Biotechnology and Bioengineering The Effect of pH Controlon Acetone-Butanol-Ethanol Fermentation by Clostridium acetobutylicum ATCC 824 with Xylose and D-Glucose and D-Xylose Mixture☆
2014-07-12WeiJiangZhiqiangWenMianbinWu2HongLiJunYangJianpingLinYijunLinLirongYangPeilinCen
WeiJiang**,Zhiqiang Wen**,Mianbin Wu2,*,Hong Li,Jun YangJianping Lin*,Yijun Lin Lirong YangPeilin Cen
1Key Laboratory of Biomass chemical engineering of Ministry of Education,Department of Chemical and Biological Engineering,Zhejiang University,Hangzhou 310027,China
2Zhejiang Key Laboratory of Antifungal Drugs,Zhejiang,Taizhou 318000,China
3Lancaster Environment Centre,Lancaster University,Lancaster LA1 4YQ,UK
Biotechnology and Bioengineering The Effect of pH Controlon Acetone-Butanol-Ethanol Fermentation by Clostridium acetobutylicum ATCC 824 with Xylose and D-Glucose and D-Xylose Mixture☆
WeiJiang1,**,Zhiqiang Wen1,**,Mianbin Wu1,2,*,Hong Li3,Jun Yang1,Jianping Lin1,*,Yijun Lin1, Lirong Yang1,Peilin Cen1
1Key Laboratory of Biomass chemical engineering of Ministry of Education,Department of Chemical and Biological Engineering,Zhejiang University,Hangzhou 310027,China
2Zhejiang Key Laboratory of Antifungal Drugs,Zhejiang,Taizhou 318000,China
3Lancaster Environment Centre,Lancaster University,Lancaster LA1 4YQ,UK
A R T I C L E I N F o
Article history:
Received 11 April2013
Received in revised form 5 December 2013
Accepted 2 January 2014
Available online 17 June 2014
Clostridium acetobutylicum ATCC 824
Xylose
Mixed sugar
pH control
D-Glucose,L-arabinose,D-mannose,D-xylose,and cellobiose are saccharification products of lignocellulose and important carbon sources for industrial fermentation.The fermentation efficiency with each of the five sugars and the mixture of the two most dominant sugars,D-glucose and D-xylose,was evaluated for acetonebutanol-ethanol(ABE)fermentation by Clostridium acetobutylicum ATCC 824.The utilization efficacy of the five reducing sugars was in the order of D-glucose,L-arabinose,D-mannose,D-xylose and cellobiose.D-Xylose, the second most abundant component in lignocellulosic hydrolysate,was used in the fermentation either as sole carbon source or mixed with glucose.The results indicated that maintaining pH at 4.8,the optimalpH value for solventogenesis,could increase D-xylose consumption when it was the sole carbon source.Different media containing D-glucose and D-xylose at different ratios(1:2,1:5,1.5:1,2:1)were then attempted for the ABE fermentation.When pH was at 4.8 and xylose concentration was five times that of glucose,a 256.9%increase in xylose utilization and 263.7%increase in solvent production were obtained compared to those without pH control. These results demonstrate a possible approach combining optimized pH control and D-glucose and D-xylose ratio to increase the fermentation efficiency of lignocellulosic hydrolysate.
©2014 The Chemical Industry and Engineering Society of China,and Chemical Industry Press.Allrights reserved.
1.Introduction
Due to energy shortage and climate change in the past decades, more intensive researches have been carried out on the conversion of abundant lignocellulosic biomass to sustainable biofuels and chemicals such as ethanol and butanol.Butanol has many promising characteristics as a renewable liquid fuel compared with other fermentation derived fuels[1],such as low vapor pressure,low water solubility,low volatility and high energy density[2].These beneficial properties allow its blending with conventional fuels at any ratio[3].
Butanol can be produced by acetone-butanol-ethanol(ABE) fermentation with a number of substrates,such as cane molasses [4],corn starch[5],potatoes,soy molasses,and cassava[6],using Clostridium acetobutylicum or Clostridium beijerinckii.However, with the rising price of these substrate materials,the cost becomes a major factor that limits mass production of butanol[7].Moreover, ethical concerns contradict the use of food and feed products as a biofuelsource[3].
In order to produce butanolcost-competitively,identifying and evaluating low cost substrates may be a way to achieve the goal[8].Lignocellulose,the most abundant sustainable raw material worldwide,is a natural candidate,which composes of three major polymers:cellulose, hemicellulose and lignin.However,plant cell walls are recalcitrant to degradation.Many pretreatment technologies have been developed to remove lignin and crack recalcitrant characteristics of lignocellulose[9]. The sequentialenzymatic hydrolysis can convert cellulose and hemicellulose into soluble oligomeric and mono-meric sugars[10].Hydrolyzation of cellulose produces hexoses,such as D-glucose,D-mannose,and D-galactose, while forhemicellulose,hydrolysis products are pentoses,such as D-xylose and L-arabinose[11].Itis known that C.acetobutylicum is one of the few strictanaerobic microorganisms thatare able to utilize a wide variety of carbohydrates to produce desirable products(ethanol,butanol and acetone).
