Energy,Resources and Environmental Technology Experimental and Modeling Study on de-NOxCharacteristics of Selective Non-catalytic Reduction in O2/CO2Atmosphere☆
2014-07-12HuiLiKuihuaHanHongtaoLiuChunmeiLu
Hui Li,Kuihua Han*,Hongtao Liu,ChunmeiLu
Energy,Resources and Environmental Technology Experimental and Modeling Study on de-NOxCharacteristics of Selective Non-catalytic Reduction in O2/CO2Atmosphere☆
Hui Li,Kuihua Han*,Hongtao Liu,ChunmeiLu
NationalEngineering Laboratory for Coal-fired Pollutants Emission Reduction,School of Energy and Power Engineering,Shandong University,Jinan 250061,China
A R T I C L E I N F o
Article history:
Received 4 March 2013
Received in revised form 16 September 2013
Accepted 12 December 2013
Available online 16 June 2014
Selective non-catalytic reduction
Denitrification
Ammonia
Kinetic modeling
O2/CO2
SO2
Additives
An experimental study of the rmalde-NOxusing NH3as reductant in O2/CO2atmosphere with the effect of SO2and different additives was performed in a drop tube furnace.Results show that the optimum temperature window is 841-1184°C,and the optimum reaction temperature is about900°C with a de-NOxefficiency of95.4%.A certain amount of SO2has an inhibiting effect on NOreduction.The effect of additives,including Na2CO3,C2H5OH and FeCl3,on NOreduction by NH3is also explored.The addition of Na2CO3and FeCl3is useful to widen the temperature window and shift the reaction to lower temperature for the efficiency is increased from 30.5%to 74.0% and 67.4%respectively at 800°C.Qualitatively,the modeling results using a detailed kinetic modeling mechanism represent well most of the process features.The effect of Na2CO3,C2H5OH and FeCl3addition can be reproduced well by the Na2CO3,C2H5OH and Fe(CO)5sub-mechanism respectively.The reaction mechanism analysis shows that the effects of these additives on NOreduction are achieved mainly by promoting the production of OH radicals at lower temperature.
©2014 The Chemical Industry and Engineering Society of China,and Chemical Industry Press.Allrights reserved.
1.Introduction
Nitrogen oxides(NOx),responsible for air pollution and harming human health are mainly emitted from coal plants.The recently released Emission Standard of Air Pollutants for Thermal Power Plants (GB13223-2011)by the Chinese government is implementing more and more stringent NOxregulation.The selective non-catalytic reduction(SNCR)process is widely used for NOxreduction by injecting into fuelgas nitrogenous compounds containing-NH-or-CN-function groups such as ammonia(NH3)[1,2],urea[(NH2)2CO][3,4]or cyanuric acid[(HNCO)3][5,6].Ammonia based SNCR techniques are cost effective,space saving,simple of fl owsheetand with high compatibility compared with other NOxcontrol technologies.However,SNCR cannot achieve 90%of NOxreduction out of the laboratory.The reduction efficiency of ammonia based SNCR is about 30%-75%depending on the facility type and scale,operating conditions,reductant mixture composition and many other variables[7].Selective catalytic reduction(SCR) has higher efficiency(60%-95%)but is also highly costly[8].Hybrid NOxreduction technologies can be an effective method to meet the more stringentemission standard,such as hybrid coalreburning/SNCR and hybrid SCR/SNCR processes[9-11].The O2/CO2combustion technology is an innovative combustion technology which can control CO2,SO2and NOxemissions simultaneously[12-14].Previous studies have shown that there are several factors in using O2/CO2atmosphere that contribute to the decrease in NOxemission level.One is the elimination of thermal NOxand prompt NOxbecause of the absence of N2.The other is the high CO2concentration which can inhibit conversion of fuel-N to NO.The third and most important factor is the interaction between the recycled NOxand the fuel-N released from coal which may further decrease NOxformation[15,16].These factors can also influence the SNCR process. Combining the O2/CO2atmosphere technique with SNCR will reduce NOxemissions further and can meet the increasingly stringent standards.
Many studies of SNCR process in air atmosphere have been performed but there is hardly any study on the O2/CO2atmosphere.In this study,new experimental results were presented for NO reduction characteristics through a drop tube furnace to provide further insight into the NOxreduction in O2/CO2atmosphere.Computational kinetic modeling using a detailed chemicalkinetic mechanism was then performed to analyze the de-NOxprocess so as to provide a direct comparison of modeling prediction with experimental measurements.
2.Experimental Methods
The experiments performed in this study were conducted in a drop tube furnace shown in Fig.1.The experimental setup consists of a gas feeding system,an additive feeding system,a reaction system and a gas analysis system.The reaction system includes an electricity-heated corundum flow reactor which has an inner diameter of 4 cm and a length of104 cm.12 carborundum sticks are located along the reactor to control the reaction temperature in the 800-1200°C range.Theactual temperature distribution along the reactor length under typical conditions is shown in Fig.2.The length of the constant temperature zone was 55 cm which was regarded as the reaction zone,and the preheating zone was about 35 cm.The sampling probe was initially located in 85 cm from the inlet of the reactor.A water-cooled system was used to cool the inlet of the reactor and the gas sampling probe.
