基于复杂多壁碳纳米管衍生物为载体的痕量铝离子电极的研究及其在环境中的应用
2014-04-27贾峰柴雅琴
贾峰,柴雅琴
(1.运城市疾病预防控制中心,山西运城 044100)
(2.西南大学化学化工学院,重庆 400715)
基于复杂多壁碳纳米管衍生物为载体的痕量铝离子电极的研究及其在环境中的应用
贾峰1,柴雅琴2*
(1.运城市疾病预防控制中心,山西运城 044100)
(2.西南大学化学化工学院,重庆 400715)
该文报道了一种新的基于复杂多壁碳纳米管衍生物为载体的铝离子碳糊电极。引入的碳糊电极,在很大程度上改善了铝离子电极的线性范围、检出限、pH值范围及响应时间等。此外,该电极具有较高选择性和较长的使用时间(超过3个月)。该文课题组选择分离溶液法(SSM)测定铝离子碳糊电极的选择性系数,该电极对铝离子具有很好的选择性。重要的是,该文课题组采用交流阻抗法和紫外-可见光谱技术研究了电极的响应机理。最后,该电极被成功地应用于环境样品中铝离子浓度的测定。
离子选择性电极;碳糊电极;多壁碳纳米管;纳米金
0 引言
铝占地壳质量的8%,是自然界中含量丰富的金属元素之一。伴随着科技发展和生活水平的不断提高,愈来愈多的铝制品广泛的应用于生产和生活当中,由于铝的熔点比较低质地软,所以是饮料的理想材料[1~3]。过去的20年已经有许多研究重点关注生命体的毒理作用,在人体中铝被认为在帕金森疾病、阿尔茨海默尔疾病、透析方便扮演很重要的角色[4~8]。骨质疏松产生的原因之一是铝的过量,并且过量的铝能够改变血脑屏障的功能,会严重影响人体健康[9~10]在医学研究中已经得到证明。能够实现对铝检测的方法主要有火焰原子吸收(FAAS)[11~14]、溶出伏安法[15~16]、电感耦合等离子体发射光谱法(ICP-OES)[17~18]、电热原子吸收光谱法(ET-AAS)[19~21]、分光光度法[22~23]、电感耦合等离子体质谱(ICP-MS)[24]。但是以上所述的方法操作复杂、价格昂贵、费时。所以,工业生产中对铝愈来愈多的使用以及铝会严重危及人体健康的问题,急需来建立一种快速灵敏、经济、方便的分析手段。
