肝细胞癌患者TACE术前后血清miR-21表达变化及临床意义
2014-06-09王以浪王亚非张亮缪亚军陈卓周陈印滇丁文彬
王以浪,王亚非,张亮,缪亚军,陈卓,周陈,印滇,丁文彬
·肿瘤介入Tumor intervention·
肝细胞癌患者TACE术前后血清miR-21表达变化及临床意义
王以浪,王亚非,张亮,缪亚军,陈卓,周陈,印滇,丁文彬
目的探讨HCC患者经皮肝动脉化疗栓塞(TACE)术前、后血清miR-21表达变化及临床意义。方法以反转录定量PCR(RT-PCR)法检测HCC患者TACE术前、后及正常者血清m iR-21水平,以酶联免疫吸附法(ELISA)检测血清AFP水平。结果HCC患者血清miR-21水平为正常人的(12.9± 3.5)倍(t=19.430 7,P<0.01),TACE术后1个月为正常参考值的(7.2±1.7)倍,较术前显著降低(t= 9.493 7,P<0.01)。血清miR-21水平与肿瘤大小、癌栓及HBV感染相关。TACE术后1个月血清miR-21水平在部分缓解、稳定和进展组中分别为正常人的(4.0±0.3)、(6.0±1.5)和(8.6±1.5)倍,各组间差异有统计学意义(F=38.168,P=0.000)。miR-21诊断HCC的ROC-AUC值为0.910±0.041,显著高于AFP的0.860±0.037(t=6.304 2,P<0.01)。miR-21检测HCC的特异度(88.1%)显著高于AFP(69.0%,χ2=4.525 3,P=0.033)。结论TACE术后m iR-21水平明显降低,能较好预测TACE术疗效,是HCC的潜在分子标记物。
肝细胞癌;经导管动脉化疗栓塞术;miRNA-21;血清
肝细胞癌(HCC)进展快,临床症状隐匿,诊断时病程多在晚期,丧失手术机会[1]。经导管选择性肝动脉化疗栓塞(TACE)是晚期HCC的首选疗法[2-3]。
1 材料与方法
1.1 临床资料
收集2009年1月—2012年12月我院诊治的HCC患者42例,其中男34例,女8例;年龄为35~74岁,平均(53±9)岁。所有患者诊断符合《原发性肝癌规范化诊治专家共识》[12]。肿瘤直径≥5 cm 32例,<5 cm 10例(多发肿瘤统计最大者直径);伴有门静脉及分支癌栓34例,无癌栓8例。AFP≥400μg/L 16例,<400μg/L 26例。HBsAg阳性36例,阴性6例。纳入标准:初治患者,无手术切除指征,未接受化疗、放疗以及分子靶向治疗;无TACE禁忌,肝功能Child-Pugh A~B级;总病灶占肝脏体积<70%,门静脉主干通畅。介入治疗术中碘化油乳剂为:表阿霉素60mg、奥沙利铂50 mg联合碘化油混成乳剂(用量根据肿瘤大小及血供情况,总量不超过30m l)。TACE时将导管超选择插入肝固有动脉以远的肿瘤供血动脉,栓入碘化油乳剂。收集42例我院门诊正常体检者血清标本作为正常对照组。
