Enhanced Photocatalytic Properties of Silver Oxide Loaded Bismuth Vanadate☆
2014-07-12LianweiShanJinboMiLiminDongZhidongHanBoLiuKeyLaboratoryofGreenChemicalEngineeringandTechnologyofCollegeofHeilongjiangProvinceCollegeofChemicalandEnvironmentalEngineeringHarbinUniversityofScienceandTechnologyHarbin150040C
LianweiShan*,Jinbo Mi,Limin Dong,Zhidong Han,Bo LiuKey Laboratory of Green Chemical Engineering and Technology of College of Heilongjiang Province,College of Chemical and Environmental Engineering,Harbin University of Science and Technology,Harbin 150040,China
2College of Material Science and Engineering,Harbin University of Science and Technology,Key Laboratory of Materials Research and Application of Heilongjiang Province,Harbin 150040,China
Enhanced Photocatalytic Properties of Silver Oxide Loaded Bismuth Vanadate☆
LianweiShan1,2,*,Jinbo Mi2,Limin Dong2,Zhidong Han2,Bo Liu11Key Laboratory of Green Chemical Engineering and Technology of College of Heilongjiang Province,College of Chemical and Environmental Engineering,Harbin University of Science and Technology,Harbin 150040,China
2College of Material Science and Engineering,Harbin University of Science and Technology,Key Laboratory of Materials Research and Application of Heilongjiang Province,Harbin 150040,China
A R T I C L E I N F o
Article history:
Received 2 January 2014
Received in revised form 17 April2014
Accepted 22 April2014
Available online 17 June 2014
In this work,BiVO4powders were synthesized by a sol-gelmethod,and the BiVO4gels with different calcination temperature were investigated by X-ray diffraction(XRD).Absorption range and band gap energy,which are responsible forthe observed photocatalystbehavior,were investigated by UV/vis diffuse reflectance spectroscopy(DRS)for pure and silver oxide loaded BiVO4.Photocatalytic properties of the prepared samples were examined by studying the degradation of the methylorange.When using NaClO2as an electron acceptor,the possible photocatalytic mechanism has been discussed by photocatalytic reactions.With the help of electron acceptor,the results show clearly that the BiVO4loaded silver oxide exhibited superior photocatalytic activity in simulated dye wastewater treatment.
©2014 The Chemical Industry and Engineering Society of China,and Chemical Industry Press.Allrights reserved.
1.Introduction
For solving urgentenergy and environmentalissues that confront mankind today,the photocatalytic splitting of water into hydrogen and oxygen and the photocatalytic degradation of organic pollutants by using visible light are promising techniques[1].The conversion of solar energy into chemical energy requires efficient photocatalysts, typically semiconductors,that facilitate the important steps of light absorption,charge separation etc.[2].Many semiconductor materials have shown photoelectrochemical activity,vast majority of them have limited utility due to being prone to photocorrosion, high charge carrier recombination,and poor band gap energies unsuitable to capturing visible light photons[3].Well-known TiO2has been widely used and investigated for the photodegradation of organic pollutants in the water owing to its cheapness,strong oxidizing power and nontoxicity[4].However,the band gaps of TiO2are too large(>3.2 eV)to absorb a significant fraction of visible light[5].
As compared to the wide band gap semiconductor materials, bismuth vanadate(BiVO4)is an ideal visible light responsing photocatalysts for recycling polluted water under visible-light irradiation[6].BiVO4has three main crystal forms:monoclinic scheelite, tetragonalzircon,and tetragonalscheelite structure.The monoclinic scheelite and tetragonal scheelite possess similar scheelite crystalline structure.However,the Bi-O polyhedron in the monoclinic scheelite is more distorted by a 6s2lone pairofBi3+than that of tetragonal scheelite phase[7].The band gaps of monoclinic scheelite,tetragonalscheelite and tetragonal zircon were 2.4,2.6 and 2.9 eV,respectively[8]. Thus monoclinic scheelite may be a promising photocatalyst for the photodegradation of organic pollutants.
