SELDI技术对开放性脊柱裂胎鼠羊水蛋白质组学的初步分析
2014-02-27刘振江袁正伟赵群
刘振江,袁正伟,赵群
(中国医科大学附属盛京医院1.小儿外科;2.卫生部小儿先天畸形重点实验室,沈阳110004)
SELDI技术对开放性脊柱裂胎鼠羊水蛋白质组学的初步分析
刘振江1,袁正伟2,赵群1
(中国医科大学附属盛京医院1.小儿外科;2.卫生部小儿先天畸形重点实验室,沈阳110004)
目的利用表面增强激光解析/离子化飞行时间质谱(SELDI-TOF-MS)技术对脊柱裂胎鼠模型的羊水进行初步的蛋白质组学分析。方法将25只雌性Wistar大鼠随机分为脊柱裂组(n=15)和对照组(n=10)。于妊娠第10日(E10)8时,脊柱裂组孕鼠经胃管注入全反式维甲酸与矿物油的混合物(140 mg/kg);对照组孕鼠给予等量的橄榄油。于妊娠第17日(E17)取胎鼠,立体显微镜下抽取羊水(0.2 mL/只)并确定开放性脊柱裂胎鼠。选取60只脊柱裂胎鼠和55只对照组胎鼠的羊水离心取上清。采用PBSⅡC型蛋白质芯片阅读机读取数据。采用Ciphergen protein chip 3.1.1软件对数据进行统计分析。SELDI数据结果采用分类与回归树分析建立诊断模型,用于区分脊柱裂组胎鼠与对照组胎鼠。结果脊柱裂胎鼠羊水中共检测到55个差异蛋白峰,有统计学意义的差异蛋白峰有9个。包括5种蛋白峰高表达,优化相对分子质量(m/z)分别为11 658,27 387,7 898,11 603,13 831 Da;4种蛋白峰低表达,m/z分别为5 124,14 702,5 403,13 626 Da。选取m/z 13 831 Da和m/z 17 798 Da用于建立决策分类树诊断模型,诊断灵敏度93.33%,特异度96.36%,阳性预测值96.55%,阴性预测值96.36%。结论SELDI技术可初步筛选出脊柱裂胎鼠羊水中的差异蛋白峰,这些蛋白可能是脊柱裂胎鼠羊水中的特异性羊水标志物。
神经管缺陷;脊柱裂;表面增强激光解析/离子化飞行时间质谱;羊水;蛋白标志物
先天性开放性脊柱裂是一种常见的先天畸形,也是先天性神经管缺陷(neural tube defects,NTDs)最重要的类型,其发病机制尚不明确。目前多认为是遗传因素与环境因素相互作用的结果。严重的显性脊柱裂常伴有脊髓脊膜膨出,甚至脊髓外翻,致死率和致残率很高。我国每年有8~10万NTDs患儿出生。因此,早期发现NTDs具有重要意义。母亲血清甲胎蛋白(maternal serum alpha-fetoprotein,MSAFP)和羊水甲胎蛋白(amniotic fluid alpha-fetoprotein,AFAFP)是筛查脊柱裂等NTDs的传统标记物,但是检出率较低。约75%~80%的开放性脊柱裂畸形的MSAFP水平升高[1~5]。而AFAFP的假阳性率为63%[6]。在临床已经确诊的NTDs中仅有80%病例出现AFAFP水平增高[7]。而羊水乙酰胆碱酯酶的敏感度在孕期也会降低,在具有其他诊断的病例和血液污染的病例均会出现假阳性[8,9]。本研究采用维甲酸制作脊柱裂胎鼠模型,利用表面增强激光解析/离子化飞行时间质谱(surface enhanced laser desorption/ionization time-of-flight mass spectrometry,SEDLI-TOF-MS)技术寻找脊柱裂胎鼠羊水的蛋白质谱规律,以期发现对于脊柱裂早期诊断有效的标记物。
1 材料与方法
1.1 材料
1.1.1 实验动物及分组:雌性Wistar大鼠(体质量250~280 g)25只,购自中国医科大学实验动物中心。分为脊柱裂组(n=15)和对照组(n=10)。饲养条件:室温20~25℃,湿度60%~70%,昼夜12 h循环。雌鼠和雄鼠交配后次日8时阴道涂片,有阴道栓精子者确定妊娠第0日(E0)。在妊娠第10日(E10)8时,脊柱裂组孕鼠经胃管注入全反式维甲酸与矿物油的混合物(140 mg/kg)。对照组孕鼠给予等量的橄榄油[10]。妊娠第17日(E17),给予10%水合氯醛(300 mg/kg)腹腔注射麻醉。取出胎鼠,抽取羊水(0.2 mL/只)并确定开放性脊柱裂胎鼠。选取60只脊柱裂胎鼠和55只对照组胎鼠的羊水作为研究对象。所有羊水立即于4℃离心10 min(4 000 r/ min)。吸取上清,按每管50 μL分装,置于-80℃保存备用。所有的动物实验均得到中国医科大学附属盛京医院医学伦理委员会的批准。
