间充质干细胞在肝脏疾病应用的研究现况
2014-05-05金银鹏傅青春陈成伟
金银鹏 傅青春 陈成伟
间充质干细胞在肝脏疾病应用的研究现况
金银鹏 傅青春 陈成伟
间充质干细胞(MSC)属于成体干细胞的一种,是一类具有自我更新和多向分化能力的多能干细胞。其来源丰富,免疫原性低,目前体内外实验均发现MSC可促进损伤肝脏修复,改善症状,提高存活率。通过调节肝脏局部和全身炎症反应和免疫紊乱发挥治疗作用。本文就MSC治疗肝脏疾病的研究现况进行综述。
间充质干细胞;肝病;机制
干细胞是在适当条件下具有自我复制、自我更新以及多向分化能力的细胞群。间充质干细胞(mesenchymal stem cells,MSC)作为干细胞的重要成员,在肝脏疾病相关的基础和临床试验逐年增多,研究成果令人关注。本文对研究进展作一综述。
一、MSC定义和生物学功能
MSC最初被认为是骨髓细胞中一群可贴壁生长,具有克隆能力和成骨分化能力的一个亚群[1-2]。1991年Caplan等[3]人把这种可在体外向中胚层细胞分化的细胞群定义为“MSC”,1999年,Pittenger等[4]发现其多向分化潜能。国际细胞治疗协会(ISCT)提出的鉴别MSC的最低标准为:(1)可贴塑料瓶壁生长;(2)可在体外向脂肪细胞、骨细胞、软骨细胞分化;(3)细胞表面应表达CD73、CD90、CD105,不表达CD45、CD19和HLA-DR[6]。MSC与多种表面标志相关,如CD51、CD271[7]、CD146[8]等,LepR或可作为骨髓MSC的特征性标志[9]。
MSC不仅存在于骨髓,还广泛分布于全身各处组织中,近年来已先后从脐血[10]、牙髓[11]、滑膜[12]、脂肪[13]、胎盘[14]、脐带[15-16]、皮肤[17]、羊水[18]、母乳[19]等组织中分离出MSC。由于不同物种、不同组织来源的MSC表面标志、增殖分化潜能及其介导的组织再生机制不尽相同,如脂肪MSC在贴壁前只表达CD90,不表达CD105,贴壁生长之后两种标志均表达[20];脐带血管周围的MSC和骨髓MSC相比具有更广的分化潜能。未来MSC鉴定标准可能需要根据物种、组织来源进行相应的定义,并可能需要分选MSC的各个亚细胞群,运用基因、分子生物学工具,探讨特定表型MSC所介导的分子治疗机制。
MSC免疫原性较低,低表达主要组织相容性复合体(MHC)Ⅰ类分子,不表达MHCⅡ类分子、Fasl和T细胞协同刺激分子B7等,因此为异基因移植提供生存并发挥其生物学功能的条件。
二、MSC在肝脏疾病的研究现况
体内外实验均发现MSC可促进损伤肝脏修复,改善症状,提高存活率。目前使用MSC进行临床试验的肝脏疾病包括急性肝衰竭[21]、慢加急性肝衰竭[22]、肝纤维化[23]、肝硬化[24]、自身免疫性肝病[25]、遗传代谢性肝病和终末期肝病[26]等(表1)。这些研究多为Ⅰ、Ⅱ期临床试验,少数已进入Ⅲ期临床试验。
三、MSC在肝病应用的机制
