白光LED用全色荧光粉Ba1.3Ca0.65-xSiO4:0.02Eu2+,0.03Mn2+,xGd3+的研究
2014-03-25陈永杰耿秀娟
黄 志, 陈永杰, 陈 琳, 耿秀娟, 杨 英
(沈阳化工大学 辽宁省稀土化学及应用重点实验室, 辽宁 沈阳 110142)
白光LED具有体积小、耗电量低、使用寿命长、亮度高、响应快、环保等优点,被誉为第4代照明光源,是未来照明光源的发展方向.目前市场上的LED成品主要是采用“蓝光芯片InGaN+黄色荧光粉”组合成白光,但因其缺少红光部分,造成显色指数较低.而“(近)紫(外)光芯片+白光LED荧光粉”得到的白光具有颜色稳定、显色指数和流明效率高的优点,逐渐成为研究的热点[1-4].
相比铝酸盐荧光粉抗湿性差、在水溶液中极易水解的特点,硅酸盐荧光粉具有良好的化学稳定性和热稳定性,光谱覆盖范围广,发射效率高(输出量子效率高于90 %),原料价廉等优点,引起了广泛的关注.Huang C H等[5]合成了近紫外激发的(Ca0.96Eu0.01Mn0.03)4Si2O7F2暖白光荧光粉,其发射光谱由蓝光(460 nm)和橙红光(576 nm)组成,且具有较佳的发光特性.Liu W R等[6]合成了近紫外激发的新型正白光荧光粉KCaY(PO4)2:1 %Eu2+,4 %Mn2+,其发射光谱由蓝光(480 nm)和红光(652 nm)组成,发光特性为CIE(0.314,0.329)和Tc=6 507 K.李郎楷等[7-8]合成了近紫外激发的白光荧光粉BaMgSiO4:0.02Eu2+,0.03Mn2+和Ba0.905Ca0.845Mg0.25SiO4:0.02Eu2+,0.025Mn2+,其发射光包括了红绿蓝三色,可作为白光LED用全色荧光粉,且调节Mn2+浓度,能够合成不同色温的冷、暖白光色.Bandi V R等[9]制备了新型Ca3Y2Si3O12:Dy3+,Ce3+白光荧光粉,其发射光谱分别由389 nm、473 nm、580 nm组成,色坐标CIE(0.349,0.33)非常接近于自然光CIE(0.33,0.33).
在稀土离子掺杂的荧光粉研究中,加入敏化剂是提高荧光粉发光性能的有效方法之一.其中3价稀土离子Dy3+、Tb3+、Er3+、Y3+、Gd3+等作为敏化剂来改善荧光粉的发光性能,已有许多报道[10-12],但Gd3+敏化Ba1.3Ca0.65SiO4:0.02Eu2+,0.03Mn2+荧光粉的发光行为尚未见报道.为此,本文主要研究Gd3+的掺杂对Ba1.3Ca0.65SiO4:0.02Eu2+,0.03Mn2+相对发光强度的影响.
1 实 验
1.1 制备
原料:碳酸钙(CaCO3,分析纯,analytical reagent,A.R.),碳酸锰(MnCO3,A.R.),碳酸钡(BaCO3,A.R.),氧化硅(SiO2,A.R.),氧化钆(Gd2O3,99.9 %,质量分数,下同),氧化铕(Eu2O3,99.99 %).采用固相法合成荧光粉Ba1.3Ca0.65-xSiO4:0.02Eu2+,0.03Mn2+,xGd3+(x=0~6 %,摩尔比,以下同),按照化学计量比称取原料,并添加摩尔分数为7 %的氯化钡(BaCl2·2H2O,A.R.)作助熔剂,放入研钵中进行研磨,加入少量的无水乙醇使物质混合均匀,再将此混合物放入烘箱中烘干,再研磨并装入瓷舟中.将瓷舟置于高温管式炉中,程序升温到400 ℃时,开始通入H2/N2(摩尔体积Vm(H2)/Vm(N2)为1/10)还原气体,到达1 000 ℃时保温1.5 h,再自然降温至400 ℃,停止通气,取出样品进行研磨,即得到粉末样品.反应式为:
1.3BaCO3+(0.65-x)CaCO3+0.01Eu2O3+
0.03MnCO3+SiO2+0.5xGd2O3→
Ba1.3Ca0.65-xSiO4:0.02Eu,0.03Mn,xGd+
(1.98-x)CO2↑;x=0~0.06
1.2 表征
采用Bruker D8型X射线衍射(XRD)仪测试样品的晶体结构.Cu靶,管电压为40 kV,波长λ=0.154 06 nm,扫描步长0.02°.用Hitachi F-4600型荧光分光光度计测试样品的激发-发射光谱,其中狭缝2.5 nm,工作电压400 V,扫描速度1 200 nm/min.用杭州远方公司的PMS-50(增强型)紫外-可见-近红外光谱分析系统测试光谱和色温、色坐标等参数(扫描步长5 nm,激发波长365 nm).所有测试均在室温下进行.
2 结果与讨论
2.1 晶体结构分析
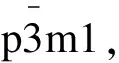