1.1.2 材料:三氟乙酸,醋酸钠,二硫苏糖醇,3-[3-(胆酰胺基丙基)二甲氨基]丙磺酸盐(CHAPS)、尿素、乙腈、饱和芥子酸(SPA),均购自Sigma公司。CM10蛋白质芯片购自美国Ciphergen公司。
1.2 方法
取出羊水样品,4℃融解。4℃,10 000 r/ min,离心2 min。取10 μL羊水,用20 μL U9缓冲液(9 mol/L Urea,2%CHAPS,50 mmol/L Tris-HCl,pH9.0加DTT)稀释。充分混匀样品。加入170 μL结合缓冲液。总稀释倍数为20倍。
CM10芯片每孔加入200 μL结合缓冲液(50 mmol/L NaAC,pH4.0),室温震荡(250 r/min)5 min,甩掉缓冲液。重复1次。每孔中再加入100 μL处理好的样品,室温震荡(250 r/min)60 min。甩出样品。重复2次。每孔加入200 μL去离子水,立刻甩出。芯片自然风干后,加SPA 0.5 μL;待风干后,重复加SPA 0.5 μL 1次。待干后,即可上机测定。
1.3 数据采集与分析
采用PBSⅡC型蛋白质芯片阅读机读取数据。条件设置:设定检样激光强度225,检测敏感度9。优化相对分子质量范围1~30 kDa。收集位置20~80,每5点收集10次,每个样品收集总点数是130次。在每次实验数据收集前,用All-in-one蛋白芯片校正仪器,使蛋白质分子量误差<0.1%。应用Biomarker Wizard(美国Ciphergen公司)软件分析处理所有峰谱,形成蛋白质指纹图谱。所有的图谱都进行了标准化,统一到它们自己全部的离子总和(峰面积的总和)。设有意义蛋白峰信噪比(S/N)>5,最小峰强度>1.6。分析相对分子质量(m/z)1~30 kDa
范围内的蛋白峰,计算出蛋白峰均值,标准差和变异系数(CV<10%)。采用t检验比较配对样本的蛋白峰,计算P值。P<0.05有统计学意义。
1.4 分类回归树(classification and regression tree,CART)
CART模式是应用问卷形式将数据分为2个节点。分裂数据的决策取决于在某一蛋白峰强度水平的截断值的存在或者不存在。例如,当为了将数据分成2个节点,问题是“质量A≤X吗?”。所有回答“是”的蛋白峰被划分到左侧的节点,所有回答“否”的蛋白峰被划分到右侧的节点。以此类推,直到终节点产生为止。终节点的分类取决于在这个节点的大多数样品数量的临床分组(如脊柱裂组和对照组)。
2 结果
2.1 羊水蛋白质谱分析
在m/z 1~30 kDa范围内,共检测到55个差异蛋白峰,有统计学意义的差异蛋白峰有9个。与对照组相比,脊柱裂组胎鼠的羊水中有5种蛋白峰高表达,m/z分别为11 658(图1),27 387,7 898,11 603,13 831 Da(图2);4种蛋白峰低表达,m/z分别为5 124(图3),14 702,5 403,13 626 Da(图2)。

图1 脊柱裂组羊水蛋白指纹图谱(与对照组羊水蛋白质谱相比,脊柱裂组中m/z 11 658Da的蛋白峰表达升高)Fig.1 Amniotic fluid protein mass spectrum(SELDI profile showed a peak with average mass of 11 658 Da that were up⁃regulated in spina bifida compared with healthy control amniotic fluid)

图2 脊柱裂组羊水蛋白指纹图谱(与对照组羊水蛋白质谱相比,脊柱裂组中m/z 13 831 Da的蛋白峰表达升高,13 626 Da的蛋白峰表达降低)Fig.2 Amniotic fluid protein mass spectrum(SELDI profile showed a peak with average mass of 13 831 Da that were up⁃regulated in spina bifida compared with healthy control amniotic fluid.SELDI profile showed a peak with average mass of 13 626 Da that were down⁃regulated in spina bifida compared with healthy control amniotic fluid)