以往人们认为MSC对于损伤肝脏的作用在于通过其“干性”(stem)发挥细胞替代作用从而参与组织再生;然而,仅有少量干细胞向肝细胞的转分化[27]或者干细胞与肝细胞的极低频率的融合[28]并不能很好地解释其促进肝脏组织再生的机制。近年来大量的临床前期实验发现,MSC具有高度活跃的代谢能力,不但可以分泌细胞外基质的组分重塑胞外基质,还可通过旁分泌作用分泌一系列细胞因子激活内源性干细胞、通过免疫调节减轻炎症反应、抑制受损肝细胞凋亡、促进新生血管形成等,从而促进肝脏组织的再生[29-30],有人很形象的称MSC为“细胞因子工厂”。并提出MSC转录组、分泌组和蛋白质组的概念,认为MSC并非通过直接细胞替代,而是通过其分泌蛋白及因子发挥组织再生促进作用[31](图1)。
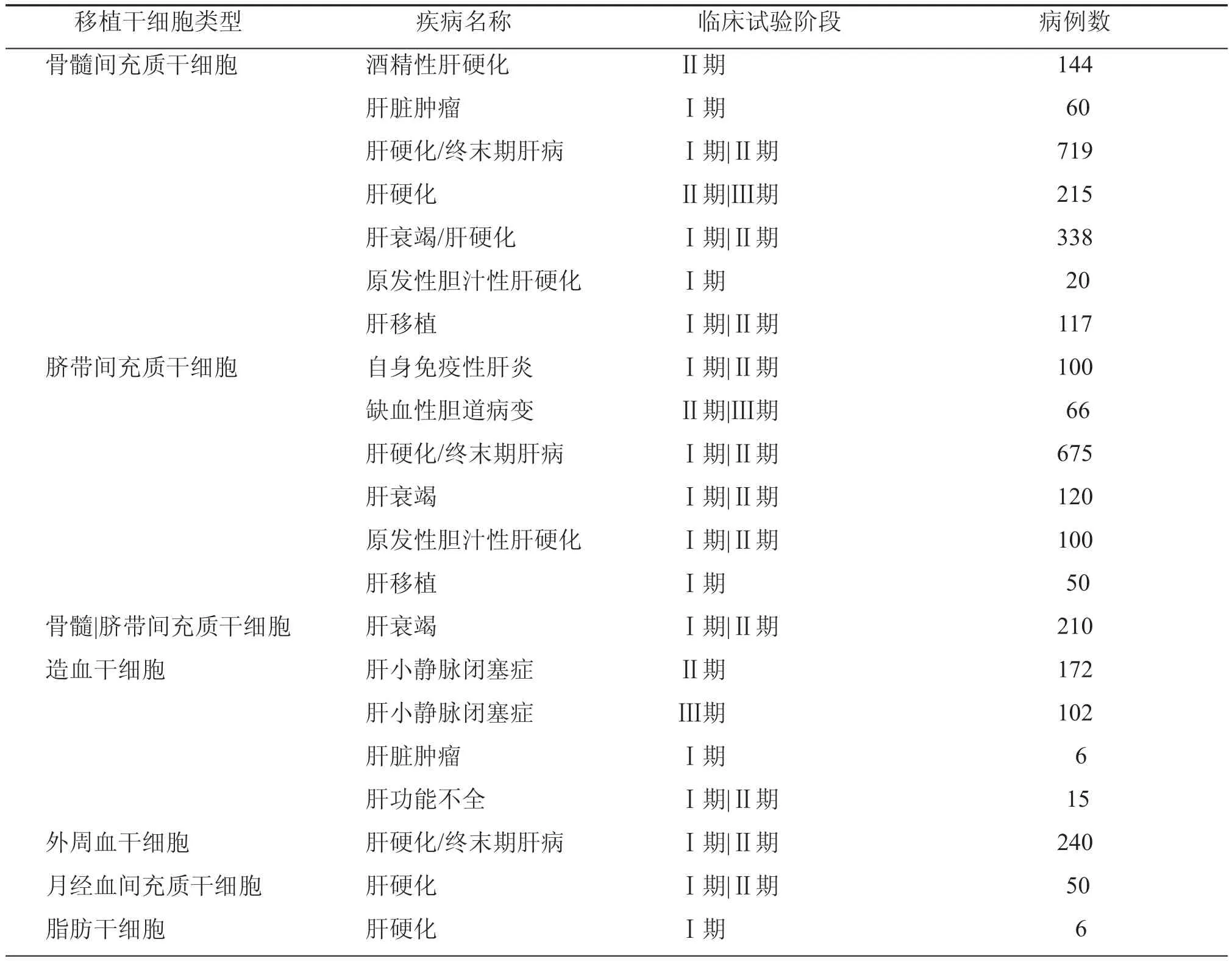
表1 clinicaltrials.gov注册干细胞临床试验
MSC治疗肝病的分子机制是目前研究的热点。人MSC表面表达有大量功能性的Toll样受体(TLR)如TLR3和TLR4[32],Waterman等[33]发现微环境中的细胞因子浓度决定了MSC的最终表型,根据MSC分泌细胞因子、趋化因子、转化生长因子-β(TGF-β)、下游效应分子SMAD3/SMAD7水平以及细胞迁移能力的不同,可以把MSC分为炎症促进型(1型)和免疫抑制型(2型)。炎症初期,肝脏局部低浓度干扰素-γ(IFN-γ)和肿瘤坏死因子-α(TNF-α)可激活MSC表面的TLR4,使其分化成为炎症促进型MSC,分泌趋化因子如CXCL9、CXCL10、MIP-1α和MIP-1β等并进一步激活T淋巴细胞,启动局部炎症反应[34-35];而随着炎症进一步加重,MSC暴露于高水平IFN-γ和TNF-α环境,细胞表面的TLR3被激活而分化为免疫抑制型MSC,通过分泌吲哚胺2,3-双加氧酶(IDO)、前列腺素E2(PGE2)、一氧化氮(NO)、肝细胞生长因子(hepatocyte growth factor,HGF)、血红素加氧酶(HO)、TGF-β等抑制T细胞的激活,TGF-β的分泌也可促进CD4+CD25+FoxP3+Treg细胞的形成,发挥免疫抑制作用。此外,MSC分泌的白细胞介素-6(IL-6)可促进单核细胞向M2型巨噬细胞转化,M2型巨噬细胞分泌大量趋化因子18(CCL18)间接促进Treg细胞的形成[36-37]。在缺少IL-6的环境中,MSC促进单核细胞向M1型巨噬细胞转化,M1型巨噬细胞分泌大量炎症因子如IFN-γ和TNF-α进一步促进MSC向免疫抑制型MSC的转化,从而避免过度免疫反应的发生[38](图2)。
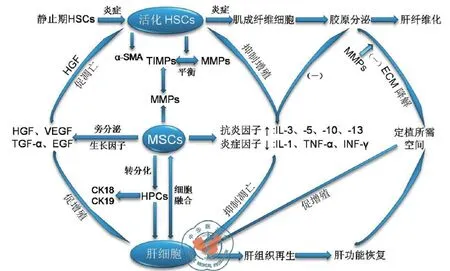
图1 间充质干细胞在肝脏组织再生中的作用
急性肝衰竭时,肝细胞短时间内大量坏死,肝功能急剧恶化,接受损伤肝组织或外来微生物释放的分子信号,如内毒素、脂多糖、热休克蛋白等刺激,MSC表面的TLRs被激活,促进MSC向肝脏的迁移并通过MEK、P38、NF-κB等通路,分泌HGF、表皮细胞生长因子(EGF)、成纤维细胞生长因子(FGFs)等细胞因子[39-41],HGF是向肝细胞诱导分化的关键因子,与MSC膜表面特异性受体c-Met结合,是肝细胞最强的促有丝分裂剂;通过下调NF-κB信号通路,使T淋巴细胞周期停滞于G0/G1期,降低其免疫应答能力[42-43]。MSC通过TLR信号调节,在病原体清除和损伤组织修复过程中扮演重要的角色,未来可能因其增强宿主防御的能力被广泛应用于临床治疗[44-45]。