1.2 方法
1.2.1 血清miR-21反转录定量PCR法检测抽取不同时期患者静脉血各5 m l,加入含有EDTA试管中。离心,抽提总RNA(美国Molecular Research Cente公司)。合成第一链cDNA(以色列Fermentas公司)。miR-21和miR-16反转录定量PCR引物由上海英骏生物公司合成(表1)。采用EzOmicsTM一步实时反转录定量PCR法检测血清miRNA(百奥迈科生物技术有限公司)。反转录定量PCR反应体系:50 ng RNA,qPCR混合物12.5μl,miRNA-21反转录引物(10μmol/L)0.5μl,miRNA-21正、反向引物各0.5μl(10μmol/L),RNA酶抑制剂,加ddH2O至25μl。每个样本进行3次检测取平均数。以miR-16为内参照基因,miR-21相对表达量采用2-ΔΔCt计算[8]。该方法是目的基因与内参基因的Ct值比较后(ΔCt),再将HCC组与正常组比较(ΔΔCt)。ΔCt=(CtmiR-21-CtmiR-16),ΔΔCt=[(CtmiR-21-CtmiR-16)HCC-(CtmiR-21-CtmiR-16)正常人],2-ΔΔCt的数值反映血清miR-21相对于正常人血清水平的倍数(定量PCR扩增仪、凝胶成像分析仪和核酸蛋白分析仪购自美国Bio-Rad公司)。
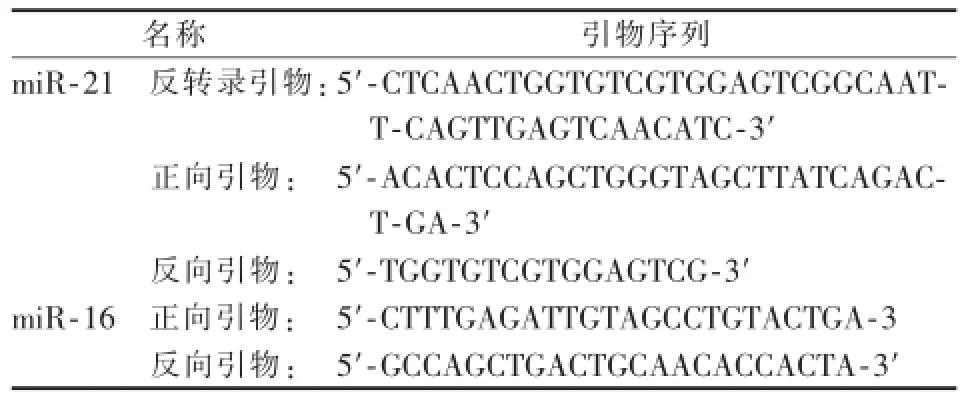
表1 反转录定量PCR引物序列
1.2.2 ELISA法血清AFP定量检测ELISA操作按说明书进行,用自动酶联仪在450 nm波长测量各孔的A值。绘制标准曲线,计算各样品中AFP的浓度。
1.2.3 疗效评价方法所有患者均在术前1周内和治疗后4周于本院接受CT(Siemens sensation 64排螺旋CT)或MR(Siemens symphony 3.0 T)多期扫描。用RECIST标准评价HCC治疗后反应。包括完全缓解(CR)、部分缓解(PR)、进展(PD)和稳定(SD)。CR指所有靶病灶消失,PR指靶病灶最大径总和缩小30%以上,PD指靶病灶最大径总和增加20%以上和(或)有新病灶出现,SD指病灶变化介于PD和PR之间。
1.3 统计学处理
您说像他这样,我怎么敢把大事儿交给他办?他自己也挺苦恼:“国家的事儿不归我想,家里的事儿全被你想了,我还能想点儿啥?”