Many researchers have studied the preparation and photocatalytic properties ofBiVO4by different methods,for example,fl ame spray pyrolysis[9],precipitation[10],metallo-organic decomposition[11],hydrothermalmethod[12],and sol-gelmethod[13].Tokunaga et al.studied selective preparation of monoclinic and tetragonal BiVO4by hydrolyzing a nitric acid solution of Bi(NO3)3and Na3VO4with bases(Na2CO3and NaHCO3)atroomtemperature[7].BiVO4exhibits different photocatalytic properties owing to difference of synthesis factors.The larger sizes of BiVO4show good precipitation performance and can easily be recovered in water purification.Its disadvantages are small specific surface area, long migration distance for excited electron-hole pairs,and increasing energy-wastefulrecombinations[14,15].Nonmetalatom(F,N etc.)into the lattice of a parent metaloxide can result in the narrowing of band gap via the hybridization of X 2p and O 2p orbitals[16,17].In order to improve the charge separation efficiency of the photogenerated electrons and holes,some noble metals including gold,silver and platinum are used to modify photocatalyst because of their high Schottky barrier among the metals facilitating the electron capture[18,19].
In view of the above considerations,a facile method is used to prepare visible-light-driven BiVO4photocatalysts in this work.Furthermore,the influence of loaded silver oxide,electron acceptors on photocatalytic properties of bismuth vanadate,is also investigated.
2.Experimental
2.1.Preparation of BiVO4
Bismuth vanadate was synthesized by sol-gelmethod with bismuth nitrate(Bi(NO3)3·5H2O),ammonium metavanadate(NH4VO3),citric acid(C6H8O7),nitric acid(HNO3),ammonia(NH3·H2O),methylorange (MO,C14H14N3SO3Na),and deionized water/distilled water(H2O).
In accordance with 1:1 molar ratio(Biin excess of5%compensating for loss in the annealing),raw materials(5.145 g Bi(NO3)3·5H2O and 1.182 g NH4VO3at 1:2 molar ratio)were weighed.3.843 g C6H8O7was added to the Bi(NO3)3solution prepared in 20 ml HNO3(1 mol·L-1)in advance,then 80 mldeionized water was added.After that,pH was adjusted with aqua ammonia(25%mass concentration) till it is equal to 7,and this was labeled as solution A.NH4VO3and 3.843 g C6H8O7were dissolved in 100 mldeionized water to get solution B with the concentration of 0.1 mol·L-1.Solution A of 100 ml was added slowly into solution B of equalvolume,and then pHwas adjusted to 7.After in water bath for 6 h,drying,ashing and grinding the precursor powders were obtained.Then,they were calcined in different temperatures(400°C,450°C,500°C,550°C)at the ambient atmosphere.They were labeled as T40,T45,T50,and T55,respectively.
The impregnation Ag loading was carried out by the following procedures:the BiVO4precursor powder samples were impregnated in silver nitrate solution(11.5 mmol·L-1)which Ag loading(the mass fraction based on bismuth vanadate)was 0.77%,1.15%,1.91%, 3.06%,and 4.60%,respectively.The dried BiVO4precursor powder adhering AgNO3on the surface was calcined at 500°C for 4 h.
2.2.Characterization
The crystallization behavior of the powders was analyzed by X-ray diffraction(XRD;ModelD/MAX-3B,Tokyo,Japan)excited by Cu Ka radiation,a sampling intervalof 0.02°,and a scan speed of 4(°)·min-1. Diffuse reflectance spectra were obtained on a Shimadzu UV-2401PC UV/vis scanning spectrophotometer equipped with a diffuse reflectance accessory.Fourier transform infrared spectra(FTIR)were acquired by FTS165 over the region 400-4000 cm-1.
2.3.Photocatalytic test
The methylorange solution 100 ml(5 mg·L-1)was mixed with 0.1 g ofeach sample respectively and then placed in a 200 mlglass beaker, dark stirring 30 min magnetically,to make the powders of bismuth vanadate fully mixed and suspended.The photocatalytic activity of each sample was tested under the simulating sunlight(500 W,Xe lamp).Appropriate amount of solution was sampled to centrifuge for 20 min by a centrifugal separator,and then used a UV757CRT UV-visible spectrophotometer(Jiangdong Precision Instrument Co., China)to measure the absorbance of supernatant.Every 30 min, the above steps were repeated to test the absorbance in order to determine the degradation rate of methyl orange(MO).NaClO2(NCO)was used as an electron acceptor.