此外,MSC可抑制树突状细胞成熟,减少B淋巴细胞的活化与增殖,抑制NK细胞的增殖与细胞毒性作用,上调抗炎细胞因子白介素-10(IL-10)水平促进调节性T淋巴细胞生成,分泌IL-1受体拮抗剂(IL-1Ra)抑制前炎症细胞因子如IL-1的生成等[46],从而减轻肝脏组织炎症反应。Parekkadan等[47]通过静脉注射MSC条件培养液治疗肝衰竭大鼠,肝脏组织学检查显示肝小叶旁白细胞浸润明显减少,大鼠肝细胞凋亡减少90﹪,增殖期肝细胞数量增加3倍,体外实验也证实MSC条件培养液具有抗肝细胞凋亡和促肝细胞有丝分裂的作用,提示MSC分泌细胞因子抑制免疫细胞增殖和向肝脏的迁移发挥其抗炎作用,分泌基质细胞衍生因子-1(SDF-1)、血管内皮生长因子(VEGF)等发挥其抗细胞凋亡作用[48]。
四、MSC在肝纤维化中的研究
MSC在肝纤维化疾病的应用仍存在争议,目前已证实基质金属蛋白酶(MMP)和基质金属蛋白酶组织抑制因子(TIMP)的失衡在肝纤维化的发生发展中起着重要的作用。MMP可通过降解多余细胞外基质,避免肝纤维化形成。Iimuro和Siller-López等[49-50]分别使用过表达MMP1和MMP8的腺病毒治疗肝纤维化大鼠,发现2周后大鼠肝纤维化程度明显减轻,Ⅰ型胶原、活化肝星状细胞(HSC)均减少,肝细胞再生明显。MMP2、MMP3、MMP9和HGF表达上调,而TGF-β表达下调。
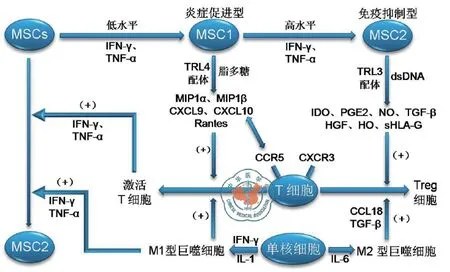
图2 间充质干细胞的免疫调节作用
Higashiyama等[51]使用骨髓MSC治疗肝纤维化小鼠,发现MSC通过高表达MMP-9和MMP-13降解细胞外基质,抑制胶原蛋白沉积和TGF-β1、平滑肌肌动蛋白(α-SMA)表达,减轻肝纤维化程度,分泌HGF诱导HSC凋亡,分泌IL-10抑制HSC增殖和基质合成。Zhao等[52]使用骨髓MSC治疗肝纤维化大鼠,血清中IL-10浓度明显升高,而IL-1β、IL-6、TNF-α、TGF-β等因子明显低于对照组。然而,Carvalho等[53]发现MSC进入肝纤维化患者或小鼠体内后倾向于分化为HSC和肌成纤维母细胞,促进肝纤维化。
五、原位MSC研究的重要性
目前针对MSC免疫调节能力的研究都是基于体外培养的细胞,将其移植到体内并希望其发挥生物学功能,充足的细胞体内原位试验是必需的。然而,由于体内MSC数量太少、缺乏合适的动物模型以及种属间作用机制差异,原位MSC的研究进展缓慢,但原位研究无疑较体外细胞实验数据更具有说服力。体内MSC较体外培养的细胞可能具有更高的转录活性,提示其具有更广的分化潜能和更多的Wnt相关基因表达[54]。Sacchetti等[55]指出,人体原位的CD146阳性MSC存在于内皮下的网状外膜细胞。