2 结果
2.1 RNA质量检测及反转录定量PCR扩增
提取外周血总RNA,用紫外分光光度计进行浓度和纯度检测,所测A值均在1.8~2.0之间。经1%琼脂糖凝胶电泳证实RNA各条带显示清晰,合成第一链cDNA,以特异性引物对miR-21和miR-16进行实时荧光定量PCR反应,其扩增曲线见图1。
图1 反转录定量PCR扩增曲线
2.2 正常人群和HCC患者血清miR-21水平比较
各组血清miR-21水平见表2。HCC患者血清miR-21水平为正常人群的(12.9±3.5)倍,TACE术后1周血清miR-21水平较术前升高,术后1个月较术前降低。
表2 正常人群和HCC患者血清miR-21水平比较(±s)

表2 正常人群和HCC患者血清miR-21水平比较(±s)
注:与TACE术前1 d组比,a:t=19.430 7,P<0.001;b:t=3.600 8,P<0.001;c:t=9.493 7,P<0.001
标志正常对照组(n=42)HCC组(n=42)术前1 d术后1周术后1个月miR-21 Ct 27.3±3.1 23.3±2.4 23.1±2.4 24.7±2.9 miR-16 Ct 24.6±2.4 24.3±2.2 24.4±2.6 23.6±2.2 2-ΔΔCt1.1±1.8a12.9±3.5 17.1±6.7b7.2±1.7c
2.3 HCC患者血清miR-21水平与临床特征关系分析
对miR-21表达与临床特征分析显示(见表3),miR-21表达与肿瘤大小、有无癌栓及HBV感染相关,与肿瘤数目、AFP水平无相关性。
HCC患者介入术后1个月行CT或MRI检查,以RECIST标准评价HCC患者介入术后近期疗效,见表4。各组间miR-21水平差异有统计学意义,miR-21水平随疗效改善而降低。
表3 HCC患者血清miR-21水平与临床特征分析(±s)

表3 HCC患者血清miR-21水平与临床特征分析(±s)
注:a:t=1.387 4,P=0.173 0;b:t=4.840 1,P=0.000 0;c:t=5.776 6,P=0.000;d:t=0.783 4,P=0.438 0;e:t=5.856 2,P=0.000 0
参数例数miR-21 Ct miR-16Ct 2-ΔΔCt肿瘤数目单发10 23.4±2.4 24.3±2.5 12.9±3.1a多发32 23.2±2.2 24.3±2.0 14.9±4.2肿瘤直径/cm≥5 32 23.1±2.1 24.3±2.2 16.0±3.7b<5 10 23.7±2.3 24.2±2.6 9.8±2.9癌栓有34 23.1±1.9 24.5±2.3 18.4±4.9c无8 23.9±2.6 24.1±2.5 8.0±2.6 AFP/(μg/L)≥400 16 23.4±2.0 24.4±2.1 13.9±3.3d<400 26 23.1±2.3 24.2±1.9 14.9±4.5 HBsAg阳性36 23.0±2.2 24.4±2.4 18.3±4.4e阴性6 23.9±2.5 24.0±2.1 7.5±2.1
表4 TACE术后miR-21水平与近期疗效关系(±s)

表4 TACE术后miR-21水平与近期疗效关系(±s)
注:F=38.168,P=0.000
疗效(n)miR-21水平PR(9)4.1±0.3 SD(8)6.0±1.5 PD(25)8.6±1.5
2.5 HCC患者血miR-21与AFP检测ROC曲线下面积(AUC)比较
以正常人群血清的miR-21与AFP表达水平为对照组,绘制ROC曲线(图2)。miR-21 ROC AUC为0.910±0.041,显著高于AFP的0.860±0.037(t=6.304 2,P<0.01)。
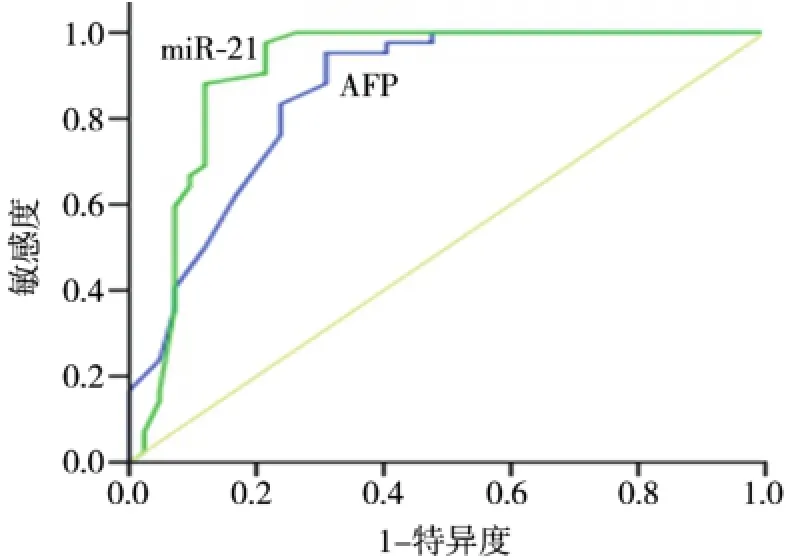
图2 miR-21与AFP的ROC