图1 Ba1.3Ca0.65SiO4:0.02Eu2+,0.03Mn2+和 Ba1.3Ca0.62SiO4:0.02Eu2+,0.03Mn2+,0.03Gd3+ 荧光粉的XRD谱图Fig.1 The XRD patterns of Ba1.3Ca0.65SiO4: 0.02Eu2+,0.03Mn2+ and Ba1.3Ca0.62SiO4: 0.02Eu2+,0.03Mn2+,0.03Gd3+ phosphors
2.2 激发光谱和发射光谱分析
图2为460 nm波长监测的荧光粉Ba1.3Ca0.65-xSiO4:0.02Eu2+,0.03Mn2+,xGd3+(x=0,3 %)的激发光谱,其激发光谱呈宽带状,主要由4个峰值283 nm、338 nm、365 nm和405 nm构成.荧光粉在275~410 nm之间有较强的吸收,与近紫外光InGaN芯片(350~410 nm)匹配.可以看出,此材料的激发光谱中存在近紫外峰,与近紫外光激发的白光荧光粉模式相符.敏化剂Gd3+的掺杂,对其峰值波长没有较大的影响,但明显增强了激发光谱的强度.波长为365 nm的芯片较283 nm、338 nm和405 nm芯片生产工艺成熟且廉价,故选用365 nm波长作为样品的激发波长.

a:Ba1.3Ca0.65SiO4:0.02Eu2+,0.03Mn2+ b:Ba1.3Ca0.62SiO4:0.02Eu2+,0.03Mn2+,0.03Gd3+
图3为365 nm激发下的不同Gd3+敏化剂掺杂的Ba1.3Ca0.65-xSiO4:0.02Eu2+,0.03Mn2+,xGd3+(x=0~6 %)荧光粉的发射光谱.可以看出荧光粉在425~560 nm的蓝绿光区域有宽的发射,归属于Eu2+的5d—4f跃迁发射,且此宽波带呈不对称分布,说明Eu2+至少存在两个发光中心,可能是Eu2+取代基质晶体结构中的不同Ba2+(Ca2+)格位而产生的.其560~700 nm的橙红光波带归属于Mn2+取代与其半径相近的Ca2+格位的4T1—6A1跃迁发射.