从人骨髓中分离出的CD146、CD271阳性MSC表达高水平的成纤维细胞生成集落单位(CFU-F)活性,而且CFU-F活性只来源于CD271阳性的细胞[56]。Daquinag等[57]人发现体内脂肪MSC表面高表达δ核心蛋白聚糖,推测其配体抵抗素通过与δ核心蛋白聚糖受体的结合决定脂肪MSC的命运,然而,当体外培养脂肪MSC时,其表面并无δ核心蛋白聚糖表达。受体内复杂多变的微环境影响,体外实验数据并不代表细胞在体内的真实情况,这也提示了原位MSC研究的重要性。
MSC在再生医学领域的应用明显滞后于实验室研究,MSC移植数量不足时可能影响疗效,但细胞数量过多或多次移植可能引起免疫反应或肺栓塞等并发症;细胞培养基、培养环境中的氧张力、细胞冻融至移植的时间、细胞的组织来源、是否对干细胞进行基因修饰、自体来源或异体来源细胞、细胞在人体内的存活时间等均对细胞的安全性和效能有较大影响。在目前国内MSC相关的临床试验中,很大部分试验并未在国家监管机构或临床试验网站注册,细胞移植后也未对患者进行长期随访观察。然而,目前已知MSC对肿瘤生成具有一定促进作用,在肿瘤部位炎症的趋化作用下,MSC可趋化至现有肿瘤组织并参与其生长[58];Ben-David等[59]近期发现体外培养的MSC具有基因不稳定性,通过染色体核型分析发现MSC发生染色体变异的概率为4﹪;尽管其他研究者认为低概率染色体核型的变异与肿瘤的生成并无必然联系,人体免疫系统对肿瘤细胞的作用不可忽视,而且目前并未见人体内MSC直接转换为肿瘤细胞的报道;但是无论MSC移植后能否向肿瘤细胞转换,同实验室研究者密切合作,在目前诸多临床前期实验的基础上设计科学、严谨的临床试验,判断其干细胞移植风险(获益比),对接受干细胞治疗的患者长期疗效及移植中、移植后并发症进行完善的评估,并建立完善的干细胞移植受试者数据库,对于未来MSC在肝脏疾病的广泛应用,将具有极高的指导意义。
干细胞产业方兴未艾,据Visiongain调研,2011年干细胞治疗的市场份额达27亿美元,2016年预计将达88亿美元。2012年美国FDA批准了Duke大学医学院的脐血造血干细胞静脉注射混悬液(DuCord)和Clinimmune实验室公司的脐带血HPC,最近又批准治疗Ⅰ型糖尿病的研究进入临床。以干细胞技术为核心的再生医学,将成为继药物治疗、手术治疗后的另一种疾病治疗途径。国内于2013年3月下发征求意见稿的《干细胞临床试验研究管理办法(试行)》、《干细胞临床试验研究基地管理办法(试行)》和《干细胞制剂质量控制和临床前研究指导原则(试行)》在年内有望发布正式文件。对于指导科学遵循医学伦理学,严谨进行干细胞试验,促进干细胞技术从实验室向应用转化,形成新的干细胞研究和产业格局,将具有积极的推动作用。
1 Friedenstein AJ,Petrakova KV,Kurolesova AI,et al.Heterotopic of bone marrow.Analysis of precursor cells for osteogenic and hematopoietic tissues[J].Transplantation,1968,6(2):230-247.