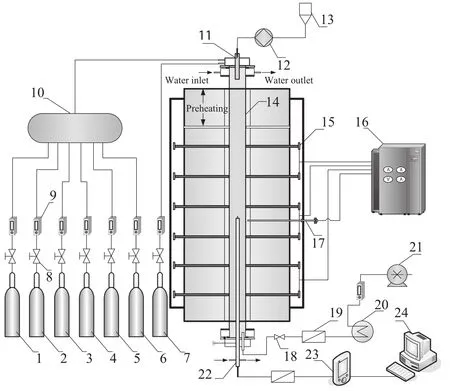
Fig.1.experimental system schematic.1—CO2;2—O2;3—NO;4—NH3;5—Ar;6—N2;7—SO2;8—pressure reducing valve;9—flow meter;10—mixer;11—additive inlet;12—peristaltic pump;13—additive solution;14—corundum tube;15—carborundum stick;16—temperature controller;17—thermocouple;18—sphericalvalve;19—filter;20—water-cooled heat exchanger;21—vacuum pump;22—sampling probe;23—gas analyzer;24—computer.

Fig.2.Measured temperature distribution along the reactor as a function of axial position (initial conditions:O2/CO2/NH3/NO,gas inlet temperature=20°C,gas flow rate in the reaction zone=2 m3·h-1,φNO=600μl·L-1,φO2=3%,CO2balanced,residence time=1.2 s,feed[NH3]/[NO]ratio=1.5).
Supplies of O2,CO2,N2and Ar were bottled standard gases with purity of99.5%.The concentration of NO and NH3was 10%and balanced respectively by Ar and CO2streams from gas bottles.All the gases were premixed in a mixture before entering the reactor.The gas inlet temperature was 20°C.The flow rates were measured and controlled by flow meters.Optima 7,a German-made multiple gas analyzer was used to continuously analyze the concentrations of O2,CO2,NO,NO2and SO2through the sampling probe.The gas flow rate and sampling probe position were controlled to change the residence time.The gas flow rate was 2 m3·h-1with a residence time of 1.2 s in the reaction zone.The NO initial concentration was 600μl·L-1and O2was 3%.The [NH3]/[NO]molar ratio(R)was 1.5.This study also concerned on the influence of SO2and NH3in combination with aqueous additives such as Na2CO3,C2H5OHand FeCl3on NO reduction.Allthe additives were analytically pure and dissolved by the deionized water.The additive initial concentration injected into the reactor was 200μl·L-1.The additive was injected into the reactor through the additive inlet by a peristaltic pump and it was gasified immediately since its flow rate was as low as 10 ml·min-1.The initialconcentration of NO2was around 30μl·L-1with 600μl·L-1of NO,and the outlet concentration was below 10μl-1.The gas analyzer could notanalyze the concentration of N2O.
According to the modeling results and previous experimental results by other researchers[17,18],the concentration of N2O was negligible, and thus the effect of N2O was neglected.The NO reduction efficiency was calculated by

whereηwas the NO reduction efficiency,andφNO,0andφNO,1were the inlet and outlet concentrations of NO respectively.
3.Kinetic Modeling
Many works have been done to establish the chemicalkinetic mechanism of SNCR in traditional combustion atmosphere.The MG99 model [19]has been proved to be reliable to predictthermalde-NOxprocess.In this study,the kinetic mechanismis builtstarting from the MG99 model complemented with the reactions taken from the Leeds model[20]todescribe the interactions between SO2and nitrogenous compounds.The additive reactions that come from the Rota model[21],Zamansky model[22]and Rumminger model[23]are also included into the mechanism.Some gas phase reactions are modified according to the experimental results to better reveal the realprocess nature.On the whole, the mechanism contains 570 elementary gas phase reactions and 114 chemical species.A plug-flow reactor(PFR)module which runs in conjunction with Chemkin 4.0 is used to perform the kinetic model calculations.The length of the model reactor is 55 cm which is the same with the reaction zone and the contribution of reaction in the preheating zone is neglected for its low temperature.
Sensitivity Analysis(SA)in Chemkin is a powerful and systematic way to determine quantitatively the relationship between the various elementary reactions and reacting species that appear in the model.In this study,the fi rst-order sensitivity analysis of NO concentration at the reactor outlet has been performed to find out which elementary reaction exerts the most influence on NO reduction.The sensitivity coefficient S is basically the ratio of the change in exiting NO concentration per change of the rate constant kiof elementary reaction i in the chemical system,while all other parameters remain constant:

The value of S stands for the intensity of influence of reaction i on NO removal.Its positive value means that reaction i produces NO and on the contrary,the negative value means that it contributes to NO reduction [24-26].
4.Results and Discussion
4.1.Axial NO distribution and NH3consumption efficiency
According to the modeling results,NH3and NO will not react under 750°C.Since there are some points with temperature higher than 750°C in the preheating zone,some influence on NO reduction may be resulted by the prolonged reaction zone.This influence was tested in the modified model by prolonging the reaction zone according to the temperature distribution.Fig.3 shows the original and modified results of NO reduction efficiency.The efficiency in the modified model is at most2.5%higher(experimentat 800°C)than that with the original results as shown in Fig.3.Some previous modeling results with the similar system[27,28]with the influence of the preheating zone neglected also reproduce well the experimentaldata.It is reasonable and reliable to neglect the influence of the preheating zone in the present work.