与之前测定铝的研究的PVC膜离子选择性电极相比碳糊电极较具有低的阻抗,稳定的响应,不需要内充溶液等优点[25~32],从而引起了愈来愈多的科学工作者的关注。在之前的报道中,应用不同载体构建的铝离子选择性电极已相继报道[33~34]。然而,这些已报道的铝离子电极大部分都存在线性范围不宽、受其它阳离子的干扰等缺点。该文中,该文课题组利用半胱氨酸(C)和羧基化的多壁碳纳米管(MWCNTs)以及纳米金成功的合成了半胱氨酸功能化的多壁碳纳米管(Au-C-g-MWCNTs)(见图1),成功地构建了高选择性高灵敏度的铝离子碳糊电极。拥有较大的比表面积、良好的导电性是多壁碳纳米管(MWCNTs)的特性,从而能够结合更多的C。同时,Au-C-g-MWCNTs具有供电子原子N、S、O的存在为构建的疏水环境和高度稳定的配体提供了有利条件。因而,可以很好的改善电极的选择性以及灵敏度。通过实验表明:该文中构建的以Au-C-g-MWCNTs为载体的铝离子电极克服了已报道其它铝离子电极的缺陷,很好的改善了电极的性能。
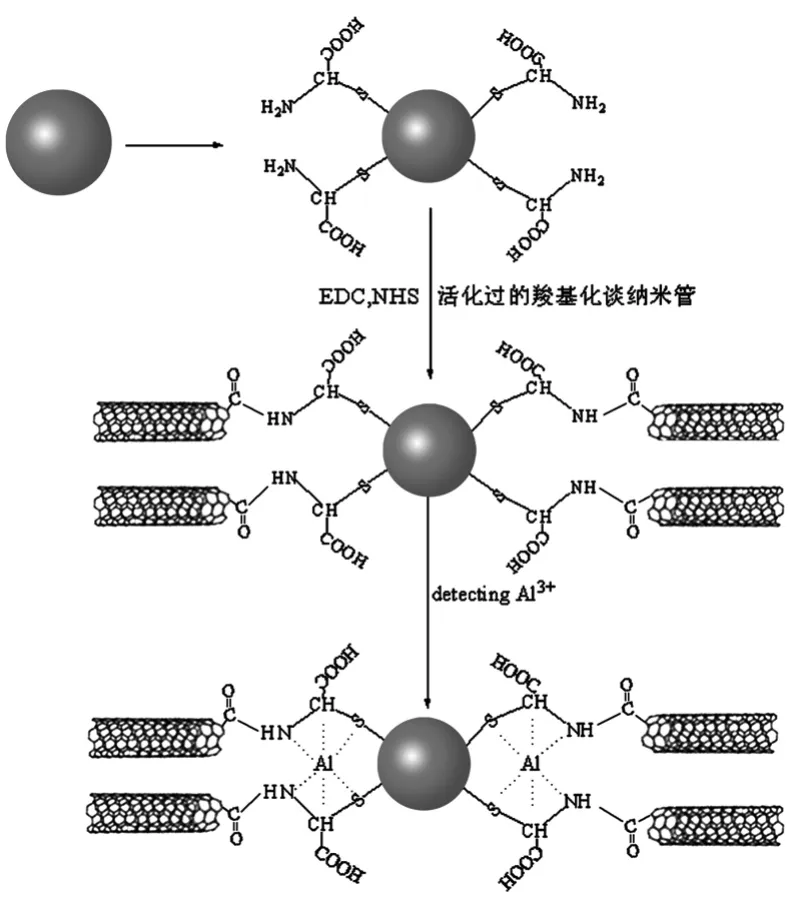
图1 载体(Au-C-g-MWCNTs)的制备方法Fig.1Au-C-g-MWCNTs were prepared according to the above procedures
1 实验部分
1.1 仪器与试剂
马釜炉(上海精密仪器公司),紫外–可见分光光度计(美国PE公司LaMbda17),傅里叶红外光谱仪(Mattson RK-6000,美国Perkin-Eimer公司),MP230酸度计(瑞士Mettler-Toledo公司);PHS-3C型离子酸度计(上海大中分析仪器厂);IM6e型交流阻抗测试系统(德国Zahner Elektrik公司);JBZ-14H型磁力搅拌器(上海大中分析仪器厂)。MWCNTs(纯度>95%,成都有机化学股份有限公司),半胱氨酸(分析纯,重庆市北碚区川东试剂厂),石墨粉(1~2 μm粒径,Sigma-aldrich Co.,中国上海),石蜡油(试剂纯,中国医药集团上海化学试剂有限公司),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(EDC)和N-羟基琥珀酰亚胺(NHS)(分析纯,重庆化学试剂厂),硝酸铁(分析纯,上海试剂一厂),甲醇(分析纯,重庆东方化学试剂厂)。
所用其它试剂均为分析纯,所用水为二次去离子水经KMnO4处理重蒸馏。
1.2 载体的合成
纳米金的制备(20~30 nm)按照文献[35]制备。