2 Friedenstein AJ,Chailakhjan RK,Lalykina KS,et al.The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells[J].Cell Tissue Kinet,1970,3(4):393-403.
3 Caplan AI.Mesenchymal stem cells[J].J Orthop Res,1991,9(5):641-650.
4 Pittenger MF,Mackay AM,Beck SC,et al.Multilineage potential of adult human mesenchymal stem cells[J].Science,1999,284(5411):143-147.
5 Horwitz EM,Le Blanc K,Dominici M,et al.Clari fi cation of the nomenclature for MSC:The International Society for Cellular Therapy position statement[J].Cytotherapy,2005,7(5):393-395.
6 Dominici M,Le Blanc K,Mueller I,et al.Minimal criteria for defining multipotent mesenchymal stromal cells.The International Society for Cellular Therapy position statement[J].Cytotherapy,2006,8(4):315-317.
7 Jones EA,Kinsey SE,English A,et al.Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells[J].Arthritis Rheum,2002,46(12):3349-3360.
8 Sacchetti B,Funari A,Michienzi S.Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment[J].Cell,2007,131(2):324-336.
9 Zhou BO,Yue R,Murphy MM,et al.Leptin-receptorexpressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow[J].Cell Stem Cell,2014,15(2):154-168.
10 Erices A,Conget P,Minguell JJ,et al.Mesenchymal progenitor cells in human umbilical cord blood[J].Br J Haematol,2000,109(1):235-242.
11 Gronthos S,Mankani M,Brahim J,et al.Postnatal human dental pulp stem cells (DPSCs)in vitroandin vivo[J].Proc Natl Acad Sci USA,2000,97(25):13625-13630.
12 De Bari C,Dell'Accio F,Tylzanowski P,et al.Multipotent mesenchymal stem cells from adult human synovial membrane[J].Arthritis Rheum,2001,44(8):1928-1942.
13 Zuk PA,Zhu M,Ashjian P,et al.Human adipose tissue is a source of multipotent stem cells[J].Mol Biol Cell,2002,13(12):4279-4295.
14 In't Anker PS,Scherjon SA,Kleijburg-van der Keur C,et al.Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta[J].Stem Cells,2004,22(7):1338-1345.
15 Wang HS,Hung SC,Peng ST,et al.Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord[J].Stem Cells,2004,22(7):1330-1337.
16 Sarugaser R,Lickorish D,Baksh D,et al.Human umbilicalcord perivascular (HUCPV) cells: a source of mesenchymal progenitors[J].Stem Cells,2005,23(2):220-229.
17 Shih DT,Lee DC,Chen SC,et al.Isolation and characterization of neurogenic mesenchymal stem cells in human scalp tissue[J].Stem Cells,2005,23(7):1012-1020.
18 Nadri S,Soleimani M.Comparative analysis of mesenchymal stromal cells from murine bone marrow and amniotic fl uid[J].Cytotherapy,2007,9(8):729-737.
19 Patki S,Kadam S,Chandra V,et al.Human breast milk is a rich source of multipotent mesenchymal stem cells[J].Hum Cell,2010,23(2):35-40.
20 Yoshimura K,Shigeura T,Matsumoto D,et al.Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates[J].J Cell Physiol,2006,208(1):64-76.