2.6 miR-21与AFP诊断HCC的灵敏度和特异度比较
取以正常人群为对照的ROC曲线约登指数最大点,确定miR-21和AFP诊断HCC的最佳临界值分别为4.95倍和13.5μg/L。两者诊断HCC灵敏度及特异度见表5。
3 讨论
近来多项研究表明外周血miRNA可以作为恶性肿瘤的标记物,为研究肿瘤发病机制、早期诊断、临床疗效评估及监测复发、转移提供了重要工具。
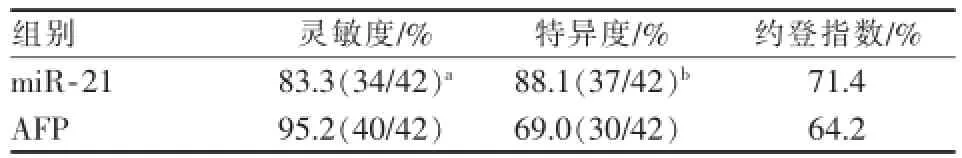
表5 miR-21与AFP诊断HCC的灵敏度和特异性比较
miRNA是一类非编码的微小RNA,其与靶基因mRNA 3′非编码区的碱基互补配对结合,通过多种途径影响靶基因的表达而发挥生理、病理作用。其主要作用方式有:抑制mRNA翻译;促使多聚核糖体脱落,抑制蛋白翻译延伸;募集蛋白酶降解翻译中的蛋白;促进mRNA降解[13]。miR-21靶基因包括数种抑癌基因,如p53、PTEN/AKT信号通路、RAS信号通路、FasL和TPM1等,miR-21可抑制靶基因表达,参与肿瘤细胞增殖、细胞周期调控、凋亡和侵袭转移,起着癌基因的作用[14-15]。
miRNA在外周血中可以稳定存在,耐RNA酶降解,易被定量PCR等方法检测到。本研究显示HCC患者外周血中miR-21水平明显高于正常人群,具有较高的诊断价值。有研究表明miR-21在HCC组织中的水平是癌旁组织的近3倍[16]。本文检测miR-21表达水平相对较高可能与两项研究标本分别是外周血及病理组织有关,此外,欧美国家HCC病因常见为HCV感染及酒精性脂肪肝等,而我国多为HBV感染。
AFP作为临床最常用的HCC标记物,具有较高的灵敏度,但也存在30%左右的假阴性,此外慢性HBV患者及生殖系肿瘤患者AFP常增高,导致了部分假阳性。本文研究显示HCC患者miR-21表达与AFP无明显相关性,miR-21 ROC AUC为0.910,显著高于AFP的0.860。miR-21和AFP诊断HCC的最佳临界值分别为4.95和13.5μg/L,此时两者检测HCC均具有较高灵敏度(83.3%和95.2%),而特异度分别为88.1%和69.0%,miR-21明显高于AFP。可见miR-21可与AFP在HCC诊断中起到互补作用,提高诊断准确率。
本研究显示HBsAg阳性者外周血miR-21水平较HBsAg阴性者显著增高。我国HCC患者90%以上为HBV相关性HCC,HBx蛋白在HBV诱发HCC形成过程中扮演了重要的作用[17-18]。HBx通过上调miR-21抑制凋亡基因表达,促进HCC形成[19]。此外,我们研究还发现肿瘤越大及伴有门静脉癌栓者,其血清miRNA-21水平越高。可能与肿瘤负荷高,HCC细胞内成熟的miR-21被脂蛋白或脂质包被成外切酶体,主动分泌至胞外进入外周血液循环有关。
TACE术后1周外周血miR-21水平较术前增高,有研究表明TACE术后7~10 d出现HCC病灶明显坏死,坏死HCC细胞内成熟的miR-21大量释放到外周血中,致血miR-21水平明显升高。TACE术后1个月miR-21较术前显著降低,可能为HCC病灶TACE术后HCC细胞负荷减少,致外周血miR-21水平下降有关。提示TACE术后检测患者血清miR-21来评估治疗效果时间窗应该在1个月后。分析外周血miR-21水平与近期疗效关系显示,miR-21水平随HCC病灶缩小、减少而降低,提示其可以作为TACE术疗效监测指标。
综上所述,本研究显示HCC患者外周血miR-21水平明显增高,与HBV感染、肿瘤大小及门脉癌栓相关,与AFP在诊断HCC中有互补作用,TACE术后miR-21水平明显降低,能较好预测TACE术疗效,可作为临床诊断、治疗HCC的潜在分子标记物。
[1]El-Serag HB.Epidemiology of viral hepatitis and hepatocellular carcinoma[J].Gastroenterology,2012,142:1264-1273.e1.