3.Results and Discussion
3.1.Characterization
To obtain the monoclinic phase BiVO4,dried gelpowders were heattreated atvarious temperatures as shown in Fig.1.Itillustrates the evolution course of BiVO4phase for the gel powders with the increasing sintering temperature.After sintering at400°C for 4 h,monoclinic BiVO4(JCPDS card No:14-0688)appears.When the sintering temperature is further increased from 450 to 500°C,the diffraction peaks ofmonoclinic and tetragonalscheelite BiVO4phases become stronger.The peak position of BiVO4loaded silver oxide and pure BiVO4is quite consistent.Thus,the presence of silver oxide has little effect on BiVO4crystalstructure.

Fig.1.XRDpatterns of BiVO4(atdifferentcalcined temperatures for 4 h).(a)T40,(b)T45, (c)T50,(d)T50 loaded 1.91%silver oxide(by mass,labeled TA50).
Fig.2 shows infrared spectra ofBiVO4before and afterphotocatalytic test.As shown by spectrum(a),the bending vibration peak at1385 cm-1corresponds with stretching vibration of M-OH(metal-OH) [V-OH or Bi-OH].H-O-H bending vibration band is observed at 1622 and 3429 cm-1.The main absorption bands(732,830 cm-1) are the V-O vibration peaks,attributed to the normalmodes ofvibration forυ3(VO4)andυ1(VO4)shown in the inset of Fig.2.After the fresh sample was being used in the photocatalytic property test,type infrared spectra were showed as a curve(b).It can be also found that the freewater or crystallization water was absorbed on the surface of BiVO4. In addition,two absorption bands(at1030 and 2926 cm-1)are related to the stretching vibrations of C-Nand C-H.It may be attributed to the adsorption or incompleted degradation of MO.
The UV-vis diffuse re fl ectance absorption spectra of the pure BiVO4and Ag2O-loaded BiVO4samples are shown in Fig.3,and the mass fraction of Ag2O loaded accounts for 1.9%(by mass)of bismuth vanadate.As can be seen from the figure,the band gap of BiVO4is 2.32 and 2.40 eV for pure and Ag-loaded BiVO4,respectively.UV absorption can be attributed to O2pband transition and the V3dband.The visible light absorption can be considered to the valence band which formed by transition from Bi6svalence band to V3dconduction band ofvanadium[20].The absorption edge shifts to the long-wavelength direction after Ag2O loaded,the“red shift”phenomenon appeared.
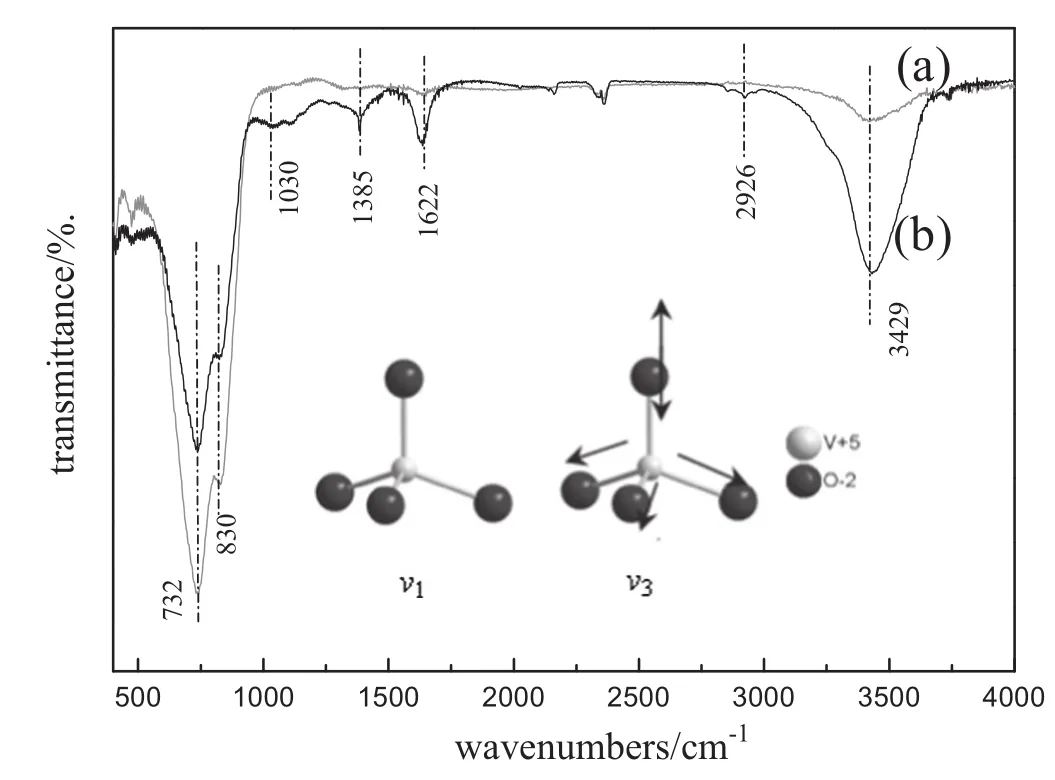
Fig.2.Infrared spectra ofT50.(a)Before photocatalytic test and(b)after 180 min photocatalytic test in 5 mmol·L-1NCO.