Since D-glucose and D-xylose are the major hydrolysates oflignocellulose,it is important to convert them into solvents as efficient as possible.However,microorganisms tend to selectively utilize a preferred sugar first and consume other sugars sequentially[12],termed carbon catabolite repression.This phenomenon makes the fermentation process complex and often reduces the productivity and yield on the target biomass.In order to improve xylose utilization of mixed sugar and achieve a high yield of solvent,some experiments have been conducted with different D-glucose/D-xylose ratios(1.5:1,2:1).Although previous work has identified that higher ratio of xylose to glucose is in favor of using xylose[13],very little information is available on the effect of pH control on ABE fermentation with different ratios of xylose to glucose.
It has long been observed that C.acetobutylicum utilizes sugars by undergoing biphasic fermentation,termed acidogenesis and solventogenesis.During acidogenic phase,acetic acid and butyric acid are produced simultaneously,reducing pH in culture media. When the acid accumulates to a certain level,re-assimilation of these organic acids leads to the formation of ABE[8],and then the pH value increases gradually.6-13 mmol·L-1of undissociated butyric acid is required for the initiation of butanolproduction[14].The optimum solventproduction occurs atpH 5.0 in a study to compare fermentation performance by C.acetobutylicum ATCC 824 at pH 4.5,5.0, and 5.5[15].
Since acid formation and reassimilation are closely dependenton pH of culture and acid accumulation is essential for the solventproduction, pH should be controlled appropriately after the acidogenic phase. However,no attention has been paid on the timing of pH control. Calcium carbonate or Na OH is usually added at the beginning of the fermentation.
The objectives of this work are to identify the utilization efficiency of 5 major sugars in lignocelluloses hydrolysate and effects of pH on both xylose utilization efficiency and solvent production using either xylose alone or glucose and xylose mixture with different ratios.
2.Materials and Methods
2.1.Microorganism and medium
C.acetobutylicum ATCC 824 was bought from CGMCC.
Spores of C.acetobutylicum ATCC 824 were maintained in distilled water at 4°C.The spores were heat shocked at 80°C for 10 min and transferred to TGY medium(tryptone 0.5%,glucose or other carbon source 0.1%,yeast extract 0.3%,pH 7.8)with 5%quantity.The culture was allowed to grow for approximately 16-18 h at 37°C when it was ready to be inoculated into the P2 medium.The P2 medium contained carbon source(monosaccharide such as D-glucose,D-mannose,D-xylose,L-arabinose,or the mixed sugar),yeast extract(5.0 g·L-1),L-cysteine hydrochloride(0.5 g·L-1,for keeping the reducing condition),and resazurin(3 ml·L-1,0.1 g·L-1to reveal the reducing state),including minerals,buffer and vitamins. The medium without stock solutions was sterilized at 115°C for 30 min followed by cooling to room temperature.After that,1 ml of concentrated mineralsolution(100 mldistilled water containing 3.48 g MgSO4,0.1 g MnSO4·H2O,0.1 g FeSO4·7H2O),10 ml of concentrated buffer(adding 0.75 g KH2PO4,0.75 g K2HPO4,2.0 g (NH4)2SO4,1.0 g NaClinto 100 mldistilled water),and 10 mlofvitamin solution(2.0 g asparagines dissolved into 100 ml distilled water),which were filter sterilized,were added to the original solution and made the total volume to 100 ml.Samples were taken for analysis at regular intervals.
2.2.Fermentation
Batch fermentations were performed anaerobically in 500 ml shake fl asks with 300 mlworking volume at 37°C,using D-glucose, D-mannose,L-arabinose,D-xylose,and cellobiose as sole carbon resource of P2 medium separately,with the initial concentration of 42 g·L-1,by C.acetobutylicum ATCC 824.
Then 70.0 g·L-1of D-xylose was used as the carbon source with accurate pH-controland fermented under the same culture condition.The pH-controlwas carried out after the acidogenic phase and achieved by feeding 0.8 mol·L-1NaOH.
After that,experiments were conducted with varied D-glucose/D-xylose ratios(1:2,1:5,1.5:1,2:1,g·g-1),using xylose-pregrown cells as inoculum according to earlier study[13]and controlling pH after 24 h.Meantime,control experiments were conducted at unregulated culture pH.
2.3.Analysis
Cell concentration was measured by optical density method at 600 nm.Glucose was determined by glucometer,other reducing sugars were tested by colorimetric analysis of DNS(3,5-dinitrosalicylic acid).Xylose in the mixed sugar was calculated from the difference between total sugarconcentration and glucose concentration.Gas chromatography(GC 6820),HP-INNOWAX(19091N-113)capillary chromatographic column, temperature programming,and Agilenttechnology were used to analyze the fermentation products(acetone,butanol,ethanol,acetic acid and butyric acid).Reactor productivity was estimated as the total ABE produced (g·L-1)divided by the fermentation time(g·L-1·h-1).ABE yield was calculated as grams of ABE produced per gram of sugar used.