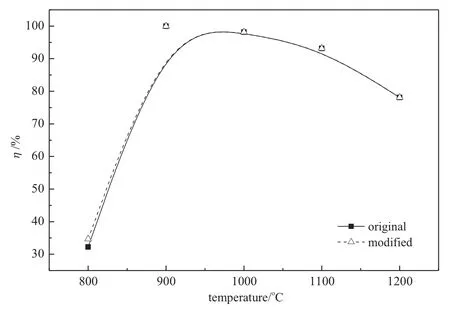
Fig.3.Original and modified modeling results of NO reduction efficiency(closed symbols and solid lines:original results;open symbols and dashed lines:modified results.Initial conditions:O2/CO2/NH3/NO,φNO=600μl·L-1,φO2=3%,CO2balanced,R=1.5).

Fig.4.NO distribution along the reaction zone as a function of axial position(closed symbols and solid lines:experimental results;open symbols and dashed lines:modeling results.Initial conditions:O2/CO2/NH3/NO,gas inlet temperature=20°C,gas flow rate in the reaction zone=2 m3·h-1,φNO=600μl·L-1,φO2=3%,CO2balanced,residence time=1.2 s,R=1.5).
Fig.4 shows the experimentaland modeling results of axial NO distribution under typical conditions in the reaction zone.They present the same trend that NO concentration decreases rapidly over the first20 cm length and then changes slowly as the NO reduction reactions are almost completed.Higher temperature leads to a higher reaction rate, but the outlet concentration of NO becomes larger due to the oxidation of ammonia.
The simulated NH3consumption efficiencies with different molar ratios are illustrated in Fig.5.The NH3consumption efficiency is calculated by

whereφNH3,0andφNH3,1are the inlet and outlet concentrations ofNH3respectively.It can be seen that the efficiency increases with temperature and NH3can be totally consumed at higher temperature.The escape concentration of NH3is much higher for higher molar ratio at lower temperature.The excessive NH3can be completely oxidized and consumed by O2when the temperature is above 1100°C.However,previous studies indicate that because of the mixing of NH3and NO and the reactions of NH3with O2,the optimal NO reduction efficiency is achieved at a higher molar ratio more than needed.The[NH3]/[NO]molar ratio is fixed to the value of1.5 according to the previous studies [2,22,27].

Fig.5.Simulated NH3consumption efficiencies with different molar ratios(initial conditions:O2/CO2/NH3/NO,gas flow rate in the reaction zone=2 m3·h-1,φNO=600μl·L-1, φO2=3%,CO2balanced,residence time=1.2 s).
4.2.Reaction temperature and atmosphere on NO reduction
Temperature is an important factor to NO reduction.In this study, temperature window is defined as the temperature range over where 80%of the maximum efficiency could be achieved.The experimental results of the effect of reaction temperature on NO reduction with a variation of atmospheres without additives in O2/CO2are illustrated in Fig.6 together with the modeling results.It is seen that as the reactor temperature increases in O2/N2,O2/Ar as well as O2/CO2,the NO reduction efficiency increases rapidly up to 900°C to reach a maximum.It is 97.5%in O2/N2,98.4%in O2/Ar and 95.4%in O2/CO2.Above 900°C,the NO reduction efficiency slowly decreases with increasing temperature.The temperature windows are all around 850-1200°C,i.e.841-1184°C in O2/ CO2,832-1200°C in O2/Ar and 843-1200°C in O2/N2.In the temperature windows of the three atmospheres,the NO reduction efficiencies show minor difference among each other.The highest efficiency is obtained in O2/Ar as well as the lowest in O2/CO2.The modeling results reproduce well the experimental data on the effect of reaction temperature on NO reduction.However,the predicted NO reduction efficiency in O2/CO2is little higher than experimental data at each temperature, possibly due to the departure from the ideal gas mixing,heat and flow conditions in the reactor leading to lower efficiency.
Table 1 shows the sensitivity analyses of the main reactions which are responsible for NO reduction at 900°C.(115),(199)and(211) which can be promoted by OH and NH2radicals have the biggest influence on NO reduction.It is seen from Fig.7 that the key step of the the rmalde-NOxprocess is the reaction between NH3and OH to form NH2.Previous studies proposed by Wu and Niu[27,29]find that at lower temperature,little NO is removed due to the lower reaction rates and lack of OH radicals.The OH radical concentration increases with increasing temperature,which leads to the increase of the NO reduction efficiency.At higher temperature,the formation of NO from ammonia oxidation is favored compared to its destruction.Ar,N2and CO2have different heat specifics at constant pressure.The temperature of the reaction gases increases fastest in O2/Ar and slowestin O2/CO2,for Ar has the smaller heat specific at constant pressure as compared with CO2.Thus,in O2/Ar the higher NO reduction efficiency in a lower temperature is achieved than that in O2/N2and O2/CO2as shown in Fig.6.