2.2 羊水蛋白质谱的CART分析
训练组羊水样品通过55个蛋白峰数据构建了CART模型,m/z 13 831(图2)和17 798 Da 2个蛋白峰被自动选择作为分裂点。将115个样品分成带有3个终节点的CART(图4)。敏感度93.33%,特异度96.36%,阳性预测值96.55%,阴性预测值96.36%(表1)。
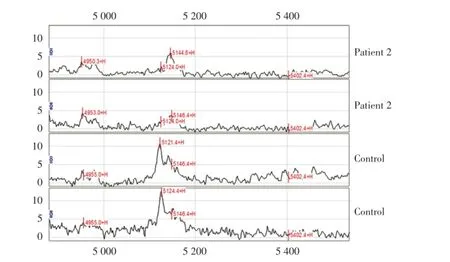
图3 脊柱裂组羊水蛋白指纹图谱(与对照组羊水蛋白质谱相比,脊柱裂组中m/z 5 124 Da的蛋白峰表达降低)Fig.3 Amniotic fluid protein mass spectrum(SELDI profile showed a peak with average mass of 5 124 Da that were down⁃regulated in spina bifida compared with healthy control amniotic fluid)

图4 在训练组病例中,脊柱裂组和对照组羊水决策分类树模式图(环形代表原始节点,方形代表终节点)Fig.4 Diagram of a decision tree for the classification of spina bifida and control amniotic fluid samples in the training dataset(circles indicated primary nodes and squares indicated terminal nodes)

表1 脊柱裂组和对照组羊水蛋白质谱的灵敏度、特异度、阳性预测值和阴性预测值Tab.1 Sensitivity,specificity,positive and negative predictive value of proteomic pattern of NTDs and control serum samples
3 讨论
NTDs的产前诊断主要依赖于超声检查,但有效的产前超声检查结果又常常受到检查者的经验、技术培训和被检查者的就诊时间等因素的限制[11]。羊水中包含多种蛋白质,这些蛋白质调节着胎儿—母体之间的相互平衡,发现蛋白质之间的失平衡将有助于诊断疾病[12]。
MSAFP和AFAFP是筛查脊柱裂等NTDs的传统标记物,能够比较敏感和特异地诊断NTDs[13]。但MSAFP[14,15]和AFAFP的特异性均较差[8]。Widlund等[6]发现AFAFP的假阳性率为63%。Kooper等[7]检测6 501例孕妇AFAFP,发现55例临床已经确诊的NTDs中仅有44例(80%)AFAFP增高,提示通过AFAFP进行NTDs的诊断筛查并不可靠。AFAFP的最佳检测时间是孕15~18周,然后随着AFAFP水平的下降其敏感度也逐渐下降[8]。AFAFP在其他的临床疾病中也会升高[16]。血液污染羊水也可导致AFAFP升高,出现假阳性[9]。羊水胆碱酯酶电泳也是一项筛查NTDs的重要方法[9]。但是乙酰胆碱酯酶电泳需要使用带有强毒性的二溴化物抑制剂来抑制特异性的乙酰胆碱,因此分析过程繁琐[17,18]。目前应用SELDI技术对羊水蛋白质组学的研究主要集中于监测羊膜早破以及羊膜腔内炎症和感染的研究诊断[12,19~24]。
为了寻找更加可靠的早期诊断NTDs的标记物,本研究首次应用SELDI-TOF-MS技术对脊柱裂胎鼠羊水进行初步的蛋白质指纹图谱描绘。结果发现:脊柱裂胎鼠羊水中共找到9个差异蛋白峰,其中有5种蛋白峰高表达,m/z分别为11 658,27 387,7 898,11 603,13 831 Da;4种蛋白峰低表达,m/z分别为5 124,14 702,5 403,13 626 Da。因此应用SELDI-TOF-MS技术能够初步筛查出脊柱裂胎鼠羊水的差异蛋白峰,这些差异蛋白峰可能是脊柱裂羊水中特异性蛋白质标志物。进一步选取13 831 Da和17 798 Da用于建立决策CART诊断模型,灵敏度93.33%,特异度96.36%,阳性预测值96.55%,阴性预测值96.36%。蛋白指纹图谱中显示的全部差异蛋白峰不能都用来建立CART。Wadsworth等[25]认为在CART的建立过程中,没有统计学差异的蛋白峰对于决策树的建立也是至关重要的。本研究验证了这一论点,虽然m/z 17 798 Da蛋白峰在羊水样品中没有统计学意义,但对于CART的构建至关重要。本研究组下一步的工作将就脊柱裂胎鼠羊水中的差异蛋白质做分离和鉴定,以期为临床早期诊断脊柱裂畸形奠定理论基础。