[2]程永德,程英升,颜志平.常见恶性肿瘤介入治疗指南[M].北京:科学出版社,2013.
[3]高嵩,朱旭,杨仁杰,等.TACE联合奥沙利铂、氟尿嘧啶、亚叶酸钙肝动脉化疗治疗中晚期原发性肝癌[J].介入放射学杂志,2012,21:377-383.
[4]梁茂全,苏洪英.肝癌化疗栓塞前后甲胎蛋白变化模式的临床意义[J].介入放射学杂志,2012,21:333-338.
[5]Wang X,Zhang A,Sun H.Power of metabolomics in diagnosis and biomarker discovery of hepatocellular carcinoma[J].Hepatology,2013,57:2072-2077.
[6]Marquardt JU,Galle PR,Teufel A.Molecular diagnosis and therapy of hepatocellular carcinoma(HCC):an emerging field for advanced technologies[J].JHepatol,2012,56:267-275.
[7]Braconi C,Henry JC,Kogure T,et al.The role of MicroRNAs in human liver cancers[J].Semin Oncol,2011,38:752-763.
[8]Wong CM,Kai AK,Tsang FH,et al.Regulation of hepatocarcinogenesis by microRNAs[J].Front Biosci(Elite Ed),2013,5:49-60.
[9]Schetter AJ,Leung SY,Sohn JJ,et al.MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma[J].JAMA,2008,299:425-436.
[10]Karakatsanis A,Papaconstantinou I,GazouliM,et al.Expression ofmicroRNAs,miR-21,miR-31,miR-122,miR-145,miR-146a,miR-200c,miR-221,miR-222,and miR-223 in patients with hepatocellular carcinoma or intrahepaticcholangiocarcinoma and its prognostic significance[J].Mol Carcinog,2013,52:297-303.
[11]Gabriely G,Wurdinger T,Kesari S,et al.MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators[J].Mol Cell Biol,2008,28:5369-5380.
[12]中国抗癌协会肝癌专业委员会.中国抗癌协会临床肿瘤学协作专业委员会和中华医学会肝病学分会.原发性肝癌规范化诊治的专家共识[J].实用肝脏病杂志,2009,12:321-328.
[13]Jansson MD,Lund AH.MicroRNA and Cancer[J].Mol Oncol,2012,6:590-610.
[14]Zhu Q,Wang Z,Hu Y,et al.miR-21 promotesmigration and invasion by themiR-21-PDCD4-AP-1 feedback loop in human hepatocellular carcinoma[J].Oncol Rep,2012,27:1660-1668.