MWCNTs的活化:用甲醇做溶剂,准确称量0.1 g MWCNTs置于烧杯中,溶解一定量的1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(EDC)和N-羟基琥珀酰亚胺(NHS)(控制EDC和NHS的摩尔比为1∶1)。超声连续进行超声8 h,用离心机离心过滤,自然晾干。
纳米金半胱氨酸多壁碳纳米管衍生物(Au-C-g-MWCNTs)的制备:将10 mL纳米金溶胶(20~30 nm)与过量的半胱氨酸置于烧杯中在室温(25℃下搅拌6 h,将反应物经过离心过滤,晾干制得纳米金与半胱氨酸的复合物(Au-C)。把10 mL经过羧基化的碳纳米管置于烧杯中,用水做溶剂,溶解一定量的1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(EDC)和N-羟基琥珀酰亚胺(NHS)(控制EDC和NHS的摩尔比为1∶1),用超声连续不断进行超声8 h,反应物经过离心机的离心,然后过滤自然晾干。将已经经过处理好的羧基化多壁碳纳米管加入到溶解有Au-C水溶液中,用超声仪连续进行超声处理12 h,用离心机对上述物质进行离心,将上层悬浮液缓慢的倒去过滤,烘干(低温),成功制得实验所需的载体Au-C-g-MWCNTs(图1)。
IR(KBr,cm-1):3 420(cm-1,νN-H),1 584(cm-1, νC=O),1 639(cm-1,νCOOH),2 585(cm-1,νS-H)。
1.3 电极的制备
用适量的石墨粉与Au-C-g-MWCNTs放入一个10 mL的小烧杯中,加入适量的丙酮溶液,用超声波连续进行超声30 min让物质充分混合均匀,然后将混合物晾干。将适量的石蜡油加入到晾干的混合物中然后将其研磨均匀制成碳糊,把碳糊填进到以预先打磨平整的一次性注射器里(内径为3 mm,高为3 cm),压紧混合物,插入铜丝,用软纸将制备好的电极底部打磨光滑。实验中制备好的电极在使用前,在pH=3.0稀硝酸溶液中连续进行24 h浸泡,实验中用饱和甘汞电极作为参比电极,试验中制备的电极电位的测定用以下化学电池来实现:
Hg–Hg2Cl2,KC1(饱和)|测试液||碳糊电极|Cu
电极的电位在25℃条件下测定,活度的计算遵循Debye–Hückel定律[36]
2 电极的结果与讨论
2.1 Au-C-g-MWCNTs为载体制备的电极对不同阳离子的响应