[1]Palomaki GE,Williams JR,Haddow JE.Prenatal screening for open neural-tube defects in Maine[J].N Engl J Med,1999,340(13):1049-1050.
[2]Burton BK,Sowers SG,Nelson LH.Maternal serum alphafetoprotein screening in North Carolina:experience with more than twelve thousand pregnancies[J].Am J Obstet Gynecol,1983,146(4):439-444.
[3]Cunningham GC,Tompkinison DG.Cost and effectiveness of the California triple marker prenatal screening program[J].Genet Med,1999,1(5):199-206.
[4]Wald NJ,Cuckle H,Brock JH,et al.Maternal serum-alpha-fetoprotein measurement in antenatal screening for anencephaly and spina bifida in early pregnancy.Report of U.K.collaborative study on alpha-fetoprotein in relation to neural-tube defects[J].Lancet,1977,1(8026):1323-1332.
[5]Cohen FL.Neural tube defects:epidemiology,detection,and prevention[J].J Obstet Gynecol Neonatal Nurs,1987,16(2):105-115.
[6]Widlund KF,Gottvall T.Routine assessment of amniotic fluid alpha-Fetoprotein in early second-trimester amniocentesis is no longer justified[J].Acta Obstet Gynecol Scand,2007,86(2):167-171.
[7]Kooper AJ,de Bruijn D,van Ravenwaaij-Arts CM,et al.Fetal anomaly scan potentially will replace routine AFAFP assays for the detection of neural tube defects[J].Prenat Diagn,2007,27(1):29-33.
[8]Muller F.Prenatal biochemical screening for neural tube defects[J].Childs Nerv Syst,2003,19(7-8):433-435.
[9]Wald N,Cuckle H,Nanchahal K.Amniotic fluid acetylcholinesterase measurement in the prenatal diagnosis of open neural tube defects.Second report of the collaborative acetylcholinesterase study[J].Prenat Diagn,1989,9(12):813-829.