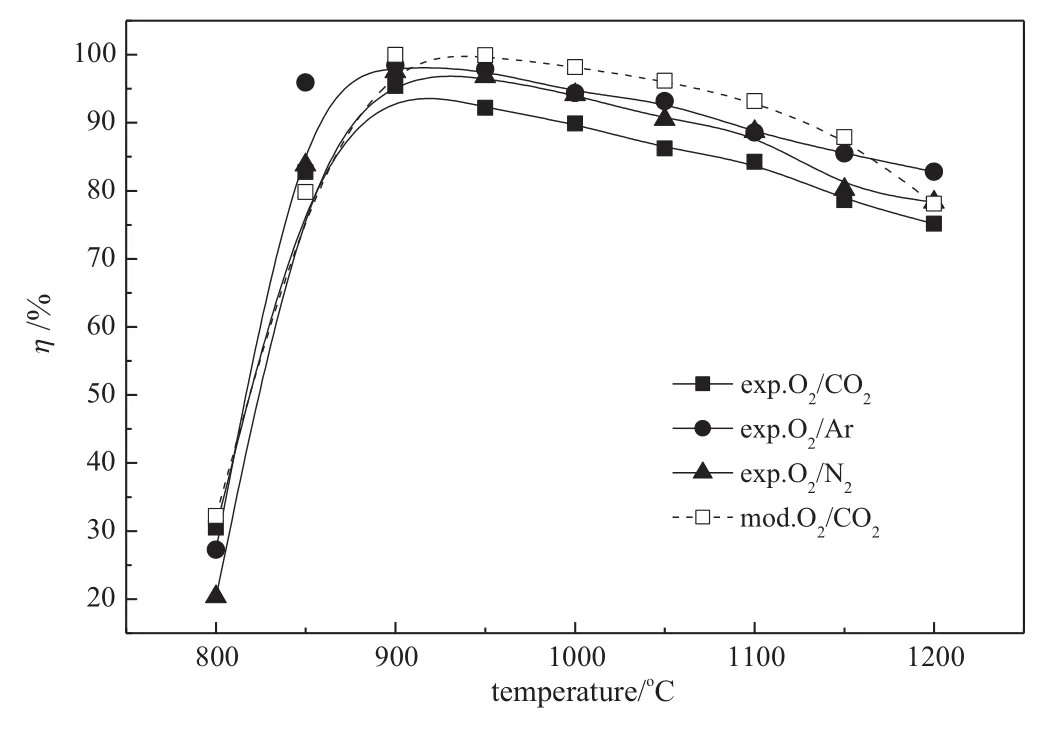
Fig.6.Temperature and atmosphere on NO reduction(closed symbols and solid lines:experimental results;open symbols and dashed lines:modeling results.Initial conditions: O2/CO2(Ar,N2)/NH3/NO,gas inlet temperature=20°C,gas flow rate in the reaction zone=2 m3·h-1,φNO=600μl·L-1,φO2=3%,residence time=1.2 s,R=1.5).

Fig.7.Reaction path diagram for the thermalde-NOxprocess.
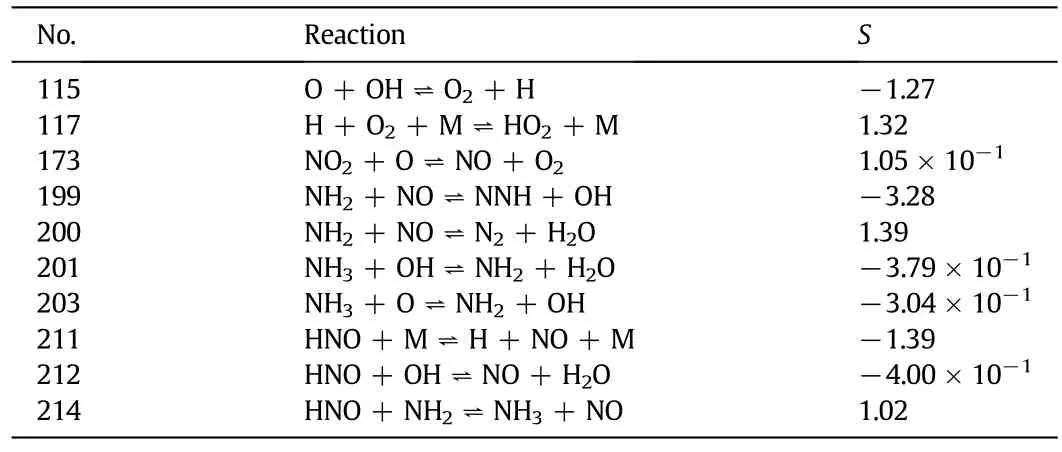
Table 1 The main reactions responsible for NO reduction at 900°C
4.3.SO2on NO reduction
Fig.8 shows the effect of 2000μl·L-1SO2added on NO reduction.It can be seen from the experimental results that SO2has an inhibiting effect on NO reduction at each experimental temperature.It decreases from 95.4%to 77.8%at the optimum temperature.The modeling presents the same trend of inhibiting effect on NO reduction,but the predicted inhibition by SO2is much weaker than the experimental observation.In this study,O2,CO2,NOand NH3are premixed before entering the reactor while SO2is added into the reactor directly.These factors may influence the deviation of modeling from experiment.Further studies need to be done to better reveal the inhibiting mechanism and confirm these assumptions.
Table 2 shows the main reactions of SO2responsible for NO reduction at 900°C.It can be seen from the table that all the reactions involved with sulfur compounds can promote the production of NO which is negative to NO reduction.Researchers[30]explained the SO2inhibition using the following sequence of reactions:

Reaction(4)reduces the O-atom concentration,resulting in a reduction of OH radical production in reaction(5).Reaction(6)would also reduce the fraction of OH radical by replacing the formation of3OH radicals via reactions(4)and(7).SO2can react with NH2radical through reaction(8).As it is discussed before,the lack of OH and NH2radicals will lead to the decline of the NO reduction efficiency.
