are summarized in Table 1.As can be seen,the ICP results showed that Ti content remained almost unchanged around 0.9%,suggesting that the effects of regeneration methods on the Ti leaching were not obvious,however,both conversion of cyclohexene and selectivity of cyclohexene oxide were gradually decreased with the reaction-regeneration recycles.Particularly,the catalytic activities of Ti/BMMs regenerated by washing with chloroform were rapidly reduced from 89%for selectivity of cyclohexene oxide as 1 recycle time to 35%as 5 recycle times,accompanied by the decreasing conversion of cyclohexene from 64%to 47%.As a comparison,the decrease of catalytic activities of Ti/BMMs regenerated by calcination was not remarkable:the selectivity of cyclohexene oxide was around 85%,and the corresponding conversion of cyclohexene was slightly decreased from 64%to 58%.
According to the above catalytic behaviors and combined with various characterizations,these results further demonstrated that the calcination method can be used to regenerate the Ti/BMM catalysts, which was efficient for removal of adsorbed molecules inside the mesoporous channels such as moisture,solvent,and reactive molecules,and on the other hand,to prevent the Ti leaching loss of tetrahedrally coordinated Tispecies[44].
4.Conclusions
In summary,the recovery and recycling properties of Ti/BMMmesoporous catalysts were investigated by means of various characterizations and their catalytic performances of the reused Ti/BMMsamples were tested by epoxidation of cyclohexene.The deactivated Ti/BMM catalysts were regenerated severalrecycle times by washing in chloroform or calcination at 450°C.The results showed that the regenerated Ti/BMMcatalysts still maintained a typical bimodal mesoporous structure and the leaching loss of the grafted Tispecies was negligible even after being regenerated 5 times.In addition,the Ti/BMM catalysts could be repeatedly used after regeneration by calcination without suffering much activity loss,while the effects of washing treatment on the catalytic activities were less favorable.The main reasons were related to the adsorbed bulky reactive molecules and multi(penta or hexa)-coordinated Tispecies existing on the mesoporous surfaces and channels of the regenerated Ti/BMM catalysts.Apparently,the calcination method is an effective approach of Ti/BMM catalyst regeneration, which is not only beneficial to removing sediment inside the mesoporous channels,but also to prevent the leaching or transforming loss of the tetrahedrally-coordinated Tispecies.
[1]A.Ruperta,Y.Shihy,“Process for making phenoxycycloalkanols”,US Pat.4754076 (1988).
[2]Y.D.Zhang,X.L.Gao,X.Chen,C.J.Wang,D.G.Jiang,Study on synthesis,characterization and catalytic activity of polystyrene-supported Mo(VI)complex in epoxidation of cyclohexene,Chin.J.Chem.Eng.11(3)(2003)318-325.
[3]M.Tamarasso,C.Perego,B.Notari,“Preparation of porous crystalline synthetic material comprised of silicon and titanium oxides”,US Pat.4410501(1983).
[4]M.A.Camblor,A.Corma,A.Martínez,J.Pérez-Pariente,Synthesis of a titaniumsilicoaluminate isomorphous to zeolite beta and its application as a catalyst for the selective oxidation of large organic molecules,J.Chem.Soc.Chem.Commun.8 (1992)589-590.
[5]J.M.Thomas,Design,synthesis,and in situ characterization of new solid catalysts, Angew.Chem.Int.Ed.38(24)(1999)3588-3628.
[6]L.L.Wang,Y.M.Liu,W.Xie,H.H.Wu,X.H.Li,M.Y.He,P.Wu,Improving the hydrophobicity and oxidation activity of Ti-MWWby reversible structural rearrangement, J.Phys.Chem.C 112(15)(2008)6132-6138.
[7]A.Corma,M.T.Navarro,J.P.Pariente,Synthesis of an ultralarge pope titanium silicate isomorphous to MCM-41 and its application as a catalyst for selective oxidation of hydrocarbons,J.Chem.Soc.Chem.Commun.2(1994)147-148.
[8]T.Blasco,A.Corma,M.T.Navarro,J.Perez-Pariente,Synthesis,characterization,and catalytic activity of Ti-MCM-41 structures,J.Catal.156(1)(1995)65-74.
[9]T.Maschmeyer,F.Rey,G.Sankar,J.M.Thomas,Heterogeneous catalysts obtained by grafting metallocene complexes onto mesoporous silica,Nature 378(6553)(1995) 159-162.
[10]K.A.Koyano,T.Tatsumi,Synthesis of titanium-containing mesoporous molecular sieves with a cubic structure,J.Chem.Soc.Chem.Commun.2(1996) 145-146.
[11]M.Morey,A.Davidson,G.Stucky,A new step toward transition metalincorporation in cubic mesoporous materials:preparation and characterization of Ti-MCM-48, Microporous.Mater.6(2)(1996)99-104.
