Preparation,Structure Characterization and Hydrodesulfurization Performance of B-Ni2P/SBA-15/Cordierite Monolithic Catalysts☆
2014-07-12PingyiWuQingyuanLiLingLanHongfeiLiuYanaJuJiaruiPiaoShengfuJiPetrochemicalResearchInstitutePetroChinaBeijing0095China
PingyiWu,Qingyuan Li,Ling Lan,HongfeiLiu,Yana Ju,Jiarui Piao,Shengfu Ji,*Petrochemical Research Institute,PetroChina,Beijing 0095,China
2State Key Laboratory of Chemical Resource Engineering,Beijing University of Chemical Technology,Beijing 100029,China
Preparation,Structure Characterization and Hydrodesulfurization Performance of B-Ni2P/SBA-15/Cordierite Monolithic Catalysts☆
PingyiWu1,2,Qingyuan Li2,Ling Lan1,HongfeiLiu2,Yana Ju1,Jiarui Piao1,Shengfu Ji2,*1Petrochemical Research Institute,PetroChina,Beijing 100195,China
2State Key Laboratory of Chemical Resource Engineering,Beijing University of Chemical Technology,Beijing 100029,China
A R T I C L E I N F o
Article history:
Received 2 January 2014
Received in revised form 17 February 2014
Accepted 24 February 2014
Available online 18 June 2014
Boron
Ni2P
SBA-15
Cordierite
Monolithic catalyst
Hydrodesulfurization
A series of B-Ni2P/SBA-15/cord monolithic catalysts were prepared by coating the slurry of the B-Ni2P/SBA-15 precursors on a pretreated cordierite support,and followed by temperature-programmed reduction in a H2fl ow.The samples were characterized by X-ray diffraction(XRD)and N2adsorption-desorption technique.The catalytic activities for the hydrodesulfurization(HDS)of dibenzothiophene(DBT)were evaluated.The results showed that Ni2P phase was present in allB-Ni2P/SBA-15/cord monolithic catalysts.The speci fi c surface areas (SBET)of the B-Ni2P/SBA-15/cord monolithic catalysts was fi rst increased to 167 m2·g-1,and then decreased to 155 m2·g-1with the increase of boron contents.The catalytic activity also showed the similar trend with the increase of boron contents.The 1.75%(by mass)B-Ni2P/SBA-15/cord monolithic catalysts exhibited the highest DBT conversion of 98.4%at 380°C.The cordierite-based monolithic catalysts showed better low temperature sensitivity for HDS of DBT in comparison with the particle catalysts.Moreover,two HDS routes, direct desulfurization(DDS)and hydrogenation(HYD),proceeded independently over B-Ni2P/SBA-15/cord monolithic catalysts and the main pathway was DDS.
©2014 The ChemicalIndustry and Engineering Society ofChina,and Chemical Industry Press.Allrights reserved.
1.Introduction
In recent years,the deep hydrodesulfurization(HDS)of oils has attracted signi fi cant attention because of the increasingly strict environmental regulations and growing environmental awareness [1,2].The research about HDS catalysts is focused on the modification of transition bimetal(CoMo and NiMo)sulfides,which are hard to meet the requirements on removalof dibenzothiophene(DBT)[3,4]. Therefore,it is urgent to develop novel HDS catalysts.Transition metal phosphide catalysts,especially Ni2P,have been reported as members of a class of desirable HDS catalysts because of their high activity and good stability[5-8].Thereinto,Lee and Oyama[9]found that the activity of Ni2P/SiO2was higher than NiMoS/Al2O3for HDS of DBT. Sun etal.[10]reported thatthe catalytic activity over Ni2P/SiO2catalyst was much better than MoP/SiO2catalysts for HDS ofDBT.In our previous study[11],we prepared a series of Ni2P/SBA-15 particle catalysts with different Nicontents,which exhibit favorable HDS activity.Besides the above,it has been reported[12]that the addition of boron(B)to bulk catalysts can improve their HDS activity and selectivity for HDS of DBT. Furthermore,Parks et al.[13]found that the sulfided Ni-B/SiO2and Ni-Mo-O-B/SiO2catalysts show good sulfur tolerance.Therefore,we prepared a series of B-Ni2P/SBA-15 particle catalysts with different B contents[14].The results showed that the addition of B can in fl uence the acidity and catalytic activity for HDS of DBT.However,the conventional particle catalysts have high pressure drops and gradient of temperature.Moreover,during the process of industrial applications, the particle catalysts take disadvantages of low mechanical strength and serious scale effect.