3.Results and Discussion
3.1.Utilization of individual reducing sugar by C.acetobutylicum ATCC 824
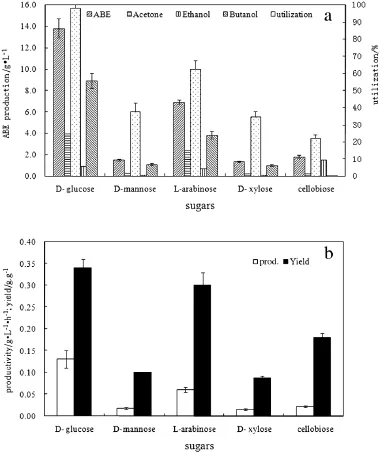
Fig.1.Production of ABE from different reducing sugars using Clostridium acetobutylicum ATCC 824.(a)Utilization of different sugars and ABE production;(b)productivity and yield.
Fig.1 compares the utilization,ABE production,reactor productivity and yield of different reducing sugars.Results indicate that it is easier forC.acetobutylicum ATCC 824 to use glucose than other reducing sugars, and the rate of sugar consumption,ABE production,and yield using different sugars are in the order of D-glucose,L-arabinose,D-mannose, D-xylose and cellobiose(Fig.1a).This agrees with previous study, using 60 g·L-1of individualsugar(glucose,xylose,arabinose,galactose)by C.acetobutylicum P260[7].This may be attributed to sugar specific mechanisms for the transcriptional regulation of transport and metabolism genes.The efficiency of transport and metabolism of glucose is far higher than that of other carbon sources,which is consistent with ABE production from glucose comparing with other sugars[16,17]. During the glucose-based fermentation,13.83 g·L-1ABE was produced with a productivity of0.13 g·L-1·h-1and a yield of0.34,far ahead of other sugars.
Although L-arabinose and xylose are two common pentoses,the induction of key genes involved in the transport and metabolism of pentose by L-arabinose is stronger than that of xylose[17],leading to a higher ABE productivity and yield.The utilization of L-arabinose was the nexthighest, the production of ABE was 6.9 g·L-1and organic acid was 3.67 g·L-1(acetate 2.14 g·L-1,butyrate 1.53 g·L-1),resulting in a yield of0.3 g·g-1and a productivity of0.06 g·L-1·h-1(Fig.1).However,with D-xylose as carbon source,the fi nal concentration of solvent was 1.33.The ability of C.acetobutylicum ATCC 824 to uptake D-mannose was similarly poor to D-xylose.The consumption of D-mannose produced only 1.09 g·L-1butanol and 1.50 g·L-1ABE.Additionally,in cellobiose fermentation,only 0.04 g·L-1butanol was measured,indicating that the strain can barely use cellobiose to produce butanol.
3.2.Effects upon xylose utilization with pH control
The intracellular trans membrane pH gradient kept constant in C.acetobutylicum is unable to maintain a constant intracellular pH automatically,so that any severe changes of external pH level will have strong effect on the growth and metabolism of cells[18].Therefore,it is important to control pH for ABE fermentation.
As seen in Fig.2,obvious differences appear between the experimental and the control group since 20 h.The pH value of control group decreases with the accumulation of organic acids and falls below 4.0 after 68 h.Since the undissociated forms of these acids are able to freely permeate through the cytoplasmic membrane,which will accumulate in the cellinterior at largeΔpH values and decrease the internal pH[19,20].Low internal pH negatively affects the cell of control group.A constantΔpH between external and internal cells can be kept by controlling external pH level.
With external pH control,the improvements in growth,xylose utilization,and total solvent production are significant.Xylose utilization increased from 37.4%to 56.0%.The final ABE concentration increased to 3-folds.The productivity and yield also increased to 3.2-folds and 2.1-folds,respectively(Fig.2e).The increase of substrate utilization and solvent production through pHcontrol with xylose as the sole carbon source is in agreement with previous study[13],in which using 10 g·L-1calcium carbonate to buffer pH,a more efficient D-xylose utilization,improved cell growth and increased ABE production were achieved.