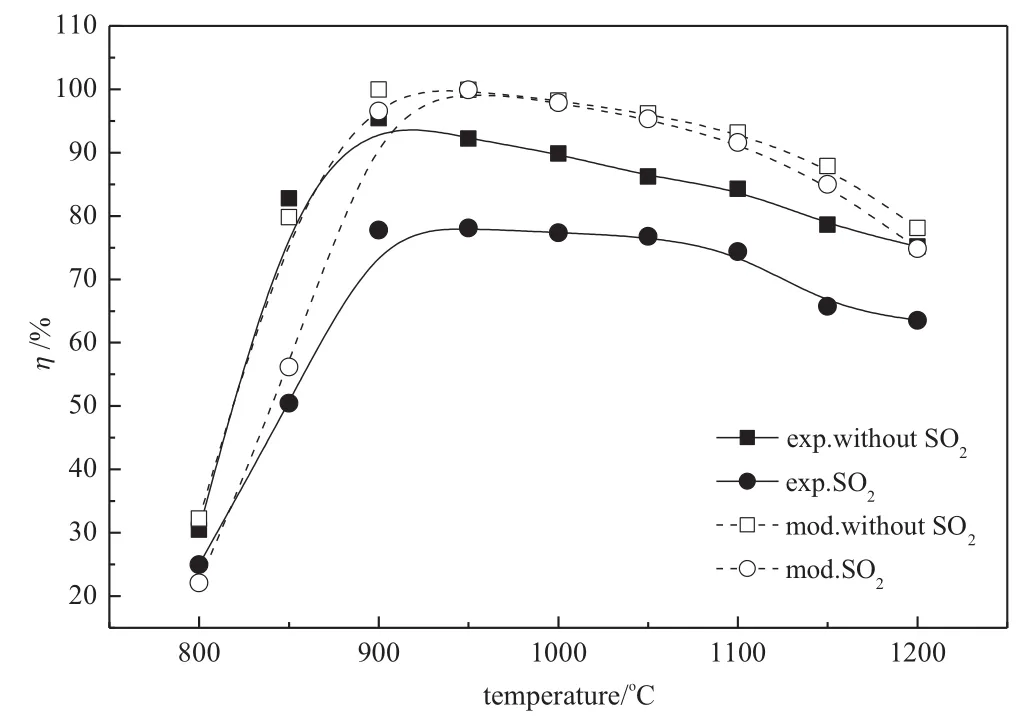
Fig.8.Inhibition of added SO2on NO reduction(closed symbols and solid lines: experimental results;open symbols and dashed lines:modeling results.Initial conditions:O2/CO2/NH3/NO,gas inlet temperature=20°C,gas flow rate in the reaction zone=2 m3·h-1,φNO=600μl·L-1,φO2=3%,CO2balanced,residence time= 1.2 s,R=1.5,φSO2=2000μl·L-1).

Fig.9.Na2CO3on NO reduction(closed symbols and solid lines:experimental results; open symbols and dashed lines:modeling results.Initial conditions:O2/CO2/NH3/NO, gas inlet temperature=20°C,gas flow rate in the reaction zone=2 m3·h-1,additive fl ow=10 ml·min-1,φNO=600μl·L-1,φO2=3%,CO2balanced,residence time= 1.2 s,R=1.5,φNa2CO3=200μl·L-1).
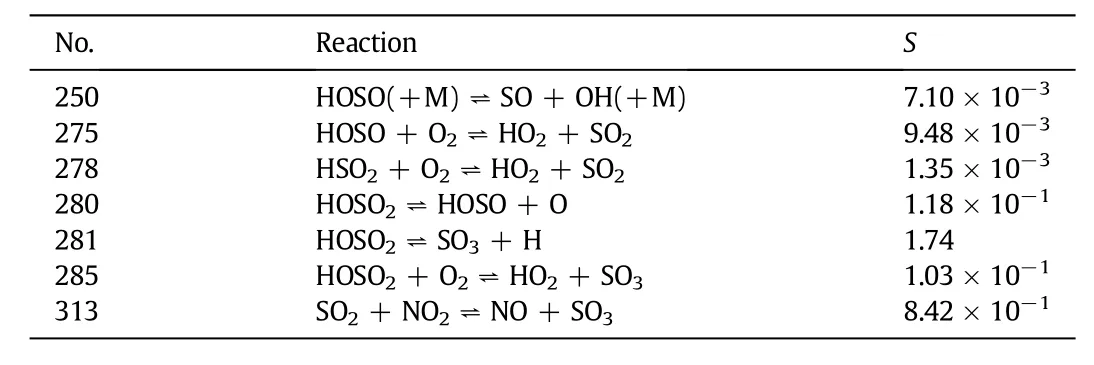
Table 2 The main reactions of SO2responsible for NO reduction at 900°C
4.4.EffectofNa2CO3
Fig.9 shows the effect of Na2CO3on NOreduction,indicating that the addition of Na2CO3widens the temperature window and shifts the optimum reaction temperature to lower points as it increases the efficiency significantly in lower temperature conditions.The NO reduction efficiency is increased from 30.5%to 74.0%at 800°C.Qualitatively the modeling results exhibit the same trend.Despite that the model under-predicts the effect of Na2CO3on NO reduction below 850°C and over-predicts it above 1050°C,it captures the shift of temperature window with the addition of Na2CO3.
Table 3 shows the sensitivity analyses of the main reactions of Na2CO3which are responsible for NO reduction at 850°C.It can be seen from the table that the reactions that involve sodium compounds are mostly positive to NO reduction and negative to few reactions. Na2CO3and the decomposition product can't react with NO but can produce OH radicals through a series of reactions.Once entering the reaction area,Na2CO3becomes a homogeneous configuration that then reacts with vapor to form NaOH,NaO,Na and many other sodium compounds.OH radicals will be produced through these reactions such as (328)and(343).The netaction is equivalent to H2O⇌H+OH resulting to an increase of H and OH radicals.At lower temperature,it can increase the NO reduction efficiency obviously as there are no enough OH radicals[31-33].