Monolithic structure catalyst,a kind of new catalyst composed by uniform structure support and active component coating layer,can provide proper channelaccording to the different reaction characteristics [15,16].Recently,the monolithic catalysts are applied to many catalysis fields,such asselective catalytic reduction(SCR)ofNOx[17,18],catalytic combustion of VOCs[19,20],and catalytic reforming[21,22],due to their advantages of low pressure drop,high heat transfer efficiency, favorable mechanical strength,and negligible serious scale effect. Thereby,the use of monolithic catalysts can efficiently extend the application range of catalytic technology industrial processes.
In this work,a series of B-Ni2P/SBA-15/cord monolithic catalysts with different B contents were prepared using cordierite as support and B-Ni2P/SBA-15 as coating.The structure of samples and the catalytic activity for HDS of DBT were investigated.The differences between the cordierite-based monolithic HDS catalysts and the particle catalysts were discussed.The aim of this study was to provide reference for the development of new type of HDS catalysts.
2.Experimental
2.1.Catalyst preparation
SBA-15 was synthesized by using the literature method[4]. B-Ni2P/SBA-15 precursors were prepared by impregnating appropriate amounts of aqueous solution of Ni(NO3)2·6H2O,(NH4)2HPO4(P/Ni molar ratio of 0.8),and H3BO3into SBA-15.In detail,9 ml of Ni(NO3)2·6H2O(0.58 g·ml-1),3 mlof(NH4)2HPO4(0.40 g·ml-1), and 2 ml of H3BO3(0.04 g/ml)were added to 2.00 g of SBA-15. Then drying at100°C for24 h and calcining at550°C for 4 h.The content of Ni2P was about 40%(by mass)and the contents of B were 0-2.10% (by mass).The B-Ni2P/SBA-15 slurry was prepared from B-Ni2P/SBA-15 precursor powder with deionized water.The concentration of the slurry was about 0.06 g·ml-1.
Commercial cordierite with a cell density of 62 channels·cm-2(300 cells per square inch)was cut to obtain samples with diameter of9 mm and length of50 mm,and each sample has 50 square channels and length of50 mm.The wall thickness of the channels was 0.3 mm. The obtained cordierite samples were pretreated in 10%(by mass) oxalic acid solution boiling for 3 h,washed with deionized water,and finally calcined at 300°C.The pretreated cordierite was dipped into the B-Ni2P/SBA-15 slurry(the grain size of the B-Ni2P/SBA-15 precursor powder was about0.15 mm),dried at room temperature in air,and then calcined at550°C for 4 h.This procedure was repeated several times to achieve the desired loading.The precursors of monolithic catalysts were thus obtained.Finally,the B-Ni2P/SBA-15/cord monolithic catalysts were prepared by temperature-programmed reduction(TPR)method in a H2flow rate of50 ml·min-1at atmospheric pressure.The temperature was increased from room temperature(RT)to 300°C at a rate of 10°C·min-1,and then from 300°C to 650°C at a rate of 1°C·min-1, and finally maintained at650°C for2 h.After TPR,the monolithic catalysts were cooled to the reaction temperature and the feed was introduced for HDS of DBT,or passivated by 1%O2/Ar(by volume)at RT for structure characterization.