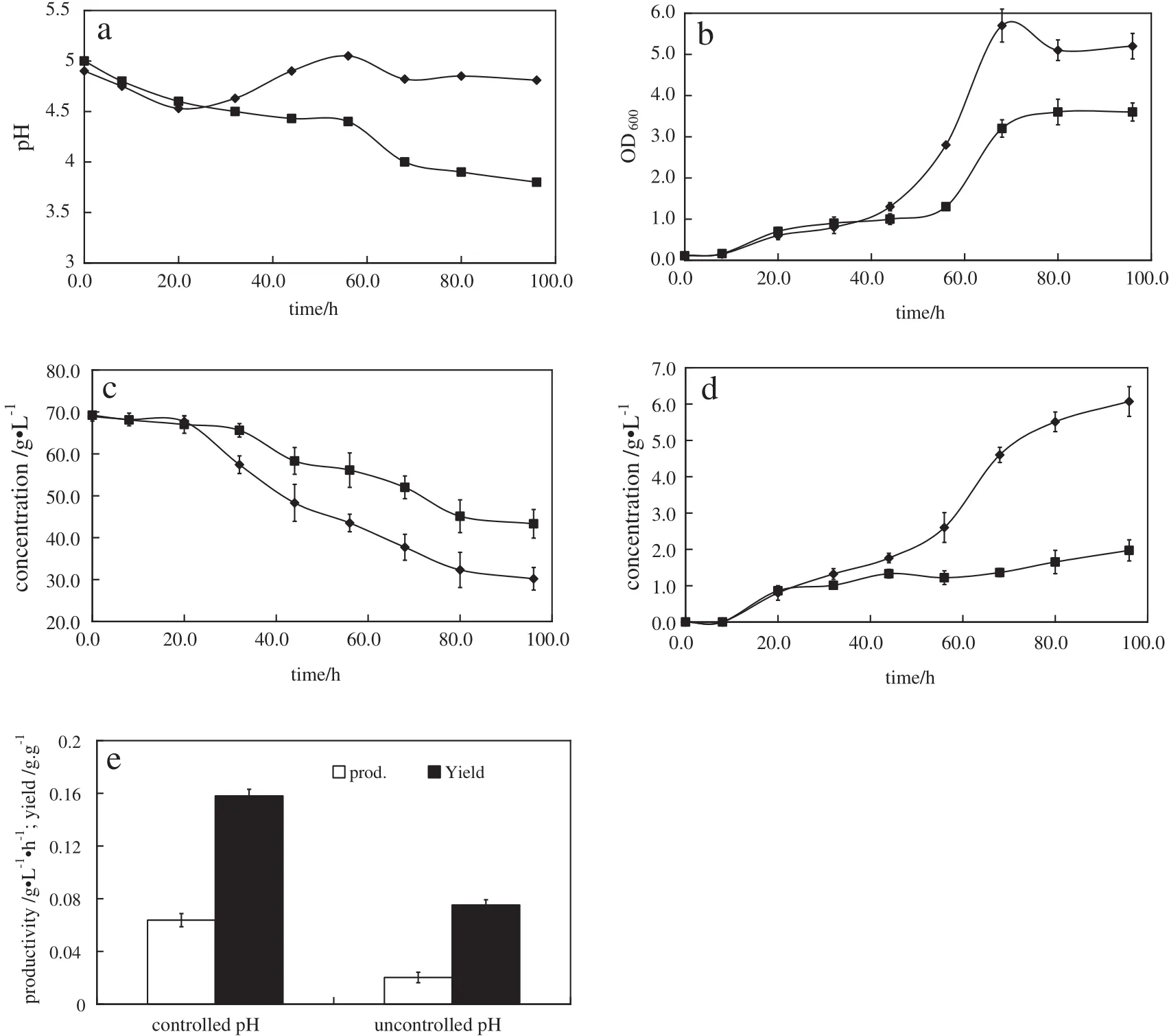
Fig.2.Batch fermentation of strain C.acetobutylicum ATCC 824 with D-xylose atcontrolled(◆)and uncontrolled(■)pH.(a)pH;(b)growth;(c)xylose consumption;(d)ABE production; (e)productivity and yield.
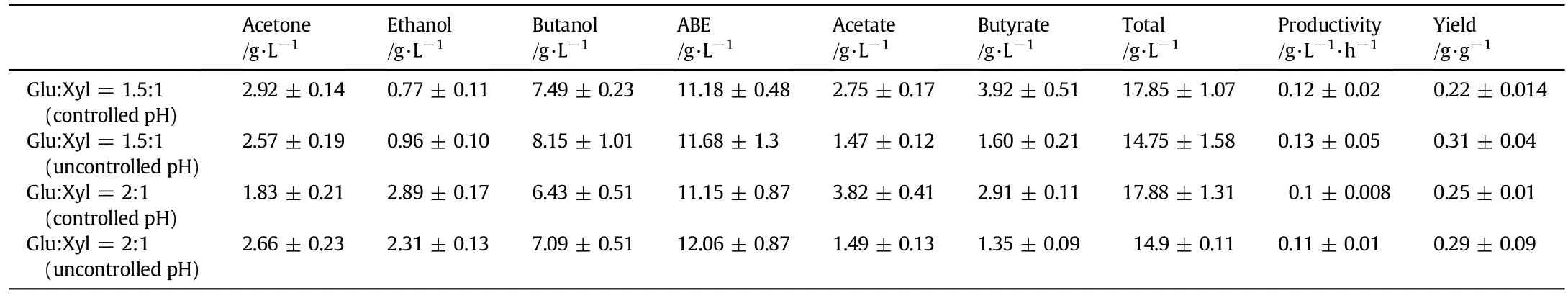
Table 1 Effect of pH controlon the fermentation by C.acetobutylicum ATCC 824 with glucose concentration higher than that of xylose
3.3.Utilization of D-glucose and D-xylose mixture
Since the hemicellulose hydrolysis may result in a mixture of D-glucose and D-xylose as the major carbohydrates,it is important to convert allresulting sugars into solvents to lower the cost of fermentation process.However,the transport and metabolism of different sugars vary considerably.C.acetobutylicum utilizes symporters and ATP-binding cassette transporters for the uptake of xylose, while glucose is primarily taken up by phosphotransferase system transporters[17].The efficiency of transport and metabolism of glucose is far higher than that of xylose,so there is severe carbon catabolite repression with glucose and xylose as carbon sources simultaneously.It has been demonstrated that the utilization of xylose is closely related with the proportion of xylose and glucose[13],so the effect ofpH control on the fermentation with different ratios of xylose and glucose should be investigated.