[10]Zhao JJ,Sun DG,Wang J,et al.Retinoic acid-induced lumbosacral neural tube defects:myeloschisis and hamartoma[J].Childs Nerv Syst,2007,23(5):549-554.
[11]Shaer CM,Chescheir N,Erickson K,et al.Obstetrician-gynecologists’practice and knowledge regarding spina bifida[J].Am J Perinatol,2006,23(6):355-362.
[12]Tsangaris GT,Karamessinis P,Kolialexi A,et al.Proteomic analysis of amniotic fluid in pregnancies with Down syndrome[J].Proteomics,2006,6(15):4410-4419.
[13]Lopez J,Mikaelian I,Gonzalo P.Amniotic fluid glial fibrillary acidic protein(AF-GFAP),a biomarker of open neural tube defects[J].Prenat Diagn,2013,33(10):990-995.
[14]Cameron M,Moran P.Prenatal screening and diagnosis of neural tube defects[J].Prenat Diagn,2009,29(4):402-411.
[15]Shan L,Fan Y,Li H,et al.Proteomic analysis of amniotic fluid of pregnant rats with spina bifida aperta[J].J Proteomics,2012,75(4):1181-1189.[16]Seppälä M,Rapola J,Huttunen NP,et al.Congenital nephrotic syndrome:prenatal diagnosis and genetic counselling by estimation of amniotic-fluid and maternal serum alpha-fetoprotein[J].Lancet,1976,2(7977):123-125.
[17]Voigtländer T,Friedl W,Cremer M,et al.Quantitative and qualitative assay of amniotic-fluid acetylcholinesterase in the prenatal diagnosis of neural tube defects[J].Hum Genet,1981,59(3):227-231.
[18]Smith AD,Wald NJ,Cuckle HS,et al.Amniotic-fluid acetylcholinesterase as a possible diagnostic test for neural-tube defects in early pregnancy[J].Lancet,1979,1(8118):685-688.
[19]Vuadens F,Benay C,Crettaz D,et al.Identification of biologic markers of the premature rupture of fetal membranes:proteomic approach[J].Proteomics,2003,3(8):1521-1525.
[20]Thadikkaran L,Crettaz D,Siegenthaler MA,et al.The role of proteomics in the assessment of premature rupture of fetal membranes[J].Clin Chim Acta,2005,360(1-2):27-36.
[21]Buhimschi IA,Zhao G,Pettker CM,et al.The receptor for advanced glycation end products(RAGE)system in women with intraamniotic infection and inflammation[J].Am J Obstet Gynecol,2007,196(2):181.e1-181.e13.
[22]Buhimschi CS,Bhandari V,Hamar BD,et al.Proteomic profiling of the amniotic fluid to detect inflammation,infection,and neonatal sepsis[J].PLoS Med,2007,4(1):e18,0084-0094.
[23]Ruetschi U,Rosen A,Karlsson G,et al.Proteomic analysis using protein chips to detect biomarkers in cervical and amniotic fluid in women with intra-amniotic inflammation[J].J Proteome Res,2005,4(6):2236-2242.
[24]Klein LL,Freitag BC,Gibbs RS,et al.Detection of intra-amniotic infection in a rabbit model by proteomics-based amniotic fluid analysis[J].Am J Obstet Gynecol,2005,193(4):1302-1306.
[25]Wadsworth JT,Somers KD,Cazares LH,et al.Serum protein profiles to identify head and neck cancer[J].Clin Cancer Res,2004,10(5):1625-1632.