检测实验中制备的载体Au-C-g-MWCNTs与众多金属离子的电位响应,实验中用Au-C-g-MWCNTs作为载体构建了工作电极来检测金属离子的电位。从图2中可以清楚的知道,相比于其它种类的金属离子,电极对Al3+显示了良好的响应性能。众所周知,电极的响应性能与载体的活性以及电极的组成密切相关。鉴于此,实验中研究了电极的组成对其响应性能的影响。以Au-C-g-MWCNTs为载体制备了Au-C-g-MWCNTs含量不同的电极,在25℃在pH=3.0铝离子的溶液的浓度范围为6.0×10-8~1.0×10-4mol/L中,对不同组成的电极对铁离子的电位响应性能进行了探究,实验结果列于表1中。由表1可知,含有质量分数为9.9%的载体Au-C-g-MWCNTs,质量分数为74.2%的石墨粉和质量分数为15.9%的石蜡油的电极对铁离子表现出了极其优异的响应性能。线性范围为6.0×10-8~ 1.0×10-4mol/L,检测下限为3.6×10-8mol/L,其斜率为18.1 mV/dec。电极在实验中用于进一步研究。
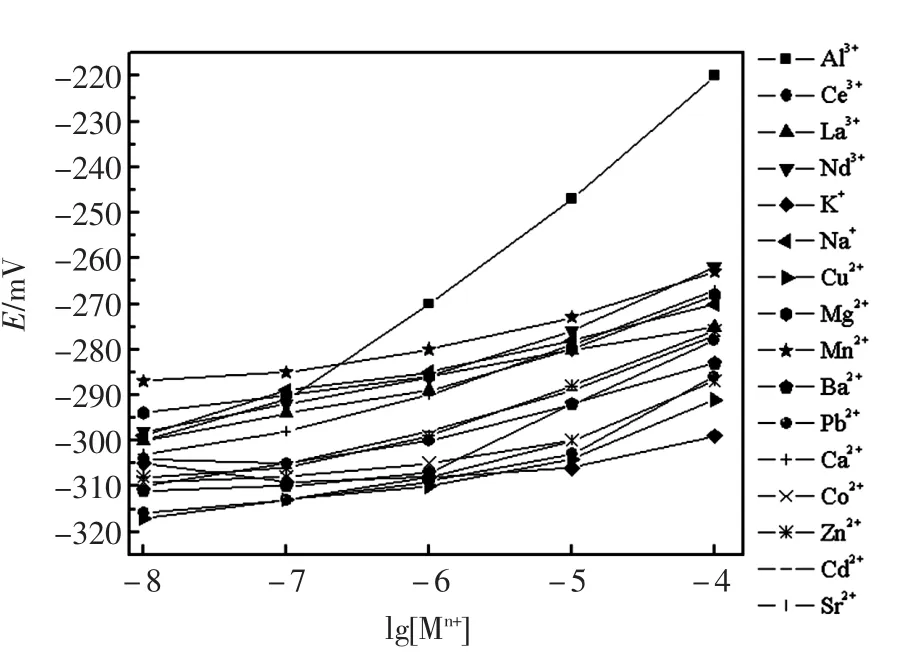
图2 电极对不同离子的选择性Fig.2Potential responses of different ion-selective electrodes
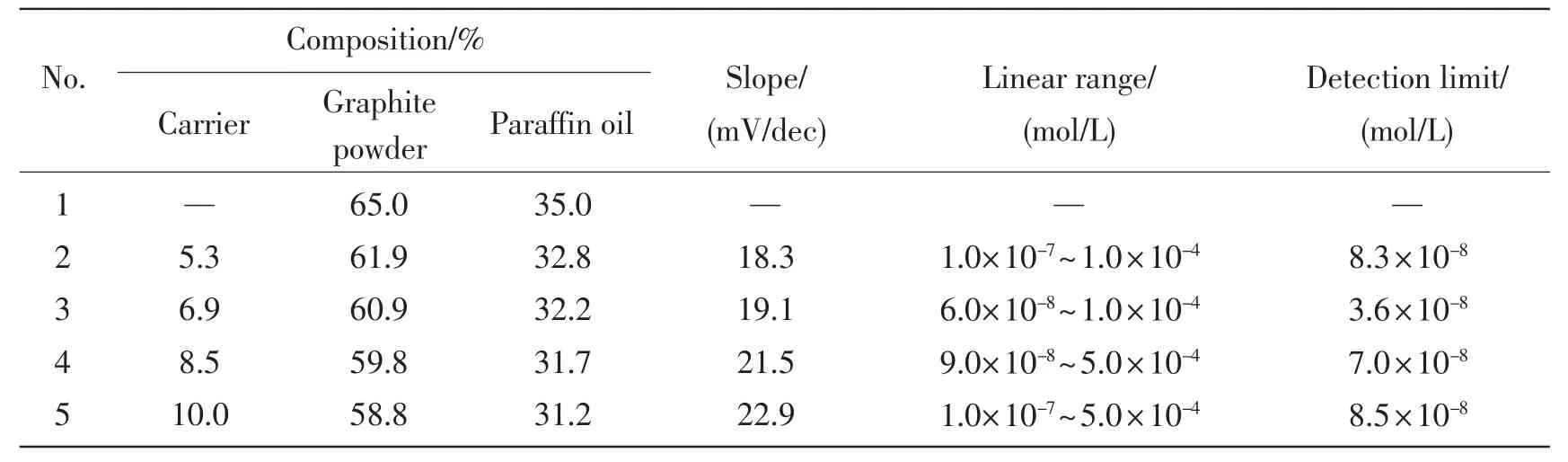
表1 碳糊电极组分的影响Tab.1The optimization of the carbon paste ingredients
2.2 电极的选择性
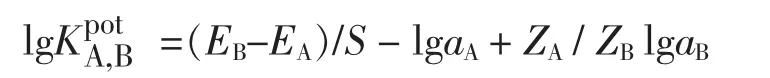
在公式中,EA代表的是1.0×10-4mol/L Al3+溶液的电位,EB代表的是1.0×10-4mol/L其它金属离子溶液的电位。用aA来表示Al3+的浓度,aB表示干扰离子的浓度。以Au-C-g-MWCNTs为载体的碳糊电极的响应斜率为S=18.9 mV/dec,ZA表示Al3+所带的电荷,ZB用来表示干扰离子所带的电荷。通过实验测得其它金属离子的选择性系数列于表2中,由表2和图2可知该电极具有极佳的选择性。
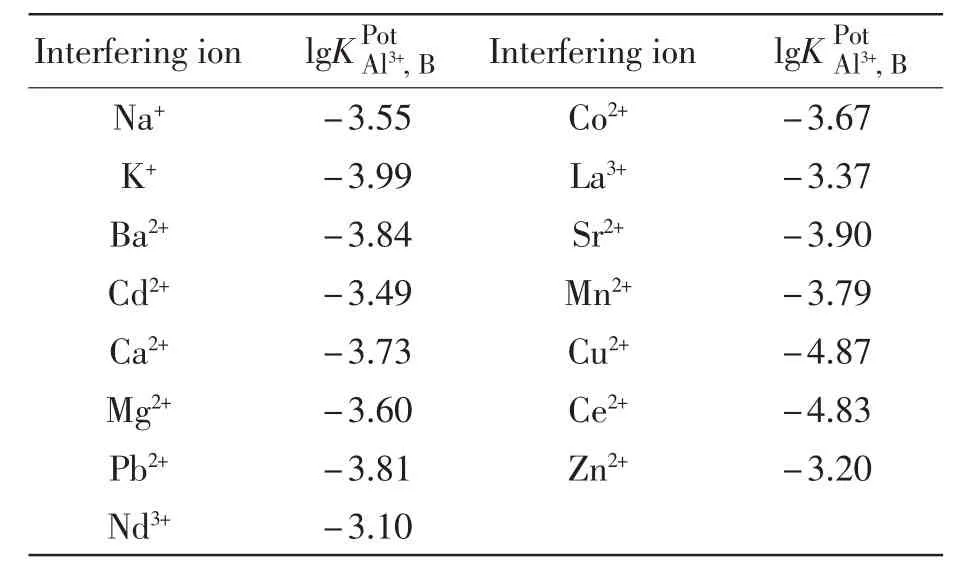
表2 电极的选择性系数Tab.2The selectivity coefficients of various interfering cations