2.2.Catalyst characterization
XRD patterns of passivated B-Ni2P/SBA-15/cord monolithic catalysts were obtained on a Rigaku D/Max 2500 VB2+/PC diffractometer with graphite monochromator,using CuKαradiation(after Kαstripping, λ=0.15406 nm)operating at 200 mA and 40 kV.N2adsorption desorption measurements for samples were performed on a Quadrasorb SI instrument.Before each measurement,the samples of30-40 mg were degassed for at least 5 h at 250°C under vacuum.BET and BJH methods were used to determine the speci fi c surface area and pore size distribution,respectively.
2.3.Catalytic activity
HDS of DBT was performed in a high-pressure fixed-bed continuous flow stainless reactor(9 mm in diameter and 50 mm length packed, volume=3.18 cm3)with a central thermocouple to measure the temperature of the catalyst bed.The monolithic catalyst was loaded in the fixed bed reactor made of quartz tube.The two ends of the catalyst packings were filled first with the quartz fiber and then with the quartz sand.The reaction feed consisting of 1%(by mass)DBT in decal in was introduced into the reactor by a piston pump.Decalin was purchased from Sinopharm Chemical Reagent Co.,Ltd.with 99.5%purity.The reaction system was a trickle bed with the gas and liquid phases fl owing concurrently downwards.The testing for the HDS reaction was operated at 3.0 MPa,300-380°C,and with a liquid hourly space velocity (LHSV,the ratio of the volume flow rate of DBT solution over that of the monolithic catalyst reactor)of1.9 h-1and a ratio of hydrogen to liquid feed of400(by volume).The liquid products were collected at 1 h intervals and analyzed off-line by a gas chromatography (SP2100,BeifenRuili Analytic Instrument(Group)Co.,Beijing) equipped with a fl ame ionization detector(FID)and a capillary column (HJ.PONA,50 m×0.20 mm×0.50μm).The main products of the reaction were biphenyl(BP)and cyclohexylbenzene(CHB).Therefore,the conversion of DBT can be used to measure the HDS performance of the monolithic catalysts.
3.Results
3.1.XRD
XRD patterns of B-Ni2P/SBA-15/cord catalyst samples with different B contents are shown in Fig.1.As observed in the fi gure,all samples have characteristic diffraction peaks at 18.0°,19.0°,21.6°,26.4°,28.4°, 29.4°,33.8°,36.8°,38.4°,and 42.9°,corresponding to the structure of ceramic.Besides,all samples have characteristic diffraction peaks at40.6°, 44.5°,47.4°,and 54.1°agreed well with a PDF reference pattern for Ni2P (no.03-0953),demonstrating the appearance of Ni2P phase.The intensity of these diffraction peaks of Ni2P phase is nearly unchanged with the increase of B contents.In our previous research,we have found that the introduction of appropriate amount of B in B-Ni2P/SBA-15 particle catalysts could effectively decrease the size of Ni2P crystals[14], thus the Ni2P phases were well dispersed on the surface of the catalyst. When the B-Ni2P/SBA-15 particle catalysts were coated on the cordierite to prepare monolithic catalysts,the Ni2P phases still maintained well dispersed.In addition,no characteristic diffraction peak of B is observed in all patterns,maybe because B is highly-dispersed state.
3.2.N2adsorption-desorption
Fig.2 shows the N2adsorption-desorption isotherms ofB-Ni2P/SBA-15/cord catalyst samples with different B contents.All samples exhibit distinct IV-type isotherm with H1 hysteretic loop,which is the typical characteristic of mesoporous structure,conforming the reservation of SBA-15 mesoporous channel.Compared with the B-Ni2P/SBA-15 particle catalysts[14],the hysteretic loops of B-Ni2P/SBA-15/cord monolithic catalysts show obvious deformation,which indicate the appearance of new pores besides the hexagonally ordered mesoporous structure of SBA-15.In our previous report[23],the N2adsorption-desorption isotherms of Mo-Ni2P/SBA-15/cord monolithic catalysts present the similar phenomenon.