[12]M.S.Morey,S.O'Brien,S.Schwarz,G.D.Stucky,Hydrothermal and postsynthesis surface modification of cubic,MCM-48,and ultralarge pore SBA-15 mesoporous silica with titanium,Chem.Mater.12(4)(2000)898-911.
[13]M.L.Peña,V.Dellarocca,F.Rey,A.Corma,S.COluccia,L.Marchese,Elucidating the local environment of Ti(IV)active sites in Ti-MCM-48:a comparison between silylated and calcined catalysts,Microporous Mesoporous Mater.44(2001)345-356.
[14]A.Vinu,P.Srinivasu,M.Miyahara,K.Ariga,Preparation and catalytic performances of ultralarge-pore TiSBA-15 mesoporous molecular sieves with very high Ticontent, J.Phys.Chem.B 110(2)(2006)801-806.
[15]Y.Chen,Y.Huang,J.Xiu,X.Han,X.Bao,Direct synthesis,characterization and catalytic activity of titanium-substituted SBA-15 mesoporous molecular sieves, Appl.Catal.A Gen.273(1-2)(2004)185-191.
[16]W.H.Zhang,J.Lu,B.Han,M.Li,J.Xiu,P.Ying,C.Li,Direct synthesis and characterization of titanium-substituted mesoporous molecular sieve SBA-15,Chem.Mater.14 (8)(2002)3413-3421.
[17]S.Wu,Y.Han,Y.C.Zou,J.Song,W.L.Zhao,Y.Di,S.Z.Liu,F.S.Xiao,Synthesis of heteroatom substituted SBA-15 by the pH-adjusting”method,Chem.Mater.16 (3)(2004)486-492.
[18]B.L.Newalkar,J.Olanrewaju,S.Komarneni,Direct synthesis of titanium-substituted mesoporous SBA-15 molecular sieve under microwave-hydrothermal conditions, Chem.Mater.13(2)(2001)552-557.
[19]F.Bérubé,F.Kleitz,S.Kaliaguine,A comprehensive study of titanium-substituted SBA-15 mesoporous materials prepared by direct synthesis,J.Phys.Chem.C 112 (37)(2008)14403-14411.
[20]F.Bérubé,F.Kleitz,S.Kaliaguine,Surface properties and epoxidation catalytic activity of Ti-SBA15 prepared by direct synthesis,J.Mater.Sci.44(24)(2009) 6727-6735.
[21]P.Wu,T.Tatsumi,Direct formation of pinacols from olefins over various titanosilicates,Chem.Mater.14(4)(2002)1657-1664.
[22]F.Bérubé,B.Nohair,F.Kleitz,S.Kaliaguine,Controlled postgrafting of titanium chelates for improved synthesis of Ti-SBA-15 epoxidation catalysts,Chem.Mater. 22(6)(2010)1988-2000.
[23]F.Bérubé,A.Khadhraoui,M.T.Janicke,F.Kleitz,S.Kaliaguine,Optimizing silica synthesis for the preparation of mesoporous Ti-SBA-15 epoxidation catalysts,Ind. Eng.Chem.Res.49(15)(2010)6977-6985.
[24]M.C.Capel-Sanchez,G.Blanco-Brieva,J.M.Campos-Martin,M.P.De Frutos,W.Wen, J.A.Rodriguez,J.L.G.Fierro,Grafting strategy to develop single site titanium on an amorphous silica surface,Langmuir 25(12)(2009)7148-7155.
[25]S.Y.Bai,X.T.Hu,J.H.Sun,B.Ren,Preparation and characterization of Tisupported bimodalmesoporous catalysts using a self-assembly route combined with a ship-in-abottle method,New J.Chem.38(5)(2014)2128-2134.
[26]J.H.Sun,Z.P.Shan,T.Maschmeyer,M.-O.Coppens,Synthesis of bimodal nanostructured silicas with independently controlled small and large mesopore sizes, Langmuir 19(20)(2003)8395-8402.
[27]J.H.Sun,Z.P.Shan,T.Maschmeyer,J.A.Moulijn,M.-O.Coppens,Synthesis of tailored bimodal mesoporous materials with independent control of the dual pore size distribution,Chem.Commun.24(2001)2670-2671.
[28]Y.Z.Li,J.H.Sun,L.Gao,X.Wu,Grafting fl uorescence molecules into the pore surface of bimodalmesoporous silicas with differentroutes,Mater.Lett.65(2)(2011)250-252. [29]S.J.Qiu,J.H.Sun,Y.Z.Li,L.Gao,Investigation of heterogeneous asymmetric dihydroxylation over OsO4-(QN)2PHAL catalysts of functionalized bimodal mesoporous silica with ionic liquid,Mater.Res.Bull.46(8)(2011)1197-1201.