21 Park CH,Bae SH,Kim HY,et al.A pilot study of autologous CD34-depleted bone marrow mononuclear cell transplantation via the hepatic artery in fi ve patients with liver failure[J].Cytotherapy,2013,15(12):1571-1579.
22 Wan Z,You S,Rong Y,et al.CD34+hematopoietic stem cells mobilization,paralleled with multiple cytokines elevated in patients with HBV-related acute-on-chronic liver failure[J].Dig Dis Sci,2013,58(2):448-457.
23 Zheng L,Chu J,Shi Y,et al.Bone marrow-derived stem cells ameliorate hepatic fibrosis by down-regulating interleukin-17[J].Cell Biosci,2013,3(1):46.
24 Xu L,Gong Y,Wang B,et al.Randomized trial of autologous bone marrow mesenchymal stem cells transplantation for hepatitis B virus cirrhosis: regulation of Treg/Th17 cells[J].J Gastroenterol Hepatol,2014,29(8): 1620-1628.
25 Wang L,Han Q,Chen H,et al.Allogeneic bone marrow mesenchymal stem cell transplantation in patients with UDCA-resistant primary biliary cirrhosis[J].Stem Cells Dev,2014,23(20):2482-2489.
26 Salama H,Zekri AR,Medhat E,et al.Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV positive patients with end stage liver disease[J].Stem Cell Res Ther,2014,5(3):70.
27 Bruckner S,Tautenhahn HM,Winkler S,et al.A fat option for the pig: hepatocytic differentiated mesenchymal stem cells for translational research[J].Exp Cell Res,2014,321(2):267-275.
28 Parekkadan B,van Poll D,Suganuma K,et al.Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure[J].PLoS One,2007,2(9):e941.
29 Hodgkinson CP,Naidoo V,Patti KG,et al.Abi3bp is a multifunctional autocrine/paracrine factor that regulates mesenchymal stem cell biology[J].Stem Cells,2013,31(8): 1669-1982.
30 Alvarez-Dolado M,Martínez-Losa M.Cell fusion and tissue regeneration[J].Adv Exp Med Biol,2011,713:161-175.
31 Ranganath SH,Levy O,Inamdar MS,et al.Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease[J].Cell Stem Cell,2012,10(3):244-258.
32 Liotta F,Angeli R,Cosmi L,et al.Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling[J].Stem Cells,2008,26(1):279-289.
33 Waterman RS,Tomchuck SL,Henkle SL,et al.A new mesenchymal stem cell(MSC) paradigm: polarization into a pro-in fl ammatory MSC1 or an Immunosuppressive MSC2 phenotype[J].PLoS One,2010,5(4):e10088.
34 Ren G,Zhang L,Zhao X,et al.Mesenchymal stem cellmediated immunosuppression occurs via concerted action of chemokines and nitric oxide[J].Cell Stem Cell,2008,2(2):141-150.
35 Li W,Ren G,Huang Y,et al.Mesenchymal stem cells:a double-edged sword in regulating immune responses[J].Cell Death Differ,2012,19(9):1505-1513.
36 Melief SM,Schrama E,Brugman MH,et al.Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages[J].Stem Cells,2013,31(9):1980-1991.
37 Eggenhofer E,Hoogduijn MJ.Mesenchymal stem celleducated macrophages[J].Transplant Res,2012,1(1):12.
38 Le Blanc K,Mougiakakos D.Multipotent mesenchymal stromal cells and the innate immune system[J].Nat Rev Immunol,2012,12(5):383-396.
39 Wang Y,Weil BR,Herrmann JL,et al.MEK,p38,and PI-3K mediate cross talk between EGFR and TNFR in enhancing hepatocyte growth factor production from human mesenchymal stem cells[J].Am J Physiol Cell Physiol,2009,297(5):1284-1293.
40 Wang Y,Wang M,Ararbanell AM,et al.MEK mediates the novel cross talk between TNFR2 and TGF-EGFR in enhancing vascular endothelial growth factor (VEGF) secretion from human mesenchymal stem cells[J].Surgery,2009,146(2):198-205.
41 Crisostomo PR,Wang Y,Markel TA,et al.Humanmesenchymal stem cells stimulated by TNF-alpha,LPS,or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism[J].Am J Physiol Cell Physiol,2008,294(3):C675-682.
42 Jones S,Horwood N,Cope A,et al.The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells[J].J Immunol,2007,179(5): 2824-2831.