Table 1 lists the pore structure parameters of B-Ni2P/SBA-15/cord catalyst samples.With the increase of B contents,the SBETof samples was first increased to 167 m2·g-1,and then decreased to 155 m2·g-1. But during this trend,the VBJHof all samples nearly kept unchanged. The SBETof 1.75%(by mass)B-Ni2P/SBA-15/cord monolithic catalyst was 167 m2·g-1,which was higher than the other modified Ni2P/SBA-15/cord monolithic catalysts[23,24].Usman et al.[12] have reported that the specific surface area of B modified MoO3/Al2O3catalysts showed the parabolic trend,and the sample with 1.2%(by mass)B contents presents the biggest specific surface area.

Table 1 Structure parameters of B-Ni2P/SBA-15/cord catalysts with different B contents

Fig.1.XRD patterns of B-Ni2P/SBA-15/cord samples with different B contents.Mass fraction of B/%:(a)0;(b)0.35;(c)0.7;(d)1.40;(e)1.75;(f)2.10.
The pore size distributions for B-Ni2P/SBA-15/cord catalyst samples are shown in Fig.3.All samples present two distinct pore size peaks. The first one is located in range of 3.5-6.0 nm,and this pore size distribution is preserved with the increase of B contents.
This is in accordance with the pore size distribution in B-Ni2P/SBA-15 particle catalysts[14],which is caused by the uniform assembling of Ni2P in the channel of SBA-15.The second one is located in the range of6.0-10.0 nm,and this pore size peak grows wider and weaker with the increase of B contents.According to our previous research about Co-Ni2P/SBA-15/cord monolithic catalysts[23],we infer that it may be formed by particle stacking during the coating process of particle catalysts on cordierite supports.

Fig.2.N2adsorption-desorption isotherms of catalysts with different B contents.Mass fraction of B/%:(a)0;(b)0.35;(c)0.7;(d)1.40;(e)1.75;(f)2.10.
3.3.Catalytic activity evaluation
Fig.4 presents the activity of B-Ni2P/SBA-15/cord monolithic catalysts for HDS of DBT.For the Ni2P/SBA-15/cord monolithic catalyst,the DBT conversion can reach 23.2%at 300°C and 97.7%at 380°C.The HDS activity of 0.35%(by mass)B-Ni2P/SBA-15/cord and 0.70%(by mass)B-Ni2P/SBA-15/cord monolithic catalysts are lower than that of Ni2P/SBA-15/cord monolithic catalyst.This may be due to the contents of B decreased the surface area ofNi2P/SBA-15/cord monolithic catalyst, and lead to the reduced DBT conversion.
For B-Ni2P/SBA-15/cord monolithic catalysts,with the increase of B contents to 1.75%(by mass),the catalytic activity of B-Ni2P/SBA-15/cord monolithic catalysts was increased.Further increase of B content did not increase the catalytic activity of B-Ni2P/SBA-15/cord monolithic catalysts. This meant that 1.75%(by mass)B-Ni2P/SBA-15/cord catalyst has the highest conversion for DBT.Especially,the DBT conversion of 1.7% (by mass)B-Ni2P/SBA-15/cord can reach 32.7%at 300°C and 98.4% at 380°C,which is higher than that of Mo and Co modified Ni2P/SBA-15/cord monolithic catalysts[23,24].
According to our previous research[25],we conclude on the relationship between HDS activity and specific surface area of catalyst:large specific surface area promotes effectively the adsorption of DBT molecules on the catalyst surface,thus improve effectively its HDS activity.Therefore,combined with the N2adsorption desorption characterization,we can conclude that the HDS activity of B-Ni2P/SBA-15/cord monolithic catalysts is attributed to the change in SBET.