4.5.Effect of C2H5OH
The experimental and modeling results obtained are shown in Fig.10.The NO reduction efficiency below 900°C is increased by C2H5OH but the reaction temperature window is not widened in the experiments.The predicted efficiency curve reproduces well the effect of C2H5OH on NO reduction.Table 4 shows the main reactions of C2H5OH responsible for NO reduction at850°C.Athigher temperature above 700°C,C2H5OH is decomposed into CHiand OH radicals.Except for the decomposition,C2H5OH can also react with O2to form OH radicals at lower temperature.As the temperature increases,the oxidation of C2H5OH and CHibecomes more complete,and it forms CO2and H2O instead of OH radicals that have little influence to the efficiency[21, 28,34].
4.6.Effect of FeCl3
The effect of FeCl3on NO reduction is illustrated in Fig.11.The mechanism of iron compounds on NO reduction is rare and not clear.The Fe(CO)5reaction sub-mechanism that came from the Rumminger model[23]was added to predict the FeCl3on NO reduction instead. The same characteristics like Na2CO3are presented by FeCl3as thetemperature window is widened for the efficiency increases significantly below 900°C.The NO reduction efficiency is increased from 30.5%to 67.4%at 800°C.The modeling study on the effect of Fe(CO)5on NO reduction exhibits a consistent trend as the experimental results of FeCl3addition.Except for the under-prediction of NO reduction at 800°C, the Fe(CO)5reaction sub-mechanism is adaptive to simulate the effect of FeCl3addition.The main reactions of Fe(CO)5which contribute to NO reduction are listed in Table 5.Fe(CO)5can decompose into CO and a series of iron compounds such as FeO2,FeO and FeOOH.Iron compounds don't react directly with NO but can produce more OH radicals through the reaction with other radicals,for example the net action of (535)and(552)is equivalent to H2O⇌H+OH.It is reasonable to indicate that FeCl3can also produce more radicals which are positive to NO reduction through a series of interactions between the iron compounds and other radicals.The accurate mechanism of NO reduction with the addition of FeCl3needs to be found out by further analyzing the reaction residuum.

Table 3 The main reactions of Na2CO3responsible for NO reduction at 850°C

Fig.10.C2H5OH on NO reduction(closed symbols and solid lines:experimental results; open symbols and dashed lines:modeling results.Initial conditions:O2/CO2/NH3/NO, gas inlet temperature=20°C,gas flow rate in the reaction zone=2 m3·h-1,additive fl ow=10 ml·min-1,φNO=600μl·L-1,φO2=3%,CO2balanced,residence time= 1.2 s,R=1.5,φC2H5OH=200μl·L-1).
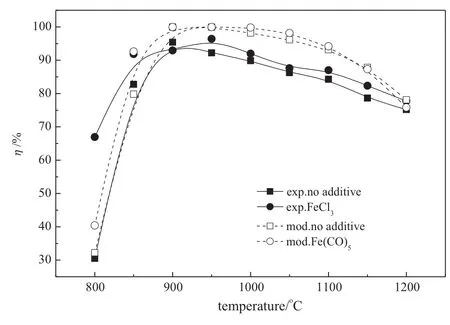
Fig.11.FeCl3on NO reduction(closed symbols and solid lines:experimental results;open symbols and dashed lines:modeling results.Initial conditions:O2/CO2/NH3/NO,gas inlet temperature=20°C,gas flow rate in the reaction zone=2 m3·h-1,additive fl ow= 10 ml·min-1,φNO=600μl·L-1,φO2=3%,CO2balanced,residence time=1.2 s,R= 1.5,φFeCl3=200μl·L-1).
5.Conclusions
New experimental results on de-NOxcharacteristics of ammonia based SNCR process were investigated in a drop tube reactor.Based on the experimental results,the conclusions can be summarized as follows. The optimum reaction temperature is 900°C with a reduction efficiency of95.4%.The temperature windows are all around 850-1200°C:841-1184°C in O2/CO2,832-1200°C in O2/Ar and 843-1200°C in O2/N2.In the temperature windows of the three atmospheres,the NO reduction efficiencies indicate little difference among each other.A certain amount of SO2has an inhibiting effect on NO reduction.The addition of Na2CO3and FeCl3is useful to widen the temperature window and shift the reaction temperature to lower one and the efficiency at 800°C is increased from 30.5%to 74.0%and 67.4%respectively.
The kinetic model using a detailed chemical kinetic mechanism was built starting from the MG99 model complemented with the submechanism taken from the Leeds model,Rota model,Zamansky model and Rumminger model.The modeling simulations reproduce well most of the process features.The Na2CO3,C2H5OH and Fe(CO)5sub-mechanism are adaptive to simulate the effect of Na2CO3,C2H5OH and FeCl3addition respectively.However,departure from the ideal mixing,heat and flow conditions leads to a lower efficiency in experiments compared to the modeling results.The main reactions responsible for NO reduction with and without additives are investigated by sensitivity analyses.The analysis on reaction mechanism shows that the reason for increasing the efficiency at lower temperature is similar for these additives.It can be attributed to the branching reactions caused by the additives,which enhance the production of OH radicals. Consequently the conversion of NH3and the reduction of NO are enhanced.