3.3.1.Effect of pH controlon the fermentation with higher ratio of glucose to xylose
Normally,glucose concentration is higher than that of xylose in most lignocellulosic hydrolysates.In this study,fermentation was initially conducted with two ratios of sugars:35 g·L-1of glucose and 23 g·L-1of xylose(1.5:1)and 40 g·L-1of glucose and 20 g·L-1of xylose (2:1).
As for glucose/xylose ratio of 1.5:1,pH regulation markedly raised the xylose utilization rate from 11.6%to 66.1%(Fig.3).However,the increased consumption of xylose did not account for the ABE production and yield.When pH was regulated,ABE production,productivity,and yield were 11.18 g·L-1,0.12 g·L-1·h-1and 0.22 g·g-1,respectively (Table 1).These were lower than those with no pH regulation,which were 11.68 g·L-1,0.13 g·L-1·h-1and 0.31 g·g-1(Table 1).On the contrary,with pH regulation for the same ratio of sugars,acetate and butyrate production always increased(Table 1).

Fig.3.Effect of pH controlon sugar utilization by C.acetobutylicum ATCC 824 xylosepregrown cells on complex medium(glucose 35 g·L-1,xylose 23 g·L-1,or glucose 40 g·L-1,xylose 20 g·L-1).
With glucose concentration higher than that of xylose,the pH controlstrategy increased the utilization ofxylose in sugar mixture,while the pH regulation effect on xylose utilization was less pronounced with a higher ratio of glucose to xylose(40 g·L-1:20 g·L-1).At this ratio and with pH control,xylose utilization rate increased from 11.5% to 31.5%in comparison with that no pH control(Fig.3).As a result of pHcontrol,fermentation produced 11.15 g·L-1ABE,which was slightly less than 12.06 g·L-1ABE obtained at uncontrolled pH(Table 1).With uncontrolled pH,the representative productivity of0.10 g·L-1·h-1and yield of0.25 g·g-1were obtained,and a productivity of0.11 g·L-1·h-1and a yield of0.29 g·g-1were measured(Table 1).
The results above may be attributed to the substantial accumulation of butanol from utilized glucose,which decreases the membrane pH gradient due to the increase of membrane fluidity.This may result in a modification of the lipid protein interactions,breaking ATPase and permease activities during xylose fermentation by C.acetobutylicum[21]. These modifications are pronounced and cause a drastic inhibition of xylose uptake especially in the presence of high concentrations of butanol.Cells in experimental group are better in xylose uptake because of higher internalpH than those in control group.However,ABE production is weakened by accumulation of acetate and butyrate,which may crash the transformation of acids to solvent[22].
3.3.2.Effectof pH controlon the fermentation with lower ratio of glucose to xylose
Because of the negative effects of pH controlon ABE production at higher ratios of glucose to xylose,further experiments were tested with the mixture of two sugars at lower ratios of glucose to xylose: 20 g·L-1of glucose and 40 g·L-1of xylose(1:2),10 g·L-1of glucose and 50 g·L-1of xylose(1:5).
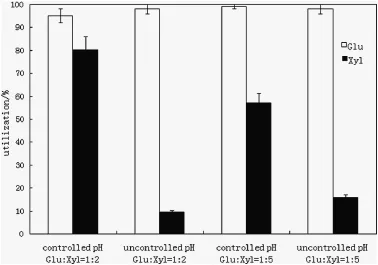
Fig.4.Effect of pH controlupon sugar utilization by C.acetobutylicum ATCC 824 xylosepregrown cells on complex medium(glucose 20 g·L-1,xylose 40 g·L-1,or glucose 10 g·L-1,xylose 50 g·L-1).