For all catalysts,the DBT conversion increases with the rise of temperature,and this trend is significant at low temperature(300-340°C)but slows down at high temperature(340-380°C).The DBT conversion of 1.75%(by mass)B-Ni2P/SBA-15/cord rapidly increases to more than 90% at 340°C.For our reported B-Ni2P/SBA-15 particle catalysts[14],the DBT conversion remains unchanged below 320°C and increases significantly above 320°C.Therefore,the B-Ni2P/SBA-15/cord monolithic catalyst exhibits better low temperature susceptibility than B-Ni2P/ SBA-15 particle catalyst at the roughly equivalent LHSV.
Fig.5 shows the BP selectivity and CHB selectivity over B-Ni2P/SBA-15/cord monolithic catalysts for HDS of DBT.For Ni2P/SBA-15/cordcatalyst,the BP selectivity and CHB selectivity are almost unchanged with the increase of temperature,which is similar with the result reported in[23,25].For B-Ni2P/SBA-15/cord catalysts,at low temperature the main product of HDS of DBT is BP.With the increase oftemperature, the CHB selectivity increases gradually and the BP selectivity decreases accordingly.Compared with the B-Ni2P/SBA-15 particle catalysts, the variation trends of the BP selectivity and CHB selectivity over B-Ni2P/SBA-15/cord monolithic catalysts are similar.The only difference is that,with the increase of temperature,the BP selectivity and CHB selectivity over B-Ni2P/SBA-15 particle catalysts maintain closer to 50%.However,the selectivity of BP over B-Ni2P/SBA-15/cord monolithic catalysts shows the increasing trend when the temperature exceed 360°C.Atthe same time,the selectivity ofBP is 10%higherthan that of CHB.
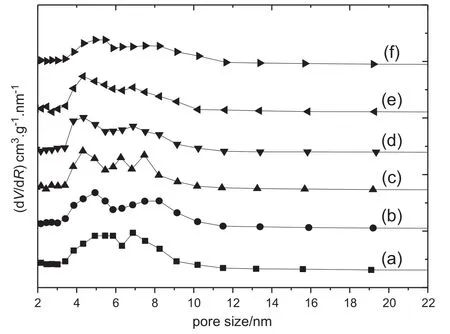
Fig.3.Pore size distributions of B-Ni2P/SBA-15/cord samples with different B contents. Mass fraction of B/%:(a)0;(b)0.35;(c)0.7;(d)1.40;(e)1.75;(f)2.10.
4.Discussions
The HDS reaction of DBT follows the well-known two parallel routes [25]schematized in Fig.6.The first pathway is called direct desulfurization pathway(DDS)and produces BP while the parallel pathway is the hydrogenation route(HYD),which proceeds through aromatic ring hydrogenation,forming various hydrogenated compounds.These hydrogenated products can be subsequently desulfurized forming CHB. Therefore,DBT conversion is usually served as the evaluation indicator of HDS.

Fig.4.Catalytic activity of catalysts with different B contents for HDS ofDBT.Mass fraction of B/%:(a)0;(b)0.35;(c)0.7;(d)1.40;(e)1.75;(f)2.10.
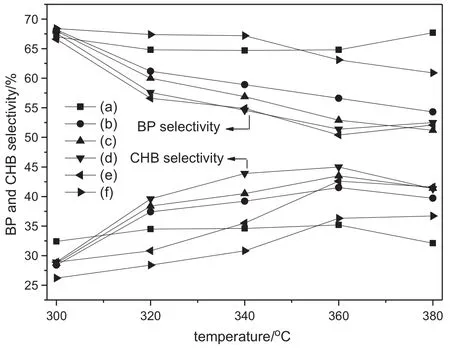
Fig.5.BP and CHB selectivity over catalysts with different B contents for HDS ofDBT.Mass fraction of B/%:(a)0;(b)0.35;(c)0.7;(d)1.40;(e)1.75;(f)2.10.