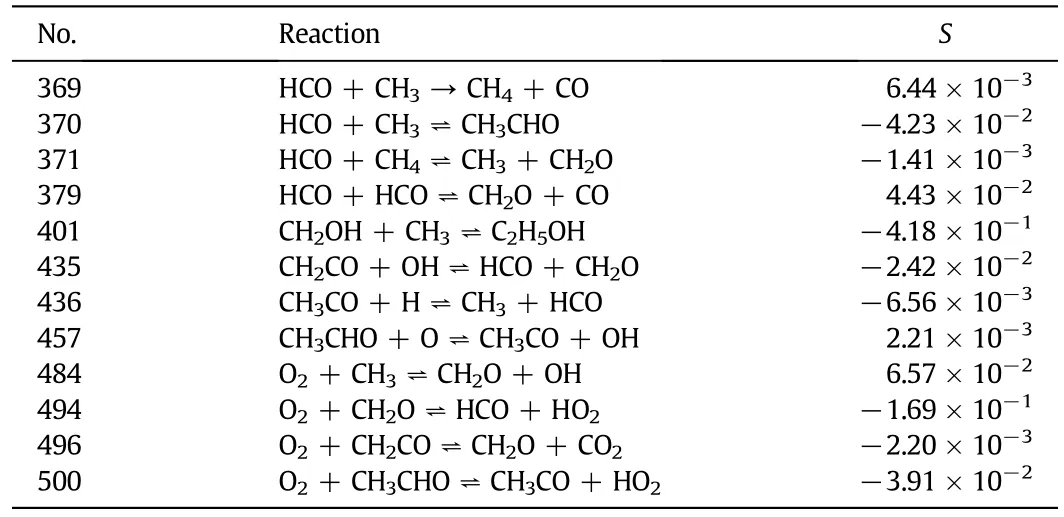
Table 4 The main reactions of C2H5OH responsible for NO reduction at 850°C

Table 5 The main reactions of Fe(CO)5responsible for NO reduction at 850°C
Nomenclature
k rate constant
R[NH3]/[NO]molar ratio in feed
S sensitivity coefficient
t time,s
αNH3consumption efficiency,%
ηNO reduction efficiency,%
φconcentration,μl·L-1
[1]C.M.Nam,B.M.Gibbs,Application of the thermal deNOxprocess to diesel engine deNOx:an experimental and kinetic modeling study,Fuel 81(2002) 1359-1367.
[2]K.H.Han,C.M.Lu,Y.Z.Wang,N.S.Niu,Z.C.Liu,W.D.Hao,Experimental study on de-NOxcharacteristics of selective non-catalytic reduction,Proc.Chin.Soc.Electron.Eng. 28(14)(2008)80-85(in Chinese).
[3]R.Rota,D.Antos,E.F.Zanoelo,M.Morbidelli,Experimental and modeling analysis of the NOxOUT process,Chem.Eng.Sci.57(2002)27-38.
[4]M.T.Javed,N.Irfan,B.M.Gibbs,Control of combustion-generated nitrogen oxides by selective non-catalytic reduction,J.Environ.Manag.83(2007)251-289.
[5]D.L.Siebers,J.A.Caton,Removal of nitric oxide from exhaust gas with cyanuric acid, Combust.Flame 79(1990)31-46.
[6]P.Glarborg,P.G.Kristensen,S.H.Jensen,K.D.Johansen,A flow reactor study of HNCO oxidation chemistry,Combust.Flame 98(1994)241-258.
[7]G.W.Lee,B.H.Shon,J.G.Yoo,J.H.Jung,K.J.Oh,The influence of mixing between NH3and NO for a de-NOxreaction in the SNCR process,J.Ind.Eng.Chem.14(2008) 457-467.
[8]W.H.J.Graus,E.Worrel,Effect of SO2and NOxcontrol on energy-efficiency power generation,Energ Policy 35(7)(2007)3898-3908.
[9]W.J.Yang,Z.J.Zhou,J.H.Zhou,H.K.Lv,J.Z.Liu,K.F.Cen,Application of hybrid coal reburning/SNCR processes for NOxreduction in a coal-fi red boiler,Environ.Eng. Sci.26(2)(2009)311-317.
[10]H.Y.Kim,S.W.Baek,C.Y.Lee,Effects of hybrid reburning system with SNCR and air staging on NOxreduction and thermal characteristics in oxygen enhanced combustion,Combust.Sci.Technol.181(10)(2009)1289-1309.
[11]W.J.Yang,Z.C.Chen,Z.J.Zhou,Z.H.Wang,J.H.Zhou,Z.Y.Huang,K.F.Cen,Costefficient nitrogen oxides controlby a hybrid selective noncatalytic reduction and selective catalytic reduction system on a utility boiler,Environ.Eng.Sci.28(5)(2011) 341-348.
[12]K.Myöhänen,T.Hyppänen,T.Pikkarainen,T.Eriksson,A.Hotta,Near zero CO2emissions in coal fi ring with oxy-fuelcirculating fluidized bed boiler,Chem.Eng.Technol. 32(3)(2009)355-363.
[13]Q.Z.Li,C.S.Zhao,W.F.Wu,X.P.Chen,W.Dong,Experimental investigation on NO emission characteristic during pulverized coalcombustion in O2/CO2environment, Proc.Chin.Soc.Electron.Eng.29(23)(2009)33-39(in Chinese).