[15]Vinciguerra M,Sgroi A,Veyrat-Durebex C,et al.Unsaturated fatty acids inhibit the expression of tumor suppressor phosphatase and tensin homolog(PTEN)viamicroRNA-21 up-regulation in hepatocytes[J].Hepatology,2009,49:1176-1184.
[16]Jiang J,Gusev Y,Aderca I,et al.Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection,cirrhosis,and patient survival[J].Clin Cancer Res,2008,14:419-427.
[17]Knoll S,Fürst K,Thomas S,et al.Dissection of cell contextdependent interactions between HBx and p53 familymembers in regulation of apoptosis:a role for HBV-induced HCC[J].Cell Cycle,2011,10:3554-3565.
[18]Kew MC.Hepatitis B virus x protein in the pathogenesis of hepatitis B virus-induced hepatocellular carcinoma[J].J Gastroenterol Hepatol,2011,26(suppl 1):144-152.
[19]Qiu X,Dong S,Qiao F,et al.HBx-mediated miR-21 upregulation represses tumor-suppressor function of PDCD4 in hepatocellular carcinoma[J].Oncogene,2013,32:3296-3305.
The changes of serum m iR-21 expression level in patientsw ith HCC before and after TACE and its clinical significance
WANG Yi-lang,WANG Ya-fei,ZHANG Liang,MIAO Ya-jun,CHEN Zhuo,ZHOU Chen,YIN Dian,DING Wen-bin.The First Peop le’s Hospital of Nantong,Natong,Jiangsu Province 226001,China
DINGWen-bin,E-mail:oncowang@163.com
ObjectiveTo investigate the changes of serum m iR-21 expression level in patients with HCC before and after transcatheter arterial chemoembolization(TACE)and to discuss its clinical significance.M ethods Before and after TACE the levels of serum miR-21 in 42 patients with HCC and 42 healthy subjectswere determined by reverse transcriptase quantitative PCR(RT-PCR),and the levels of serum AFP were also estimated by enzyme-linked immunosorbent assay(ELISA).The results were analyzed.Results The serum miR-21 level in patientswith HCC was(12.9±3.5)times of that in normal subjects(t=19.430 7,P<0.01).One month after TACE,the serum miR-21 level became(7.2±1.7)times of that of normal reference value,which was remarkably lower than thatobtained before the treatment(t=9.493 7,P<0.01).The serum miR-21 levelwas closely correlated with the tumor size,the presence of tumor thrombus and HBV infection.Onemonth after TACE the serum m iR-21 levels in patient groups showing partial response,stable disease and progressive diseasewere(4.0±0.3),(6.0±1.5)and(8.6±1.5)times,respectively,of that of normal reference value,and statistically significant difference existed between each other among the three groups(F=38.168,P=0.000).ROC-AUC value ofMiR-21 in diagnosing HCC was 0.910±0.041,which was significantly higher than thatof AFP(0.860±0.037,t=6.3042,P<0.01).The specificity ofmiR-21 in detecting HCC was 88.1%,which was remarkably higher than thatof AFP(69%,χ2=4.5253,P=0.033).Conclusion After TACE the serum MiR-21 level in HCC patients is significantly decreased,which is very helpful in predicting the therapeutic efficacy of TACE.Therefore,MiR-21 can be regarded as apotentialmolecularmarker of HCC.(J Intervent Radiol,2014,23:406-410)
hepatocellular carcinoma;transcatheter arterial chemoembolization;miRNA-21;serum
R735.7
A
1008-794X(2014)-05-0406-05
2013-11-13)
(本文编辑:俞瑞纲)
10.3969/j.issn.1008-794X.2014.05.009
226001江苏南通南通市第一人民医院肿瘤科(王以浪、王亚非、张亮、缪亚军、印滇),介入科(陈卓、周陈、丁文彬)
丁文彬E-mail:oncowang@163.com