As seen in Fig.4,pH controldid not affect glucose utilization in the two sugar ratios,while the xylose utilization was clearly higher withthe controlled pH.At glucose/xylose ratio of1:2,pH controlincreased ABE production by 48.0%.However,the final concentrations of acetate and butyrate also increased by 81.9%and 103.3%,respectively,compared to those with uncontrolled pH(Table 2).The productivity and yield were 0.10 g·L-1·h-1and 0.23 g·L-1·h-1,respectively,in controlled pH treatment,while a lower productivity(0.07 g·L-1·h-1) and a higher yield(0.34 g·g-1)were measured in uncontrolled pH treatment(Table 2).When glucose was one-fifth of xylose with pHcontrol,the production of ABE was 6.11 g·L-1,3.6-folds of that without pH control(Table 2).Furthermore,as a result of pH control,the productivity shows the same trends of increase.
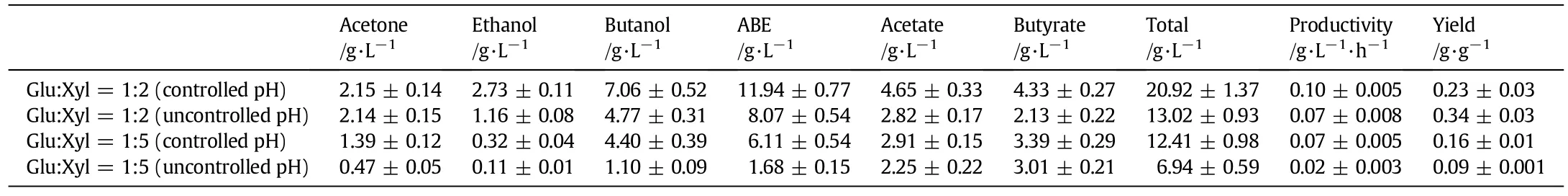
Table 2 Effect of pH controlon the fermentation with different proportions of glucose/xylose mixture(1:2,1:5)by C.acetobutylicum ATCC 824
Utilization of xylose and ABE production are promoted notably with sugar mixture by pHcontrolstrategy.Only a small amount of but anolis produced when glucose is exhausted,which will not remarkably inhibit the xylose absorption.Additionally,metabolism of glucose provides convenience(for example,induce transport and glycolytic enzymes) for uptake and metabolism of xylose[23,24].The pH control also plays a key role in maintaining a constant intracellular pH andΔpH,which could improve xylose utilization and ABE production.However,owing to the poor utilization efficacy of xylose,the concentrations of solvent are stillrather low.
Allin all,xylose is the limiting factor for the fermentation of lignocellulosic hydrolysates by C.acetobutylicum ATCC 824 due to its poor utilization efficacy and less preferred to be used when mixed with glucose.This study demonstrates that pH control could increase xylose utilization efficiency with mixed glucose and xylose regardless of their proportions.Interestingly,when xylose concentration is higher than that of glucose,pHcontroleven increases the solvent production.It is inferred that glucose could enhance xylose utilization at low but nonzero concentrations[23,25].Our data confirm it as the extent of improved xylose utilization by pH controlis more pronounced when glucose concentration is lower.The mechanisms for the improved xylose utilization can be attributed to the inductions of transport systems [23,26],glycolytic enzymes[24]and improved co-factor generation. These mechanisms may provide optimized conditions for further increasing the efficiency of lignocellulosic hydrolysate fermentation.
However,when xylose concentration is lower than that of glucose,the increased xylose consumption through pH controlleads to greater acid accumulation instead of solvent production.This suggests that pH control will make little difference in the improvement of solvent production,or increase ABE yield and productivity,as normallignocellulosic hydrolysates contain more glucose than xylose.Therefore,the efficientxylose uptake could be only achieved when glucose concentration is lower than that of xylose[27].Inspired by Bertilsson et al.'s result[27]and the findings in this study,it is possible to find a way of using lignocellulose more economically and efficiently in solvent fermentation.Firstly,lignocellulose could be pretreated by either acid or alkaline.This stage will mainly act on hemicellulose,releasing pentoses and hexoses included in hemicellulose as monomers,while most of glucose is present as glucan fiber in cellulose[27].Therefore,the glucose concentration remains low. Then fermentation is undertaken by C.acetobutylicum ATCC 824 using these mixed fermentable monosaccharides as carbon sources.Secondly, after the decomposition of lignin,enzymes(β-glucosidase,cellulase)are used to hydrolyze cellulose into glucose,which can also be used as the carbon source for C.acetobutylicum ATCC 824 without producing excessive inhibition effects(Fig.5).It is hoped that by combining successive hydrolysis and timely pH control,an increased solvent production with efficient xylose utilization and improved productivity can be achieved.
Biobutanol production from lignocellulosic biomass involves physical/chemical pretreatment,enzymatic hydrolysis,fermentation (or simultaneous saccharification and fermentation or co-fermentation) and some other unit operations[28].At present,the unsolved problems such as inefficient co-fermentation of C6 and C5 sugars associated with the utilization of lignocelluloses hinder the establishment of large scale lignocellulose-based biobutanol plants.However,this may change in the near future because of the worldwide research and development efforts aimed at applying lignocellulose as a fermentation feedstock. Furthermore,separation and hydrolysis of various components of biomass will allow complete use of crop,which confers economic as well as environmental benefits[29].