43 Choi H,Lee RH,Bazhanov N,et al.Antiinflammatory protein TSG-6 secreted by activated MSC attenuates zymosan-induced mouse peritonitis by decreasing TLR2/ NF-kB signaling in resident macrophages[J].Blood,2011,118(2):330-338.
44 Nemeth K,Mayer B,Mezey E,et al.Modulation of bone marrow stromal cell functions in infectious diseases by tolllike receptor ligands[J].J Mol Med (Berl),2010,88(1):5-10.
45 Delarosa O,Dalemans W,Lombardo E.Toll-like receptors as modulators of mesenchymal stem cells[J].Front Immunol,2012,3:182.
46 Ortiz LA,Dutreil M,Fattman C,et a1.Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury[J].Proc Natl Acad Sci USA,2007,104(26):11002-11007.
47 Parekkadan B,van-poll D,Suganuma K,et a1.Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure[J].PLoS ONE,2007,2(9):e941.
48 van Poll D,Parekkadan B,Cho CH,et a1.Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regenerationin vitroandin vivo[J].Hepatology,2008,47(5):1634-1643.
49 Iimuro Y,Nishio T,Morimoto T,et al.Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat[J].Gastroenterology,2003,124(2):445-458.
50 Siller-López F,Sandoval A,Salgado S,et al.Treatment with human metalloproteinase-8 gene delivery ameliorates experimental rat liver cirrhosis[J].Gastroenterology,2004,126(4):1122-1133.
51 Higashiyama R,Inagaki Y,Hong YY,et al.Bone marrowderived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice[J].Hepatology,2007,45(1):213-222.
52 Zhao W,Li JJ,Cao DY,et al.Intravenous injection of mesenchymal stem cells is effective in treating liver fi brosis[J].World J Gastroenterol,2012,18(10):1048-1058.
53 Carvalho AB,Quintanilha LF,Dias JV,et al.Bone marrow multipotent mesenchymal stromal cells do not reduce fi brosis or improve function in a rat model of severe chronic liver injury[J].Stem Cells,2008,26(5):1307-1314.
54 Churchman SM,Ponchel F,Boxall SA,et al.Transcriptional profile of native CD271+multipotential stromal cells: evidence for multiple fates,with prominent osteogenic and Wnt pathway signaling activity[J].Arthritis Rheum,2012,64(8):2632-2643.
55 Sacchetti B,Funari A,Michienzi S,et al.Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment[J].Cell,2007,131(2): 324-336.
56 Tormin A,Li O,Brune JC,et al.CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization[J].Blood,2011,117(19): 5067-5077.
57 Daquinag AC,Zhang Y,Amaya-Manzanares F,et al.An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells[J].Cell Stem Cell,2011,9(1):74-86.
58 Spaeth E,Klopp A,Dembinski J,et al.Inflammation and tumor microenvironments:definingthe migratory itinerary of mesenchymal stem cells[J].Gene Therapy,2008,15(10): 730-738.
59 Ben-David U,Mayshar Y,Benvenisty N.Large-scale analysis reveals acquisition of lineage-speci fi c chromosomal aberrations in human adult stem cells[J].Cell Stem Cell,2011,9(2):97-102.
Advances in treatment of liver disease with mesenchymal stem cells
Jin Yinpeng,Fu Qingchun,Chen Chengwei.Shanghai Liver Disease Research Center,Nanjing Military Command,Shanghai 200235,China
Chen Chengwei,Email: ccw2@163.com
Mesenchymal stem cell(MSC) are adult stem cells with self-renewal and multipotent differentiation capacity.Rich source and low immunogenicity of MSC enable their clinical application for damaged liver tissue.Improvement of symptoms and survival rate of patients has been observed.MSC play a therapeutic role in liver diseases by modulating local and systemic inflammatory response and immune dysfunction.This review focuses on the current status of MSC in treating liver diseases.
Mesenchymal stem cell;liver disease;mechanism
2014-09-23)
(本文编辑:蔡晓珍)
10.3877/cma.j.issn.2095-1221.2014.04.011
200235 上海,解放军第八五医院 上海南京军区临床肝病中心
陈成伟,Email: ccw2@163.com
金银鹏,傅青春,陈成伟,等.间充质干细胞在肝脏疾病应用的研究现况[J/CD].中华细胞与干细胞杂志:电子版,2014,4(4):277-283.