2.3 溶液pH对电极性能的影响
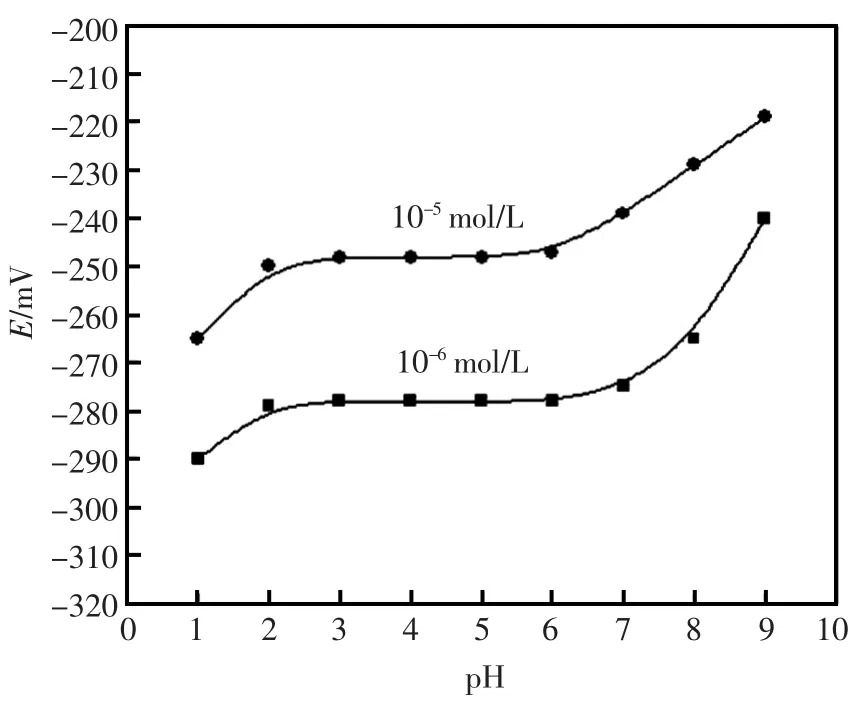
图3 电极的pH范围Fig.3Effect of pH on the response of the electrode
电极的pH使用范围是衡量电极性能的重要标准之一。该实验研究了pH1.0~10.0的Al3+离子浓度为1.0×10-5mol/L和1.0×10-6mol/L溶液,以Au-C-g-MWCNTs为载体制备的碳糊电极的pH范围。由图3可以清楚的知道,当在pH3.0~7.0的范围内电势基本保持不变,然而不在该范围之内该碳糊电极的电势有着非常明显的变化。当pH高于7.0时,电势出现明显变化的原因可能是溶液中丰富的氢氧根与溶液中的Al3+离子形成了复杂的络合物或其它化合物,在pH低于3.0时,电势发生明显变化的原因可能是溶液中氢离子浓度的增大对电极产生的严重的干扰。所以,通过实验表明适合该电极检测的pH范围是3.0~7.0。
2.4 电极的动态响应时间、稳定性、重现性
响应时间的快慢是衡量电极性能非常重要标准。该实验中测定了电极的动态响应时间是在6.0×10-8~1.0×10-4mol/L浓度范围内进行的,由图4可知,该电极在整个浓度范围内达到了响应平衡所需的时间是20 s,同时该碳糊电极的电位在5 min内基本保持不变。电极在1.0×10-5mol/L的Al(NO3)3溶液(pH=3.0)中连续进行测试了12 h,该碳糊电极的电位读数的标准偏差为1.03 mV (n=60)。
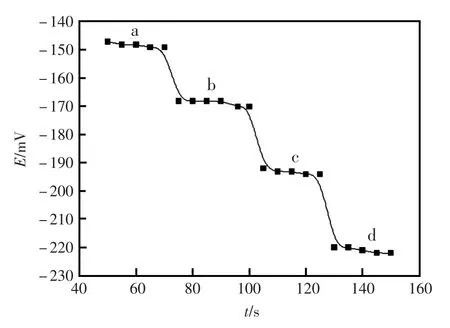
图4 电极的动态响应时间Fig.4Dynamic response time of the proposed sensor by changing the concentration of (a)1.0×10-7mol/L,(b)1.0×10-6mol/L,(c)1.0×10-5mol/L, (d)1.0×10-4mol/L
2.5 交流阻抗行为的研究
交流阻抗图5能说明电极是否与响应离子发生主客体的特异性结合。基于交流阻抗的实验,从图中可以清楚的知道Al3+参与了传输过程,同时Au-C-g-MWCNTs携带Al3+通过该碳糊电极的传输过程受到扩散控制。
2.6 紫外-可见光谱的研究