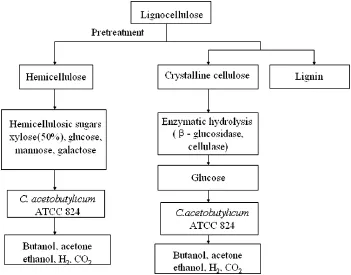
Fig.5.Schematic main steps of lignocellulose conversion.
4.Conclusions
The utilization rate of major sugars in lignocellulose by C.acetobutylicum ATCC 824 is in the order of D-glucose,L-arabinose,D-mannose,D-xylose and cellobiose.With D-xylose as the only carbon source or at higher concentration than that of glucose,pH control could improve cell growth,increase D-xylose consumption, and enhance ABE production.However,when xylose is less than that of glucose,the increased xylose consumption by pH controlleads to acid accumulation instead of solvent production.
Acknowledgments
The authors would like to give their sincere gratitude to Professor Nathan Yang(Shanghai Long March Hospital,Second Military Medical University)for the revision of the manuscript.
[1]T.C.Ezeji,H.P.Blaschek,Biofuelfrom butanol:advances in genetic and physiological manipulation of clostridia,BioWorld Eur.2(2007)12-15.
[2]Nexant Of fices,Biobutanol:the next big biofuel,Chemical Systems with Chemical Strategies,Nexant,2009.
[3]C.Weber,A.Farwick,F.Benisch,D.Brat,H.Dietz,T.Subtil,E.Boles,Trends and challenges in the microbial production of lignocellulosic bioalcohol fuels,Appl. Microbiol.Biotechnol.87(4)(2010)1303-1315.
[4]D.T.Jones,D.R.Woods,Acetone-butanol fermentation revisited,Microbiol.Rev.50 (4)(1986)484-534.
[5]T.C.Ezeji,N.Qureshi,H.P.Blaschek,Production of acetone butanol(AB)from liquefi ed corn starch,a commercial substrate,using Clostridium beijerinckii coupled with product recovery by gas stripping,J.Ind.Microbiol.Biotechnol.34(12)(2007) 771-777.
[6]K.Shetty,G.Paliyath,A.Pometto,R.E.Levin,Food Biotechnology,Second edition Taylor&Francis Group,Boca Raton,Florida,USA,2005.525-551.
[7]N.Qureshi,X.L.Li,S.Hughes,B.C.Saha,M.A.Cotta,Butanolproduction from corn fiber xylan using Clostridium acetobutylicum,Biotechnol.Prog.22(3)(2006) 673-680.
[8]N.Qureshi,T.C.Ezeji,Butanol,‘a superior biofuel’production from agriculturalresidues(renewable biomass):recent progress in technology,Biofuels,Bioprod.Biorefin. 2(4)(2008)319-330.
[9]H.S.Lü,M.M.Ren,M.H.Zhang,Y.Chen,Pretreatment of corn stover using supercritical CO2with water-ethanol as co-solvent,Chin.J.Chem.Eng.21(5)(2013) 551-557.
[10]M.J.Zhang,R.X.Su,W.Qi,R.Y.Du,Z.M.He,Enzymatic hydrolysis of cellulose with different crystallinities studied by means of SEC-MALLS,Chin.J.Chem.Eng.19(5) (2011)773-778.
[11]J.Chen,Y.Wang,G.He,H.Zhang,Z.Zhou,Bioconversion of lignocellulose to ethanol, Sci.Silvae Sin.43(5)(2007)99-105.
[12]J.Kim,D.E.Block,D.A.Mills,Simultaneous consumption of pentose and hexose sugars:an optimalmicrobialphenotype for efficient fermentation of lignocellulosic biomass,Appl.Microbiol.Biotechnol.88(5)(2010)1077-1085.
[13]A.E.Kanouni,I.Zerdani,S.Zaafa,M.Znassni,M.Lout,M.Boudouma,The improvement of glucose/xylose fermentation by Clostridium acetobutylicum using calcium carbonate,World J.Microbiol.Biotechnol.14(3)(1998)431-435.
[14]M.H.W.Husemann,E.T.Papoutsakis,So lventogenesis in Clostridium acetobutylicum fermentation related to carboxylic acid and proton concentrations,Biotechnol. Bioeng.32(7)(1988)843-852.
[15]C.Ren,Y.Gu,S.Y.Hu,Y.Wu,P.Wang,Y.L.Yang,C.Yang,S.Yang,W.H.Jiang,Identification and inactivation of pleiotropic regulator CcpA to eliminate glucose repression of xylose utilization in Clostridium acetobutylicum,Metab.Eng.12(5)(2010) 446-454.