图3 荧光粉Ba1.3Ca0.65-xSiO4:0.02Eu2+,0.03Mn2+, xGd3+的发射光谱(x=0~6 %)Fig.3 Emission spectra of phosphors Ba1.3Ca0.65-xSiO4: 0.02Eu2+,0.03Mn2+,xGd3+ (x=0~6 %)
图4为不同Gd3+含量的Ba1.3Ca0.65-xSiO4:0.02Eu2+,0.03Mn2+,xGd3+(x=0~6 %)荧光粉的发光强度的变化关系.可以看出:Gd3+的掺入,其荧光粉的发光强度明显增强.当0

图4 不同摩尔分数的Gd3+对应样品的蓝绿光和橙红光 强度变化趋势(λex=365 nm)Fig.4 The maximum of blue emission band and orange- red emission band with different Gd3+ content
图5为460 nm波长监测下的荧光粉Ba1.3Ca0.65SiO4:0.02Eu2+,0.03Mn2+的激发光谱和365 nm波长激发下Ba1.3Ca0.68SiO4:0.02Gd3+荧光粉的发射光谱.

图5 荧光粉Ba1.3Ca0.65SiO4:0.02Eu2+,0.03Mn2+的 激发光谱(λem=460 nm)和Ba1.3Ca0.68SiO4: 0.02Gd3+的发射光谱(λex=365 nm)Fig.5 The excitation spectra of phosphor Ba1.3Ca0.65SiO4: 0.02Eu2+,0.03Mn2+ and emission spectra of Ba1.3Ca0.68SiO4:0.02Gd3+
从图5可以看出:Ba1.3Ca0.68SiO4:0.02Gd3+荧光粉几乎没有发射光谱,不可能作为荧光粉的发光中心.且两光谱几乎没有重叠,说明Gd3+不可能通过共振作用将其能量传递给Eu2+或Mn2+来提高其发光强度.敏化剂Gd3+在此荧光粉中的具体敏化过程还有待进一步研究.
2.3 光色参数分析
表1为365 nm激发下的Ba1.3Ca0.65-xSiO4:0.02Eu2+,0.03Mn2+,xGd3+(x=0~6 %)荧光粉的光色参数.制备的一系列荧光粉的色坐标落在白光区域,如图6所示.

表1 荧光粉Ba1.3Ca0.65-xSiO4:0.02Eu2+,0.03Mn2+, xGd3+ (x=0~6 %)的光色参数Table 1 Light-color parameters of Ba1.3Ca0.65-xSiO4: 0.02Eu2+,0.03Mn2+,xGd3+(x=0~6 %) phosphors