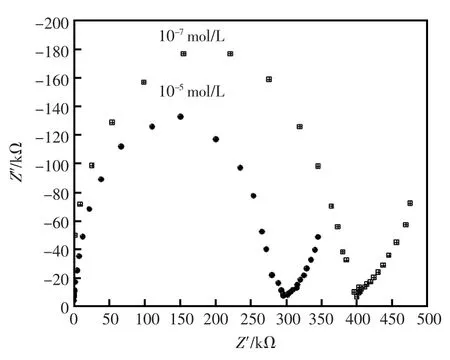
图5 电极的交流阻抗Fig.5Impedance plots of proposed sensor
该电极之所以能够对Al3+表现出优良的选择性是由于Au-C-g-MWCNTs与Al3+之间发生了特殊的作用。Au-C-g-MWCNTs中含有供电子原子N、S、O以及纳米金,同时也因为金属离子Al3+也具有空的轨道,能够接受孤对电子。用水做溶剂经过紫外分析由图6可知Al3+确实与载体发生了强烈的作用。
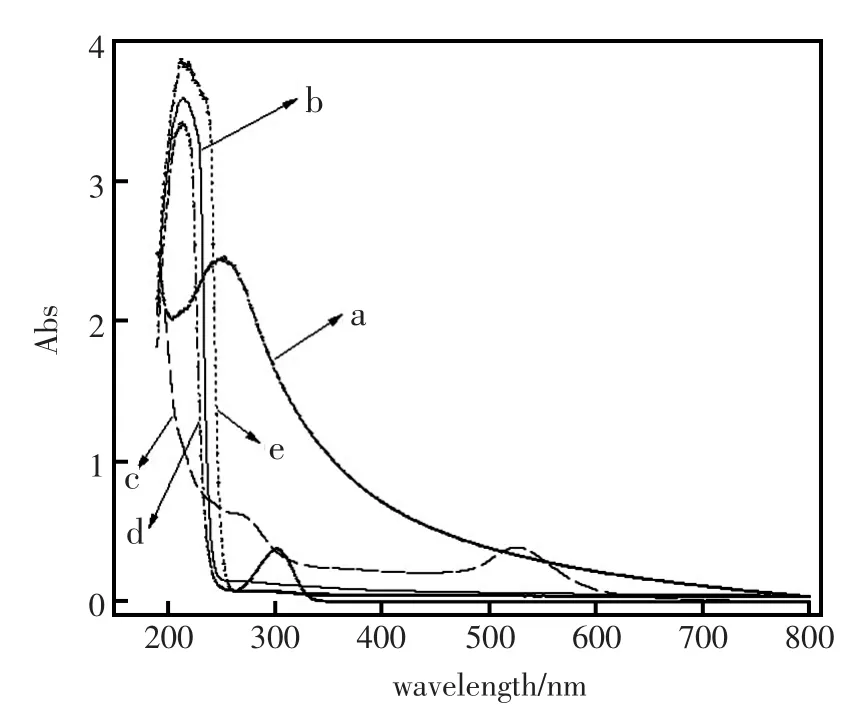
图6 紫外-可见吸收光谱图Fig.6Uv-vis absorption spectroscopy a:Au-C-g-MWCNTs,b:MWCNTs,c:Au,d:Au-C-g-MWCNTs+Al3+,e:Al3+
3 电极的初步应用
实验中用制得的电极检测水中铝离子的浓度,并且用石墨炉原子吸收光谱法的检测结果进行了比较,从表3中可以清楚的知道,用离子选择性电极法的检测结果与利用石墨炉原子吸收光谱法测定的结果基本保持一致的,该结果说明该电极能够成功的应用于实际样品中铝离子含量的检测。
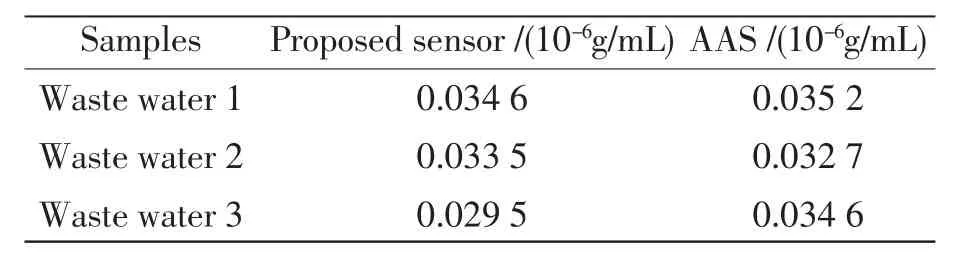
表3 水中铝离子检测Tab.3Determination of Al3+in water
4 结论
该文中成功的纳米材料引进到电化学传感器中,基于功能化的多壁碳纳米管进行修饰制得多壁碳纳米管的衍生物Au-C-g-MWCNTs,并且将其成功的用于碳糊电极的构建。以Au-C-g-MWCNTs为载体制备的电极的性能在各方面取得了较为满意的结果。通过分析研究发现,能够产生这样优异的结果可能是由于多壁碳纳米管的特异性优点,并且修饰的小分子空间位阻较小有利于金属离子的特异性结合与释放。所以,该电极有望成为铝离子痕量分析中的一名新成员。
[1]Gupta V K,Goyal R N,Jain A K,et al.Aluminium(Ⅲ)-selective PVC membrane sensor based on a Schiff base complex of N,N′-bis(salicylidene)-1,2-cyclohexanediamine[J].Electrochimica Acta,2009,54:3 218~3 224.