In general,active components of the catalysts take the decisive role in HDS of DBT[26-28].However,as a typicalgas-liquid-solid heterogeneous catalytic process,catalyst structure can be closely related to HDS activity ofDBT.Fig.7 illustrates the HDS process ofDBT catalyzed by particle catalysts and monolithic catalysts,respectively.It is well-known that the diffusion of gas reactants in solid catalyst has a significant influence to HDS activity.For traditional particle catalysts,especially B-Ni2P/SBA-15 particle catalysts,the diffusion path is relatively long and the diffusion resistance is relatively high due to the large catalyst particle size(1.4 mm in[14]).In contrast,the diffusion ofgas reactants in B-Ni2P/SBA-15/cord monolithic catalysts becomes relatively easy due to their thin coating layer(about0.1 mm)with rich accumulation pores. Furthermore,we know that elevated temperature is conducive to reinforce gas diffusion in solid catalysts[15],thus it can be used to explain the low temperature susceptibility of B-Ni2P/SBA-15/cord monolithic catalysts.
Due to the unordered accumulation of the B-Ni2P/SBA-15 particle catalysts in reactor,particle catalysts bed exhibit high pressure drop, slow mass transfer rate,and low effectiveness factor,which is to meet some the heat exchange issues in the large-scale industrial application. When the gas reactants(DBT and H2)and products(BP and CHB)fl ow through the whole particle catalysts bed,the molecular weight of DBT was bigger and the mass transfer rate was slower.Thus,the DBT had the more chance to contact with the particle catalysts.That meant nearly the same amount of BP could be continuously hydrogenated.It is known that BP will be hydrogenated to CHB in the presence of Ni2P [27]by increasing the H2consumption.

Fig.6.Reaction mechanism of the HDS of DBT.

Fig.7.Reaction scheme for HDS of DBT over B-Ni2P/SBA-15 particle catalyst and B-Ni2P/SBA-15/cord monolithic catalyst.
Based on the above results and discussions,it can be found that there are obvious differences during HDS of DBT catalyzed by particle catalysts and cordierite based monolithic catalysts.First of all,the monolithic catalysts exhibithigh catalytic efficiency at low temperature, but the particle catalysts show the same catalytic activity at relatively high temperature.
5.Conclusions
A series of the B-Ni2P/SBA-15/cord monolithic catalysts with different B contents(0-2.10%(by mass))were prepared by an impregnation method.The influence of B to catalyst structure and the HDS activity of DBT were investigated.AllB-Ni2P/SBA-15/cord monolithic catalysts exhibittwo distinct pore size distributions(3.5-6.0 nm and 6.0-1.0 nm). SBETof the monolithic catalysts showed parabolic trend with the increase of B contents while VBJHremain unchanged.The 1.75%(by mass)B-Ni2P/SBA-15/cord sample has the biggest SBETof167 m2·g-1. The HDS activities over the B-Ni2P/SBA-15/cord monolithic catalysts are closely related to the SBETof samples.The 1.75%(by mass)B-Ni2P/SBA-15/cord catalyst shows the best DBT conversion,which can reach 98.4% at 380°C.The selectivity of BP was increased at high temperature. Both routes(DDS and HYD)proceed in the present reaction of HDS. Comparing with the particle catalysts,the cordierite based monolithic catalysts was easily to apply in industry due to advantages of low pressure drop.Also,it is possible to regulate the selectivity of BP and CHB by varying the operation temperature.
[1]C.S.Song,An overview of new approaches to deep desulfurization for ultra-clean gasoline,dieselfueland jet fuel,Catal.Today 86(2003)211-263.
[2]C.Li,G.Sun,C.Li,Y.Song,Preparation,characterization,hydrodesulfurization and hydride-nitrogenation activities of alumina-supported tungsten phosphide catalysts,Chin.J.Chem.Eng.14(2)(2006)184-193.
[3]A.Duan,C.Xu,S.Lin,Effect of operation variables on hydrodenitrogenation and hydrodesulfurization over NiMo/Al2O3catalysts,Chin.J.Chem.Eng.11(6)(2003) 743-746.