[16]C.Grimmler,C.Held,W.Liebl,A.Ehrenreich,Transcriptional analysis of catabolite repression in Clostridium acetobutylicum growing on mixtures of d-glucose and d-xylose,J.Biotechnol.150(3)(2010)315-323.
[17]M.D.Servinsky,J.T.Kiel,N.F.Dupuy,C.J.Sund,Transcriptional analysis of differential carbohydrate utilization by Clostridium acetobutylicum,Microbiology 156(11) (2010)3478-3491.
[18]T.Millat,H.Janssen,G.J.Thorn,J.R.King,H.Bahl,R.J.Fischer,O.Wolkenhauer,A shift in the dominantphenotype governs the pH-induced metabolic switch of Clostridium acetobutylicum in phosphate-limited continuous cultures,Appl.Microbiol.Biotechnol. 97(14)(2013)6451-6466.
[19]M.Gottwald,G.Gottschalk,The internalpH of Clostridium acetobutylicum and its effect on the shift from acid to solvent formation,Arch.Microbiol.143(1)(1985) 42-46.
[20]D.B.Kell,M.W.Peck,G.Rodger,J.G.Morris,On the permeability to weak acids and bases of the cytoplasmic membrane of Clostridium pasteurianum,Biochem.Biophys. Res.Commun.99(1)(1981)81-88.
[21]K.Ounine,H.Petitdemange,G.Raval,R.Gay,Regulation and butanolinhibition of D D-xylose and D D-glucose uptake in Clostridium acetobutylicum,Appl.Environ. Microbiol.49(4)(1985)874-878.
[22]I.S.Maddox,E.Steiner,S.Hirsch,S.Wessner,N.A.Gutierrez,J.R.Gapes,K.C.Schuster, The cause of“acid crash”and“acidogenic fermentations”during the batch acetonebutanol-ethanol(ABE-)fermentation process,J.Mol.Microbiol.Biotechnol.2(1) (2000)95-100.
[23]J.-P.Pitkänen,A.Aristidou,L.Salusjärvi,L.Ruohonen,M.Penttilä,Metabolic flux analysis of xylose metabolism in recombinant Saccharomyces cerevisiae using continuous culture,Metab.Eng.5(1)(2003)16-31.
[24]E.Boles,S.Müller,F.K.Zimmermann,A multi-layered sensory system controls yeast glycolytic gene expression,Mol.Microbiol.19(3)(1996)641-642.
[25]N.Q.Meinander,I.Boels,B.Hahn-Hägerdal,Fermentation of xylose/glucose mixtures by metabolically engineered Saccharomyces cerevisiae strains expressing XYL1 and XYL2 from Pichia stipitis with and without over expression of TAL1, Bioresour.Technol.68(1)(1999)79-87.
[26]M.Bertilsson,J.Andersson,G.Lidén,Modeling simultaneous glucose and xylose uptake in Saccharomyces cerevisiae from kinetics and gene expression of sugar transporters,Bioprocess Biosyst.Eng.31(4)(2008)369-377.
[27]M.Bertilsson,K.Olofsson,G.Lidén,Prefermentation improves xylose utilization in simultaneous sacchari fi cation and co-fermentation of pretreated spruce,Biotechnol. Biofuels 2(2009)8.
[28]M.-R.Ricardo,V.G.Krist,S.M.Anne,S.Gürkan,A mathematical model for simultaneous sacchari fi cation and co-fermentation(SSCF)of C6 and C5 sugars,Chin.J. Chem.Eng.19(2)(2011)185-191.
[29]P.A.M.Claassen,J.B.van Lier,A.M.Lopez Contreras,E.W.J.van Niel,L.Sijtsma,A.J.M. Stams,S.S.de Vries,R.A.Weusthuis,Utilisation of biomass for the supply of energy carriers,Appl.Microbiol.Biotechnol.52(6)(1999)741-755.
☆Supported by the National Natural Science Foundation of China(20306026 and 21376215)and the National High Technology Research and Development Program of China(2012AA022302).
*Corresponding authors.
**Authors with equalcontribution to this work.
E-mailaddresses:wumb@zju.edu.cn(M.Wu),linjp@zju.edu.cn(J.Lin).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Promoting Xylene Production in Benzene Methylation using Hierarchically Porous ZSM-5 Derived from a Modified Dry-gel Route☆
- Preparation,Structure Characterization and Hydrodesulfurization Performance of B-Ni2P/SBA-15/Cordierite Monolithic Catalysts☆
- Enhanced Photocatalytic Properties of Silver Oxide Loaded Bismuth Vanadate☆
- Recovery and Recycling of Ti Supported Bimodal Mesoporous Catalysts Prepared via Ship-in-a-bottle Method in the Epoxidation of Cyclohexene☆
- Energy,Resources and Environmental Technology Experimental and Modeling Study on de-NOxCharacteristics of Selective Non-catalytic Reduction in O2/CO2Atmosphere☆
- Energy,Resources and Environmental Technology CO2Removal from Biogas by Water Washing System☆