[2]Arancibia V,Munoz C.Determination of aluminium in water samples by adsorptive cathodic stripping voltammetry in the presence of pyrogallol red and a quaternary ammonium salt[J].Talanta,2007,73:546~552.
[3]Arvand M,Asadollahzadeh S A.Ion-selective electrode for aluminum determination in pharmaceutical substances,tea leaves and water samples[J].Talanta,2008, 75:1 046~1 054.
[4]Yari A,Darvishi L,Shamsipur M.Al(Ⅲ)-selective electrode based on newly synthesized xanthone derivative as neutral ionophore[J].Analytica Chimica Acta,2006, 555:329~335.
[5]Kawahara M,Muramoto K,Kobayashi K,et al.Aluminum promotes the aggregation of Alzheimer's amyloid bet-protein in vitro[J].Biochemical and Biophysical Research Communications,1994,198:531~535.
[6]Paik S R,Lee J H,Kim D H,et al.Aluminum-Induced Structural Alterations of the Precursor of the Non-A [beta]Component of Alzheimer's Disease Amyloid[J].Archives of Biochemistry and Biophysics,1997,344: 325~334.
[7]Lin J L,Kou M T,Leu M L.Effect of long-term low-dose aluminum-containing agents on hemoglobin synthesis in patients with chronic renal insufficiency[J].Nephron Clinical Practice,1996,74:33~38.
[8]Good P F,Olanow C W,Perl D P.Neuromelanin containing neurons of the substantia nigra accumulate iron and aluminum in Parkinson's disease:a LAMMA study[J]. Brain Research,1992,593:343~346.
[9]Yoke R A,Rhineheimer S S,Brauer R D,et al.Aluminum bioavailability from drinking water is very low and is not appreciably influenced by stomach contents or water hardness[J].Toxicology,2001,161:93~101.
[10]Alfrey A C,LeGendre G R,Kaehny W D.Thedialysis encephalopathy syndrome:Possible aluminum in-toxication [J].The New England Journal of Medicine,1976,294: 184~188.
[11]Elci L,Soylak M,Ozcan B.Coprecipitation of Cu(Ⅱ),Ni (Ⅱ),Fe(Ⅲ),Cd(Ⅱ),Pb(Ⅱ),and Co(Ⅱ)in Wastewater, Sediment,and Metallic Zinc Samples with HMDTC–HMA for Flame Atomic Absorption Spectrometric Determination[J].Analytical Letters,2003,36:987~999.
[12]Lazaru A,Stafilov T.Determination of Fe,Mn,Cu,Cr and Ni in sulfide minerals from Alshar by atomic absorption spectrometry Geol[J].Prima guerra macedonica,1993, 7:73~80.
[13]Cesur H,Bati B.Determination of copper by FAAS after pre-concentration with lead-4-methy lpiperidinedithiocarbamate on microcrystalline naphthalene by solidphase extraction[J].Analytical Letters,2000,33:489~ 501.
[14]Karatepe A U,Soylak M,Elci L.Separation/pre-concentration of copper,lead,and iron in natural water samples on chromosorb105 resin prior o flame atomic absorption spectrometric determinations[J].Analytical Letters, 2003,36:797~812.
[15]Safavi A,Maleki N,Shams E,et al.Determination of copper by adsorptive stripping voltammetry of its complex with adenine[J].Electroanalysis,2002,14:929~ 934.
[16]Safavi A,Shams E.Determination of trace amounts of copper(Ⅱ)by adsorptive stripping voltammetry of its complex with pyrogallol red[J].Analytica Chimica Acta, 1999,385:265~272.
[17]Takara A,Cabello S D P,Cerutti S,et al.On-line preconcentration/determination of copper in parenteral solutions using activated carbon by inductively coupled plasma optical emission spectrometry[J].Journal of Pharmaceutical and Biomedical Analysis,2005,39:735~ 739.
[18]Bezerra M A,Santos W N L Dos,Lemos V A,et al.Online system for preconcentration and determination of metals in vegetables by inductively coupled plasma optical emission spectrometry[J].Journal of Hazardous Materials,2007,148:334~339.
[19]Stafilov T.Determination of trace elements in minerals by electrothermalatomicabsorptionspectrometry[J]. Spectrochimica Acta Part B,2000,55:893~906.
[20]Zendelovska D,Pavlovska G,Cundeva K,et al.Electrothermal atomic absorption spect-rometric determination of cobalt,copper,lead and nickel traces in aragonite following flotation and extraction separation[J].Talanta, 2001,54:139~146.
[21]Lazaru A,Stafilov T.Determination of copper in dolomite by electrothermal atomic absorption spectrometr[J]. American Laboratory,1997,6:101~103.
[22]Li Q,Zhao X H,Jiang K,et al.Study of spectrophotometric method for determination of trace copper after the separation and enrichment with solid phase extractantmicrocrystalline phenolphthalein[J].Chinese Science Bulletin,2007,52:65~70.