Transmission electron microscopy(TEM)was used to characterize the microstructure of BiVO4samples.The images in Fig.4 demonstrate that the BiVO4particles prepared under our procedure are in a size range of 50-150 nm.The SAED analyses in Fig.4(b)inset show that (011),(200),and(211)planes ofmonoclinic BiVO4.These are in good accordance with the XRD patterns in Fig.1.
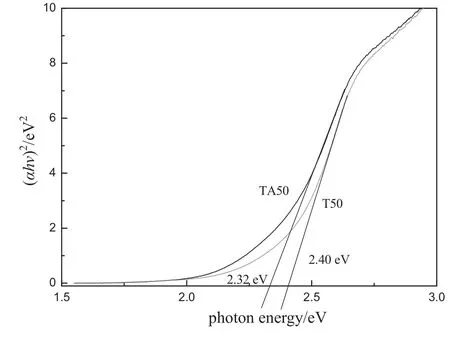
Fig.3.The UV-vis diffuse re fl ectance spectra of BiVO4and Ag2O loaded BiVO4.
3.2.Performance ofphotocatalyst
Fig.5 shows that the decolorization rate of MO with Ag2O-loaded catalystand AgNO3was used as an electron acceptor for BiVO4powders under the simulate visible-light irradiation.In the mixture of NaClO2and MO,we can see that the MO decolorization rate is 13.2%and 17.4%when concentration of NaClO2is 5 and 10 mmol·L-1,respectively.Itcan be seen that the MOdecolorization rate ofAg2O-loaded catalyst is 47.3%and pure BiVO4is 36.9%,both with 0.01 g AgNO3as an electron acceptor.They are much better than that of19.8%for the pure catalyst without AgNO3.This is mainly due to that the electron acceptorcaptures electrons of BiVO4conduction band which are excited by visible light. After NaClO2was introduced,the photocatalysts showed significantly enhanced activity.When pure BiVO4was introduced using NaClO2(5 mmol·L-1),the photocatalytic efficiency for MO is 78.4%.In the presence of NaClO2(10 mmol·L-1),the decolorization rate reaches 87.6%.In our results,TA50 exhibits the MO concentration was reduced as much as 93.6%when using NaClO2(5 mmol·L-1)as an electron acceptor in 180 min.In addition,the BiVO4loaded with Ag2O improved the absorption of visible light,so that enhanced photocatalytic properties are obtained.
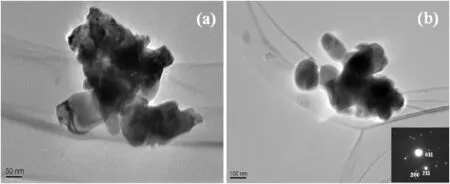
Fig.4.TEMimage of BiVO4.(a)T50,(b)TA50.
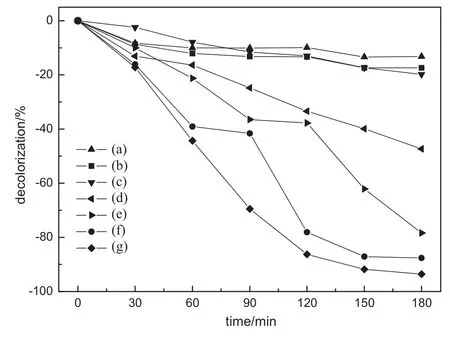
Fig.5.The decolorization rate of methyl orange.(a)5 mmol·L-1NCO+MO; (b)10 mmol·L-1NCO+MO;(c)T50,(d)TA50;(e)T50+5 mmol·L-1NCO;(f)T50+ 10 mmol·L-1NCO;(g)TA50+5 mmol·L-1NCO.