[4]P.Rayo,M.S.Rana,J.Ramírez,J.Ancheyta,A.Aguilar-Elguézabal,Effect of the preparation method on the structural stability and hydrodesulfurization activity of NiMo/SBA-15 catalysts,Catal.Today 130(2008)283-291.
[5]R.Wang,K.J.Smith,Hydrodesulfurization of 4,6-dimethyldibenzothiophene over high surface area metalphosphides,Appl.Catal.A Gen.361(2009)18-25.
[6]A.W.Burns,K.A.Layman,D.H.Bale,M.E.Bussell,Understanding the relationship between composition and hydrodesulfurization properties for cobalt phosphide catalysts,Appl.Catal.A Gen.343(2008)68-76.
[7]L.Liu,G.Li,B.Liu,D.Liu,X.Hu,Y.Liu,C.Liu,Hydrosulfurization performance study of Ni2P-modi fi ed MoS2/γ-Al2O3catalysts,J.Chem.Ind.Eng.(China)62(5)(2011) 1296-1301(in Chinese).
[8]K.S.Cho,H.R.Seo,Y.K.Lee,A new synthesis of highly active Ni2P/Al2O3catalyst by liquid phase phosphidation for deep hydrodesulfurization,Catal.Commun.12 (2011)470-474.
[9]Y.K.Le,S.T.Oyama,Bifunctional nature of a SiO2-supported Ni2P catalyst for hydrotreating:EXAFS and FTIR studies,J.Catal.239(2006)376-389.
[10]F.X.Sun,W.C.Wu,Z.L.Wu,J.Guo,Z.B.Wei,Y.X.Yang,Z.X.Jiang,F.P.Tian,C.Li, Dibenzothiophene hydrodesulfurization activity and surface sites of silicasupported MoP,Ni2P,and Ni-Mo-P catalysts,J.Catal.228(2004)298-310.
[11]X.Huang,S.Ji,P.Wu,Q.Liu,H.Liu,J.Zhu,C.Li,Structure and hydrodesulfurization performances of Ni2P/SBA-15 catalysts,Acta Phys.Chim.Sin.24(10)(2008) 1773-1779.
[12]U.Usman,M.Takaki,T.Kubota,Y.Okamoto,Effect of boron addition on a MoO3/ Al2O3catalyst:physicochemical characterization,Appl.Catal.A Gen.286(2005) 148-154.
[13]G.L.Parks,M.L.Pease,A.W.Burns,K.A.Layman,M.E.Bussell,X.Q.Wang,J. Hanson,J.A.Rodriguez,Characterization and hydrodesulfurization properties of catalysts derived from amorphous metal-boron materials,J.Catal.246 (2007)277-292.
[14]P.Zhao,S.Ji,N.Wei,Q.Ma,H.Liu,C.Li,Effect of boron promoter on the structure and hydrodesulfurization activity of Ni2P/SBA-15 catalysts,Acta Phys.-Chim.Sin. 27(7)(2011)1737-1742.
[15]M.T.Kreutzer,F.Kapteijn,J.A.Moulijn,Shouldn't catalysts shape up structured reactors in general and gas-liquid monolith reactors in particular,Catal.Today 111 (2006)111-118.
[16]J.A.Moulijn,A.Cybulski,Structured Catalysts and Reactors,Marcel Dekker, New York,1998.10-25.
[17]A.V.Boix,S.G.Aspromonte,E.E.Miró,Deactivation studies of the SCR ofNOxwith hydrocarbons on Co-mordenite monolithic catalysts,Appl.Catal.A Gen.341(2008) 26-34.
[18]Y.S.Shen,Y.Yu,T.Qiu,S.M.Zhu,Preparation and performance of ceria doped two component deNOxmonolithic catalysts at low temperature,Rare Metal Mater.Eng. 40(6)(2011)967-972.