[23]Kiriyama T,Kurada R.Anion-exchange enrichment and spectrophotometric determination of traces of gallium in natural waters[J].Fresenius'Journal of Analytical Chemistry,1988,332:338~340.
[24]Pozebon D,Dressler V L,Curtius A J.Determination of copper,cadmium,lead,bismuth,selenium(Ⅳ)in seawater by electrothermal vaporization inductively coupled plasma mass spectrometry after on-line separation[J]. Journal of Analytical Atomic Spectrometry,1998,13: 363~369.
[25]Bakker E,Qin Y.Electrochemical sensors[J].Analytical Chemistry,2006,78:3 965~3 984.
[26]Svancara I,Vytras K,Kalcher K,et al.Carbon paste electrodes in facts,numbers,and notes:A review on the occasion of the 50-years jubilee of carbon paste in electrochemistry and electroanalysis[J].Journal of Electroanalytical Chemistry,2009,21:7~28.
[27]Mikysek T,Svancara I,Kalcher K,et al.New approaches to the characterization of carbon paste electrodes using the ohmic resistance effect and qualitative carbon paste indexes[J].Analytical Chemistry,2009,81:6 327~ 6 333.
[28]Tesarova E,Baldrianova L,Hocevar S B,et al.Anodic stripping voltammetric measurement of trace heavy metals at antimony film carbon paste electrode[J].Electrochimica Acta,2009,54:1 506~1 510.
[29]Javanbakht M,Ganjali M R,Norouzi P,et al.Carbon paste electrode modified with functionalized nanoporous silica gel as a new sensor for determination of silver ion [J].Journal of Electroanalytical Chemistry,2007,19: 1 307~1 314.
[30]Javanbakht M,Badiei A,Ganjali M R,et al.Use of organofunctionalized nanoporous silica gel to improve the lifetime of carbon paste electrode for determination of copper(Ⅱ)ions[J].Analytica Chimica Acta,2007,601: 172~182.
[31]Goyal R N,Oyama M,Gupta V K,et al.Sensors for 5-hydroxytryptamine and 5-hydroxyindole acetic acid based on nanomaterial modified electrodes[J].Sensors and Actuators B,2008,134:816~821.
[32]Gholivand M B,Ahmadi F,Rafiee E.A Novel Al(Ⅲ)-Selective Electrochemical Sensor Based on N,N′-Bis (salicylidene)-1,2-phenylenediamine Complexes[J]. Electroanalysis,2006,18:1 620~1 626.
[33]Takashi I,Chihiro G,Keiichi N.Lanthanoid ion-selective solvent polymeric membrane electrode based on 1-phenyl-3-methyl-4-octadecanoyl-5-pyrazolone[J]. Analytica Chimica Acta,2001,443:41~51.
[34]Abdollah Y,Leila D,Mojtaba S.Al(Ⅲ)-selective electrode based on newly synthesized xanth one derivative as neutral ionophore[J].Analytica Chimica Acta,2006, 555:329~335.
[35]曾琦斐.纳米金的制备及其应用研究[J].嘉应学院学报(自然科学),2011,29:54~59.
[36]Kamata S,Bhale A,Fukunaga Y,et al.Copper(Ⅱ)-selective electrode using thorium disu-lfide nutral carrier [J].Analytical Chemistry,1988,60:2 464~2 467.
[37]Umezawa Y,Umezawa K,Sato H.Recommended methods for reporting Kvalues commission on analytical nomenclature[J].Pure and Applied Chemistry,1995, 67:507~518.
Studies on derivatized multi-walled carbon nanotubes application on Al3+carbon paste electrode
Jia Feng1,Chai Ya-Qin2*
(1.Yuncheng Center For Disease Control and Prevention,Yuncheng 044100,China)
(2.College of Chemistry and Chemical Engineering,Southwest University,Chongqing 400715,China)
A new derivatized multi-walled carbon nanotubes–based Al3+carbon paste electrode is reported.The introduce of carbon nanotubes in the electrode,have largely improved the performance of the Al3+electrode,such as Nernstian linear range,detection limit,pH range,response time.Moreover,it also shows a high selectivity and a long life time(more than 3 months).The selectivity coefficients of Al3+carbon paste electrode were determined by the separate solution method(SSM).The electrode is selective towards Al3+ion over a lot of other cations,as the selectivity coefficient value is slightly higher.Importantly,the response mechanism of the proposed electrode was investigated by using AC impedance and UV-visible spectroscopic techniques.Finally,the electrode was successfully applied for the determination of Al3+ion concentration in environmental samples.
ion-selective electrodes(ISEs);carbon electrode;multi-walled carbon nanotubes;nano gold
*通讯联系人,E-mail:yqchai@swu.edu.cn