3.3.Photocatalysis mechanism
On the basis of the above experimental results and electron acceptors model[20],
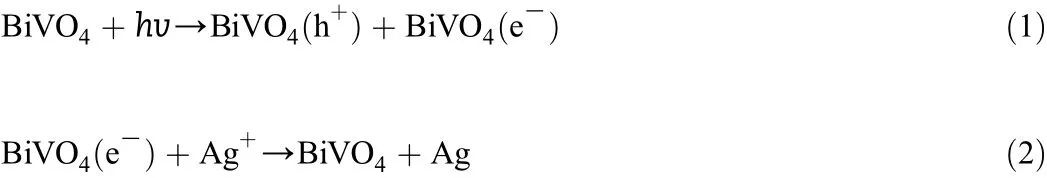
Fig.6 shows the possible photocatalytic mechanism.When the semiconductor was irradiated by sunlight,as long as the photon energy is greater or equal than the semiconductor band gap,the valence band electron is stimulated to the conduction band,which leaves photogenerated holes in the valence band.
When using NaClO2(5 mmol·L-1)as an electron acceptor,silver oxide has a role of transition electron acceptor,because Eθ(Ag+/Ag)(0.8 V)is less than Eθ(ClO2+/ClO-)(1.6 V),these Ag+obtained electrons can be captured by ClO2-anion as follows:

The photo-generated holes which do not undergo recombination with electrons will migrate to the semiconductor surface and react with MO to generate a degradation effect as follows:


Fig.6.Mechanism of the visible-light-induced MO photodegradation.(a)Silver oxide captures light-generated electrons,lightgenerated holes enter solution;(b)BiVO4absorbs a photon with energy equalto or larger than its band-gap(2.40 eV),electrons are promoted from the valence band(VB)to the conduction band(CB)of BiVO4to generate electron-hole pairs;and (c)electron acceptor ClO2-oxidizes Ag caught light generated electrons.
4.Conclusions
In this study,BiVO4was synthesized using a sol-gel method.The photocatalyst showed a structure of monoclinic type with high crystallinity.By using NaClO2as an electron acceptor,the decolorization rate of MO for BiVO4powders loaded with silver oxide increased significantly. The present study of decolorization rate of MO suggested that loaded silver oxide has a role of acting as the transition electron acceptor when the NaClO2was used as an electron acceptor.
[1]Z.G.Xiong,X.S.Zhao,Titanate@TiO2core-shellnanobelts with an enhanced photocatalytic activity,J.Mater.Chem.A 1(2013)7738-7744.
[2]L.Tong,A.Iwase,A.Nattestad,U.Bach,M.Weidelener,G.Götz,A.Mishra,P.Bäuerle, R.Amal,G.G.Wallace,A.J.Mozer,Sustained solar hydrogen generation using a dyesensitised NiO photocathode/BiVO4tandem photo-electrochemical device,Energy Environ.Sci.5(2012)9472-9475.
[3]J.Su,L.Guo,N.Bao,C.A.Grimes,Nanostructured WO3/BiVO4heterojunction films for efficient photoelectrochemical water splitting,Nano Lett.11(2011) 1928-1933.
[4]H.F.Liu,S.F.Ji,Y.Y.Zheng,M.Li,H.Yang,Porous TiO2-coated magnetic core-shell nanocomposites:preparation and enhanced photocatalytic activity,Chin.J.Chem. Eng.21(2013)569-576.
[5]H.Y.Xu,Z.Zheng,G.J.Mao,Enhanced photocatalytic discoloration of acid fuchsine wastewater by TiO2/schorl composite catalyst,J.Hazard.Mater.175(2010) 658-665.
[6]Y.Sun,B.Qu,Q.Liu,S.Gao,Z.Yan,W.Yan,B.Pan,S.Wei,Y.Xie,Highly efficient visible-light-driven photocatalytic activities in synthetic ordered monoclinic BiVO4quantum tubes-graphene nanocomposites,Nanoscale 4(2012)3761-3767.
[7]S.Tokunaga,H.Kato,A.Kudo,Selective preparation of monoclinic and tetragonal BiVO4with scheelite structure and their photocatalytic properties,Chem.Mater.13 (2001)4624-4628.
[8]G.Q.Li,Y.Bai,W.F.Zhang,Difference in valence band top of BiVO4with different crystal structure,Mater.Chem.Phys.136(2012)930-934.