图6 荧光粉Ba1.3Ca0.65-xSiO4:0.02Eu2+, 0.03Mn2+,xGd3+的色坐标Fig.6 Color coordinates of Ba1.3Ca0.65-xSiO4:0.02Eu2+, 0.03Mn2+,xGd3+ phosphors
该系列荧光粉色温均低于5 660 K,呈暖白光(≤6 000 K),显色指数在80以上,高于市场上采用“蓝光芯片+YAG”组合的白光LED的显色指数(低于78).且当x=2 %时,荧光粉Ba1.3Ca0.63SiO4:0.02Eu2+,0.03Mn2+,0.02Gd3+的色坐标CIE(0.343 1,0.331 8)最接近正白光CIE(0.333 3,0.333 3),且有较好的色温Tc=5 010 K,显色指数Ra=81.7.当x=3 %时,Ba1.3Ca0.62SiO4:0.02Eu2+,0.03Mn2+,0.03Gd3+荧光粉有着较佳的色坐标CIE(0.328 8,0.342 8)和色温Tc=5 660 K,同时也具有高的显色指数Ra=84.3,但其发射光谱强度较x=2 %的样品低.综合考虑,此系列最佳荧光粉为Ba1.3Ca0.63SiO4:0.02Eu2+,0.03Mn2+,0.02Gd3+.
3 结 论
高温固相法制备了Gd3+敏化的Ba1.3Ca0.65-xSiO4:0.02Eu2+,0.03Mn2+,xGd3+(x=0~6 %)系列单一基质白光荧光粉.与荧光粉Ba1.3Ca0.65SiO4:0.02Eu2+,0.03Mn2+相比,敏化剂Gd3+的掺入明显提高荧光粉的发射光谱强度,较佳掺杂摩尔分数为x=2 %.发光特性较佳的Ba1.3Ca0.63SiO4:0.02Eu2+,0.03Mn2+,0.02Gd3+荧光粉的色坐标CIE(0.343 1,0.331 8)与正白光CIE(0.333 3,0.333 3)相近,暖色温Tc=5 010 K,显色指数Ra=81.7,具有潜在的应用前景.
参考文献:
[1] Zhang X M,Li W L,Seo H J.A Study of the Luminescence and Energy Transfer in Ba1.6Ca0.4P2O7Codoped with Eu2+and Mn2+[J].Phys.Status.Solidi A,2011,208(12):2819-2823.
[2] Wu L,Zhang Y,Gui M G,et al.Luminescence and Energy Transfer of a Color Tunable Phosphor:Dy3+-,Tm3+-,and Eu3+-coactivated KSr4(BO3)3for Warm White UV LEDs[J].J.Mater.Chem.,2012,22:6463-6470.
[3] Li G,Zhang Y,Geng D,et al.Single-composition Trichromatic White-emitting Ca4Y6(SiO4)6O:Ce3+/Mn2+/Tb3+Phosphor:Luminescence and Energy Transfer[J].Appl.Mater.Interfaces,2012,4(1):296-305.
[4] Guo N,Zheng Y H,Jia Y C,et al.Warm-white-emitting from Eu2+/Mn2+-Codoped Sr3Lu(PO4)3Phosphor with Tunable Color Tone and Correlated Color Temperature[J].J.Phys.Chem.C.,2012,116(1):1329-1334.
[5] Huang C H,Chan T S,Liu W R,et al.Crystal Structure of Blue-white-yellow Color-tunable Ca4Si2O7F2:Eu2+,Mn2+Phosphor and Investigation of Color Tunability through Energy Transfer for Single-phase White-light near-ultraviolet LEDs[J].J.Mater.Chem.,2012,22,20210-20216.
[6] Liu W R,Huang C H,Yeh C W,et al.A Study on the Luminescence and Energy Transfer of Single-phase and Color-tunable KCaY(PO4)2:Eu2+,Mn2+Phosphor for Application in White-light LEDs[J].Inorg.Chem.,2012,51(18):9636-9641.
[7] 陈永杰,李郎楷,肖林久,等.用一步法合成Ba0.905Ca0.845Mg0.25SiO4:xEu2+,yMn2+白光荧光粉及其发光性能[J].硅酸盐学报,2011,39(6):908-912.
[8] 李郎楷,陈永杰,肖林久,等.白光LED用全色荧光粉BaMgSiO4:Eu2+,Mn2+的光谱性质[J].材料导报,2010,24(11):55-57.
[9] Bandi V R,Nien Y T,Chen I G.Enhancement of White Light Emission from Novel Ca3Y2Si3O12:Dy3+Phosphors with Ce3+Ion Codoping[J].J.APPL.PHYS.,2010,108(2):23111-23114.
[10] Huang Z,Chen Y J,Chen L,et al.Fluorescence Improvement of Ba1.3Ca0.7SiO4:Eu2+,Mn2+Phosphors via Dy3+Addition and their Color-tunable Properties[J].Ceramics International,2013,39:2709-2714.
[11] Koo H Y,Han J M,Kang Y C,et al.Ca7.97-xMg(SiO4)4Cl2:Eu0.03,Dx(D=Y,Gd,Mn) Phosphor Particles Prepared by Spray Pyrolysis[J].Jpn J Appl Phys,2008,47(1):163-166.
[12] 陈永杰,黄志,李郎楷,等.单一基质白光荧光粉Ba1.3Ca0.7SiO4的制备和发光特性[J].硅酸盐学报,2011,39(9):1406-1410.
[13] Koichiro F,Masamichi I,Tomoyuki I.Crystal Structure and Structural Disorder of (Ba0.65Ca0.35)2SiO4[J].J Solid Chem,2007,180(8):2305-2309.