[19]H.F.Lu,Y.Zhou,H.F.Huang,B.Zhang,Y.F.Chen,In-situ synthesis of monolithic Cu-Mn-Ce/cordierite catalysts towards VOCs combustion,J.Rare Earths 29(9) (2011)855-860.
[20]B.Kucharczyk,W.Tylus,Effect of washcoat modi fi cation with metaloxides on the activity of a monolithic Pd-based catalyst for methane combustion,Catal.Today 137(2008)324-328.
[21]X.Karatzas,J.Dawody,A.Grant,E.E.Svensson,L.J.Pettersson,Zone-coated Rh-based monolithic catalyst for autothermal reforming of diesel,Appl.Catal.B Environ.101 (2011)226-238.
[22]L.X.Sang,B.Sun,H.Y.Tan,C.X.Du,Y.T.Wu,C.F.Ma,Catalytic reforming ofmethane with CO2over metal foam based monolithic catalysts,Int.J.Hydrogen Energy 37 (2012)13037-13043.
[23]Y.Guo,P.Zeng,S.Ji,N.Wei,H.Liu,C.Li,Effect of Mo promoter content on performance of Mo-Ni2P/SBA-15/cordierite monolithic catalyst for hydrodesulfurization, Chin.J.Catal.31(3)(2010)329-334(in Chinese).
[24]P.Wu,L.Lan,S.Ji,N.Wei,Z.Lv,H.Liu,Co-Ni2P/SBA-15/cordierite monolithic catalysts:preparation and performance for hydrodesulfurization of dibenzothiophene, Chin.J.Inorg.Chem.28(3)(2012)565-571(in Chinese).
[25]N.Wei,S.Ji,P.Wu,Y.Guo,H.Liu,J.Zhu,C.Li,Preparation of nickel phosphide/SBA-15/cordierite monolithic catalysts and catalytic activity for hydrodesulfurization of dibenzothiophene,Catal.Today 147(2009)S66-S70.
[26]M.E.Cervantes-Gaxiola,M.Arroyl-Albiter,R.Maya-Yescas,Synthesis,characterization and catalytic activity during hydrodesulphurization of dibenzothiophene of NiMoWcatalysts supported on Al-Ti mixed oxides modi fi ed with MgO,Fuel100 (2012)57-65.
[27]C.Sepúlveda,N.Escalona,R.García,D.Laurenti,M.Vrinat,Hydrodeoxygenation and hydrodesulfurization co-processing over ReS2supported catalysts,Catal.Today 195 (2012)101-105.
[28]R.Huirache-Acuña,B.Pawelec,C.V.Loricera,E.M.Rivera-Muñoz,R.Nava,B.Torres,J. L.Fierro,Comparison of the morphology and HDS activity ofternary Ni(Co)-Mo-W catalysts supported on Al-HMS and Al-SBA-16 substrates,Appl.Catal.B Environ.125 (2012)473-485.
☆Supported by the National Basic Research Program of China(2006CB202503)and the Science Foundation of PetroChina(2010D-5006-0401).
*Corresponding author.
E-mailaddress:jisf@mail.buct.edu.cn(S.Ji).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Separation Science and Engineering Performance Prediction of Structured Packing Column for Cryogenic Air Separation with Hybrid Model☆
- Enhanced Photocatalytic Properties of Silver Oxide Loaded Bismuth Vanadate☆
- Recovery and Recycling of Ti Supported Bimodal Mesoporous Catalysts Prepared via Ship-in-a-bottle Method in the Epoxidation of Cyclohexene☆
- Promoting Xylene Production in Benzene Methylation using Hierarchically Porous ZSM-5 Derived from a Modified Dry-gel Route☆
- Energy,Resources and Environmental Technology Experimental and Modeling Study on de-NOxCharacteristics of Selective Non-catalytic Reduction in O2/CO2Atmosphere☆
- Energy,Resources and Environmental Technology CO2Removal from Biogas by Water Washing System☆