[9]R.Strobel,H.J.Metz,S.E.Pratsinis,Brilliantyellow,transparentpure,and SiO2-coated BiVO4nanoparticles made in fl ames,Chem.Mater.20(2008)6346-6351.
[10]T.Saison,N.Chemin,C.Chanéac,O.Durupthy,V.r Ruaux,L.Mariey,F.o Maugé,P. Beaunier,J.-P.Jolivet,Bi2O3,BiVO4,and Bi2WO6:impact of surface properties on photocatalytic activity under visible light,J.Phys.Chem.C 115(2011)5657-5666.
[11]A.Galembeck,O.L.Alves,Bismuth vanadate synthesis by metallo-organic decomposition:thermal decomposition study and particle size control,J. Mater.Sci.37(2002)1923-1927.
[12]A.P.Zhang,J.Z.Zhang,Synthesis and characterization of Ag/BiVO4composite photocatalyst,Appl.Surf.Sci.256(2010)3224-3227.
[13]M.Wang,Q.Liu,Y.Che,L.Zhang,D.Zhang,Characterization and photocatalytic properties of N-doped BiVO4synthesized via a sol-gel method,J.Alloys Compd. 548(2013)70-76.
[14]M.Long,W.Cai,J.Cai,B.Zhou,X.Chai,Y.Wu,Efficient photocatalytic degradation ofphenolover Co3O4/BiVO4composite under visible light irradiation,J.Phys.Chem. B 110(2006)20211-20216.
[15]Y.Liu,J.Ma,Z.Liu,C.Dai,Z.Song,Y.Sun,J.Fang,J.Zhao,Low-temperature synthesis of BiVO4crystallites in molten salt medium and their UV-vis absorption,Ceram.Int. 36(2010)2073-2077.
[16]Z.X.Zhao,H.X.Dai,J.G.Deng,Y.X.Liu,C.T.Au,Enhanced visible-light photocatalytic activities of porous olive-shaped sulfur-doped BiVO4-supported cobalt oxides,Solid State Sci.18(2013)98-104.
[17]H.Yu,X.X.Zheng,Z.Y.Yin,F.Tao,B.B.Fang,K.S.Hou,Preparation of nitrogen-doped TiO2nanoparticle catalyst and its catalytic activity under visible light,Chin.J.Chem. Eng.15(2007)802-807.
[18]X.Z.Li,F.B.Li,Study of Au/Au3+-TiO2photocatalysts toward visible photooxidation for water and wastewater treatment,Environ.Sci.Technol.35(2001)2381-2387.
[19]S.Kohtani,J.Hiro,N.Yamamoto,A.Kudo,K.Tokumura,R.Nakagaki,Adsorptive and photocatalytic properties of Ag-loaded BiVO4on the degradation of 4-nalkylphenols under visible light irradiation,Catal.Commun.6(2005)185-189.
[20]A.Kudo,K.Omori,H.Kato,A novelaqueous process for preparation of crystalformcontrolled and highly crystalline BiVO4powder from layered vanadates at room temperature and its photocatalytic and photophysical properties,J.Am.Chem.Soc. 121(1999)11459-11467.
☆Supported by the Education Department of,Heilongjiang Province(12541111),the Program for Innovative Research Team in University of Heilongjiang Province(2013TD008), the Key Laboratory of Green Chemical Engineering and Technology of College of Heilongjiang Province,Harbin University of Science and Technology and the Technology and Innovative ExperimentalProjectof Harbin University of Science and Technology.
*Corresponding author.
E-mailaddress:shlw0531@163.com(L.Shan).
Bismuth vanadate
Photocatalytic properties
Sol-gelmethod
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Separation Science and Engineering Performance Prediction of Structured Packing Column for Cryogenic Air Separation with Hybrid Model☆
- Preparation,Structure Characterization and Hydrodesulfurization Performance of B-Ni2P/SBA-15/Cordierite Monolithic Catalysts☆
- Recovery and Recycling of Ti Supported Bimodal Mesoporous Catalysts Prepared via Ship-in-a-bottle Method in the Epoxidation of Cyclohexene☆
- Promoting Xylene Production in Benzene Methylation using Hierarchically Porous ZSM-5 Derived from a Modified Dry-gel Route☆
- Energy,Resources and Environmental Technology Experimental and Modeling Study on de-NOxCharacteristics of Selective Non-catalytic Reduction in O2/CO2Atmosphere☆
- Energy,Resources and Environmental Technology CO2Removal from Biogas by Water Washing System☆