[14]L.B.Duan,C.S.Zhao,J.Y.Lu,W.Zhou,Y.J.Li,X.P.Chen,SO2/NO emission characteristics during O2/CO2coal combustion process,J.Chem.Ind.Eng.Soc.China 60(5) (2009)1270-1274(in Chinese).
[15]H.Liu,R.Zailani,B.Gibbs,Comparisons of pulverized coalcombustion in air and in mixtures of O2/CO2,Fuel84(2005)833-840.
[16]R.Zhao,H.Liu,H.Hu,X.J.Zhong,Z.J.Wang,Z.Y.Xu,J.R.Qiu,Experimental and modeling study of NO emission under high CO2concentration,Sci.Chin.Technol. Sci.53(12)(2010)3275-3283.
[17]Z.M.Lu,J.D.Lu,Influence of O2concentration on NO reduction and N2O formation in thermal deNOxprocess,Combust.Flame 156(2009)1303-1315.
[18]B.C.Moo,F.C.Chin,Low temperature SNCR process for NOxcontrol,Sci.Total Environ. 198(1997)73-78.
[19]J.A.Miller,P.Glarborg,Modeling the formation of N2O and NO2in the thermal de-NOxprocess,in:M.Bodenstein,J.Wolfrum,H.R.Volppp,R.Rannacher,J.Warnatz (Eds.),Gas Phase Chemical Reaction Systems:Experiments and Models 100 Years After,Springer Series in Chemical Physics,1996,pp.318-333.
[20]M.J.Pill,T.Turanyi,K.J.Hughes,“Irreversible mechanism mole Kelvin units”,The University of Leeds,http://gar field.chen.elte.hu/Combustion/mechanisms 2004.
[21]R.Rota,E.F.Zanoelo,Influence of oxygenated additives on the NOxOUT process ef ficiency,Fuel82(2003)765-770.
[22]V.M.Zamansky,V.V.Lissianski,P.M.Maly,L.Ho,D.Rusli,W.C.Gardiner,Reaction of sodium species in the promoted SNCR process,Combust.Flame 117(1999)821-831.
[23]M.D.Rumminger,D.Reinelt,V.Babushok,G.T.Linteris,Numerical study of the inhibition of premixed and diffusion fl ames by iron pentacarbonyl,Combust.Flame 116 (1999)207-219.
[24]A.E.Lutz,R.J.Kee,J.A.Miller,SENKIN:a FORTRAN program for predicting homogeneous gas phase chemicalkinetics with sensitivity analysis,Sandia National Laboratories Report,SAND87-8248,1988.
[25]D.M.Hamby,A review of techniques for parameter sensitivity analysis of environmental models,Environ.Monit.Assess.32(2)(1994)135-154.
[26]T.Turányi,Sensitivity analysis of complex kinetic systems.Tools and applications,J. Math.Chem.5(3)(1990)203-248.
[27]S.H.Wu,Q.X.Cao,H.Liu,Q.An,X.Huang,Experimental and modeling study of the effects of gas additives on the thermal deNOxprocess,Chin.J.Chem.Eng.18(1) (2010)143-148.
[28]L.Gasnot,D.Q.Dao,J.F.Pauwels,Experimental and kinetic study of the effect of additives on the ammonia based SNCR process in low temperature conditions,Energy Fuel26(5)(2012)2837-2849.
[29]S.L.Niu,K.H.Han,C.M.Lu,Experimental study on the effect of urea and additive injection for controlling nitrogen oxides emissions,Environ.Eng.Sci.27(1)(2010) 47-53.
[30]J.A.Silver,The effect of sulfur on the thermal deNOxprocess,Combust.Flame 53 (1-3)(1983)17-21.
[31]W.J.Yang,J.H.Zhou,J.J.Zhou,Z.C.Chen,J.Z.Liu,K.F.Cen,Action of oxygen and sodium carbonate in the urea-SNCR process,Combust.Flame 156(2009)1785-1790.
[32]K.H.Han,C.M.Lu,Kinetic modeland simulation of promoted selective non-catalytic reduction by sodium carbonate,Chin.J.Chem.Eng.15(4)(2009)512-519.
[33]S.L.Niu,K.H.Han,C.M.Lu,An experimental study on the effect of operating parameters and sodium additive on the NOxOUT process,Process Saf.Environ.89(2011)121-126.
[34]M.T.Javed,W.Nimmo,A.Mahmood,N.Irfan,Effect of oxygenated liquid additives on the urea based SNCR process,J.Environ.Manag.90(2009)3429-3435.
☆Supported by the National Natural Science Foundation of China(51206096).
*Corresponding author.
E-mailaddress:hankh@163.com(K.Han).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Promoting Xylene Production in Benzene Methylation using Hierarchically Porous ZSM-5 Derived from a Modified Dry-gel Route☆
- Preparation,Structure Characterization and Hydrodesulfurization Performance of B-Ni2P/SBA-15/Cordierite Monolithic Catalysts☆
- Enhanced Photocatalytic Properties of Silver Oxide Loaded Bismuth Vanadate☆
- Recovery and Recycling of Ti Supported Bimodal Mesoporous Catalysts Prepared via Ship-in-a-bottle Method in the Epoxidation of Cyclohexene☆
- Biotechnology and Bioengineering The Effect of pH Controlon Acetone-Butanol-Ethanol Fermentation by Clostridium acetobutylicum ATCC 824 with Xylose and D-Glucose and D-Xylose Mixture☆
- Energy,Resources and Environmental Technology CO2Removal from Biogas by Water Washing System☆