(编辑王又冬)
Amniotic Fluid Proteomic Analysisof FetalRatwith Spina Bifida Using Surface Enhanced Laser Desorption/Ionization Time-of-flight Mass Spectrometry
LIUZhen-jiang1,YUANZheng-wei2,ZHAOQun1
(1.DepartmentofPediatric Surgery,Shengjing Hospital,China MedicalUniversity,Shenyang 110004,China;2.Pediatric CongenitalDeformity Laboratory ofMinistry ofHealth,Shengjing Hospital,China MedicalUniversity,Shenyang 110004,China)
ObjectiveNeuraltube defects(NTDs)are usually identified by ultrasonography and confirmed by alpha-fetoprotein(AFP)assay and acetylcholinesterase(AchE)electrophoresis in amniotic fluid.Both of these biomarkers can be found positive in other etiologies.Amniotic fluid proteomic analysis of fetal rat with spina bifida using surface enhanced laser desorption/ionization time-of-flight mass spectrometry(SELDI-TOF-MS).
neural tube defects;congenital spina bifida aperta;surface enhanced laser desorption/ionization time-of-flight mass spectrometry;amniotic fluid;protein biomarker
R714.53
A
0258-4646(2014)01-0026-06
国家自然科学基金青年基金(81000254)
刘振江(1976-),男,副教授,博士.
赵群,E-mail:rezh2001@yahoo.com
2013-12-23
网络出版时间:
Methods25 female Wistar white rats(250-280 g)were purchased from the animal center of China Medical University.The animals were maintained in an environmentcontrolled fortemperature(20-25°C)and humidity(60%-70%)with a 12-h light/dark cycle.Adultfemale rats were mated with males of the same strain.The appearance of vaginal plugs in the female rat the morning after mating was determined E0.All animal experiments were approved by the local ethics committee.Spina bifida fetuses were induced with ATRA administration on E10.Twenty-five pregnant rats were divided into two groups,incluiding spina bifida groups(n=15)and control groups(n=10).Fifteen pregnant rats in spina bifida groups were subjected to intragastric administration of ATRA[Sigma,4%(wt/vol)in olive oil;140 mg/kg body weight]by a single gavage feeding.The same amountofolive oilwas given to the otherten pregnantrats,which were the controls.Allpregnantrats were killed on E17,and the fetusesand amniotic fluid were harvested.The fetuses were examined for spina bifida aperta,and the amniotic fluid of each fetal sac was aspirated under a stereomicroscope.We obtained 60 amniotic fluid from the fetalsacs ofembryoswith spina bifida aperta exposed to ATRA treatmentand 50 amniotic fluid from fetal sacs of normal controls.Amniotic fluid was immediately centrifuged at 4 000 r/min and 4°C for 10 minutes,aliquoted as 50 microliters,and stored at-80°C until analysis.Protein chip biosystem(PBS II C)and CM10 chip were purchased from Ciphergen Biosystems(USA).A mass spectrometerwas calibrated with chips thathad been bound with all-in-one standard proteins to setup parameters.The parametersused were:the optimal detection mass/charge size(m/z)range was between 1 and 30 kDa.The data were processed with the ProteinChip Software version 3.1.1 for t test.P<0.05 was considered statisticaIIy significant.ResultsA total of 55 qualified mass peaks(signal-to-noise ratio>5)were detected in the training set
ofratamniotic fluid between 1 and 30 kDa.Compared with the spectra ofcontrolgroups,there were 9 potentialratamniotic fluid biomarkers detected in the spectra ofthe rats with spina bifida,the protein expression washigh in 5 of9(m/z 11 658,27 387,7 898,11 603,13 831 Da)and low in the 4 of9(m/z 5 124,14 702,5 403,13 626 Da).Adecision tree classification algorithm was builtusing all55 protein peaks and 2 protein peaks at 13 831 and 17 798 Da were automatically selected as splitters.This pattern analysis process yielded a sensitivity of 93.33%,specificity of 96.36%,positive predictive value of 96.55%and negative predictive value of 96.36%.ConclusionThese discrepancy mass peaks of amniotic fluid of fetal ratwith spina bifida identified by SELD-TOF-MS may be the specific amniotic fluid protein biomarkersoffetalratswith spina bifida.