[30]L.Gao,J.H.Sun,B.Ren,Y.Z.Li,H.Zhang,Structural characterization and surface heterogeneity of bimodal mesoporous silicas functionalized with aminopropyl groups and loaded aspirin,Mater.Res.Bull.46(10)(2011)1540-1545.
[31]J.He,H.Ma,Z.Y.Guo,D.G.Evans,X.Duan,Chemical stability of Ti-modified MCM-41 catalysts in the hydroxylation of benzene in the liquid phase,Top.Catal.22(1-2) (2003)41-51.
[32]Q.F.Wang,L.Wang,J.X.Chen,Y.L.Wu,Z.T.Mi,Deactivation and regeneration of titanium silicalite catalyst for epoxidation of propylene,J.Mol.Catal.A Chem.273 (1-2)(2007)73-80.
[33]M.Guidotti,N.Ravasio,R.Psaro,G.Ferraris,G.Moretti,Epoxidation on titaniumcontaining silicates:do structural features really affect the catalytic performance? J.Catal.214(2)(2003)242-250.
[34]Y.S.Zhou,G.W.Jiang,Study on properties of composite oxides TiO2/SiO2,Chin.J. Chem.Eng.10(3)(2002)349-353.
[35]G.A.Eimer,S.G.Casuscelli,G.E.Ghione,M.E.Crivello,E.R.Herrero,Synthesis,characterization and selective oxidation properties of Ti-containing mesoporous catalysts, Appl.Catal.A Gen.298(2006)232-242.
[36]R.A.Sheldon,J.A.Van Doorn,Metal-catalyzed epoxidation of olefins with organic hydroperoxides:I.A comparison of various metalcatalysts,J.Catal.31(3)(1973) 427-437.
[37]A.Corma,From microporous to mesoporous molecular sieve materials and their use in catalysis,Rev.Chem.97(6)(1997)2373-2420.
[38]A.Bhaumik,T.Tatsumi,Organically modified titanium-rich Ti-MCM-41,efficient catalysts for epoxidation reactions,J.Catal.189(1)(2000)31-39.
[39]W.B.Fan,P.Wu,S.Namba,T.Tatsumi,Synthesis and catalytic properties of a new titanosilicate molecular sieve with the structure analogous to MWW-type lamellar precursor,J.Catal.243(1)(2006)183-191.
[40]A.Corma,H.Garcia,Lewis acids as catalysts in oxidation reactions:from homogeneous to heterogeneous systems,Chem.Rev.102(10)(2002)3837-3892.
[41]T.Blasco,M.A.Camblor,J.L.G.Fierro,J.Perez-Pariente,X-ray photoelectron spectroscopy of Ti-Beta zeolite,Microporous Mesoporous Mater.3(3)(1994) 259-263.
[42]M.C.Capel-Sanchez,V.A.Dela Peña-O'Shea,J.M.Campos-Martin,J.L.G.Fierro,TDDFT analysis of the electronic spectra of Ti-containing catalysts,Top.Catal.41 (1-4)(2006)27-34.
[43]X.W.Liu,X.S.Wang,X.W.Guo,G.Li,H.S.Yan,Regeneration oflamina TS-1 catalystin the epoxidation of propylene with hydrogen peroxide,Catal.Lett.97(3-4)(2004) 223-229.
[44]Q.C.Yuan,A.Hagen,F.Roessner,An investigation into the Ti-grafting structure on MCM-41 and epoxidation catalysis,Appl.Catal.A Gen.303(1)(2006)81-87.
☆Supported by the National Natural Science Foundation of China(21076003, 21272005),and the Funding Project for the Academic Human Resources Development in Institutions of Higher Learning under the Jurisdiction of the Beijing Municipality (PHR201107104,005000541211019/20,005000543111517).
*Corresponding author.
E-mailaddress:jhsun@bjut.edu.cn(J.Sun).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Separation Science and Engineering Performance Prediction of Structured Packing Column for Cryogenic Air Separation with Hybrid Model☆
- Preparation,Structure Characterization and Hydrodesulfurization Performance of B-Ni2P/SBA-15/Cordierite Monolithic Catalysts☆
- Enhanced Photocatalytic Properties of Silver Oxide Loaded Bismuth Vanadate☆
- Promoting Xylene Production in Benzene Methylation using Hierarchically Porous ZSM-5 Derived from a Modified Dry-gel Route☆
- Energy,Resources and Environmental Technology Experimental and Modeling Study on de-NOxCharacteristics of Selective Non-catalytic Reduction in O2/CO2Atmosphere☆
- Energy,Resources and Environmental Technology CO2Removal from Biogas by Water Washing System☆