Microbial PN1001 Remediation of Pentyl Amine and Anilin from Wastewater Pollution
2013-10-30KIMJinwooWANGLiBARRINGTONSuzelle
KIM Jin-woo, WANG Li, BARRINGTON Suzelle
(1.Mcgill University,Montreal H9X3V9,Canada;2.Shenyang university of Chemical Technology,Shenyang 110142,China)
Environmental problems are often brought about by petrochemical contaminants,being difficult to degrade in wastewater treatment facilities and by accident,leaking into the environment as a result of container failure on land and vessels breakdown at sea.To further develop its industrial potential,petrochemical companies must have the tool to confront and treat these sources of pollution.Environmental legislation is not only increasing the population’s awareness of such problems but also facilitating the introduction ofnew wastewater treatment technologies for petrochemical industry[1].Bio-control processing is a technology capable of eliminating such petrochemical pollutants.For current petrochemical wastewater treatment,the most widely used bio-control systems use aerobic,anaerobic and aerobic /anaerobic processes.
The complex bio-denitrification system(CBDS)is a process which has recently demonstrated significant potential for gentrification of this wastewater[2].Unlike other denitrification systems,CBDS operates as O/A/O(Oxidization and Nitrification/Anoxic denitrification/Oxidization)activated sludge process.This process varies the flow rate,energy input and reactor volume according to a pre-determined and periodic operating strategy.During this research a strain PN1001 was isolated from the activated sludge of CBDS.This strain had been acclimatized,reproduced using an enrichment culture and identified.It was found to degrade petrochemical wastewaters contained pentyl amine and aniline[2].Refinery wastewater can seriously affect human health and ambient conditions when discharged into river bodies.
The objective of the present research was to explore and improve a bio-system for the treatment of wastewater produced by the crude oil refineries.These wastewaters are richer in nitrogen compounds than crude oil wastewaters from other countries,because they have different geological structure conditions and components of petroleum when formed[3].The biological nitrogen degradation or bio-denitrification of petrochemical wastewater containing pentyl amine and aniline was studied by adopting various process configurations and by using the previously isolated strain PN1001.The project also investigates the characteristics of this strain and the optimal conditions leading to the acceptable biodegradation of nitrogen rich compounds found in petrochemical wastewaters.The result will help further project find a solution for the treatment of this kind of wastewater.
1 Materials and Methods
1.1 Source of Strain
A sample of activated sludge was taken from a CBDS bio-reactor treating the wastewater of an oil refinery containing pentyl amine and aniline in Northeastern China.This sludge was used as inoculum for a laboratory reactor containing pentyl amine,aniline and their mixture and allowed to ferment for one month to isolate the strain.Petrochemical wastewater containing pentyl amine and aniline was also taken from the oil refinery on two different days and analyzed using methods complying with standard methods for the examination of water and wastewater.The chemical composition of this wastewater is presented in Table 1.
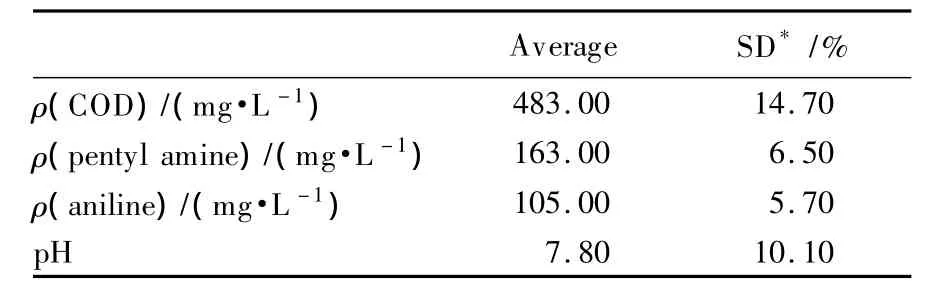
Table 1 Chemical characteristics of the pollutants
1.2 Strain Isolation
The first step consisted in preparing selective culture media made up of 0.1%(mass fraction,same as following)glucose,0.1%beef extract paste,0.05 % yeastextractpaste,0.05 %K2HPO4.Each medium received pentyl amine,aniline or a 1∶1(mass ratio)mixture of both pollutants,for a final medium concentration of 3 000 mg/L.The culture media received 1.8%to 2.0%agar gel,was sterilized and then cooled to 55 ℃.
The second step consisted in isolating the culture.A sample of 5 mL of activated sludge fermented for a month,was mixed with a solution containing either pentyl amine,aniline or their 1∶1 mixture,at a concentration of 1 000 mg/L.Then,100 mL inoculated samples were incubated at 30℃ for 8 h.Under sterile conditions,0.5 mL of the incubated samples were removed using a pipette and placed in 15 Petri dishes along with the agar described above containing pentyl amine,aniline or their 1∶1 mixture at concentrations of 1 000,1 500,2 500 and 3 000 mg/L.The petri dishes were incubated at 30℃for 48 h.
Once incubated,the petri dishes showed colonies,and those with the highest level of mono-colonies were sampled and transferred to other Petri dishes containing medium with 2 000 mg/L of pentyl amine,aniline or their 1∶1 mixture.Bacteria contained in these Petri dishes were cultured at 30℃,and their colonies were separated repeatedly until a pure strain was obtained after over 100 steps by dye check under microscope observation.
Once isolated,the strain was inoculated using a selective medium containing the pollutants at 2 000 mg/L concentration,and incubated while shaking at 30℃for 24 h.To verify that the proper strain was isolated,its degradation efficiency was assayed.The isolated strain demonstrated a strong affinity for degrading pentyl amine and aniline and was therefore named as PN1001.This strain was stored at 4℃in stock culture media by using inclined peptone beef extract paste.According to the literature[4-6]and[7],the strain was identified.
1.3 Testing for Optimal Condition
To identify ideal fermentation conditions for the strain PN1001,the degradation of pentyl amine,aniline and a 1∶1 mixture of both pollutants(where each pollutant made up 50%of the indicated concentration)was measured under several variable conditions.Those conditions tested for were:pH,temperature,retention time,pollutant concentration,and aeration.Table 1 summarizes the experimental factors used for this test.
For all optimal fermentation tests,duplicate 50 mL samples of the selective medium were enriched to the specified concentration of pentyl amine,aniline or their 1∶1 mixture.Each sample was placed in individual 250 mL conical flasks,sterilized and then inoculated with 1 mL of inoculum containing 109cells/mL of the strain PN1001.As control,another set of duplicate 50 mL solutions was prepared in a similar fashion,but without being inoculated.The flasks were incubated for a specified retention time,during which or at the end of which remaining levels of pentyl amine and aniline were quantified using HPLC(high performance liquid chromatography and ion chromatography)(Table 2).For the temperature test,COD was also monitored.
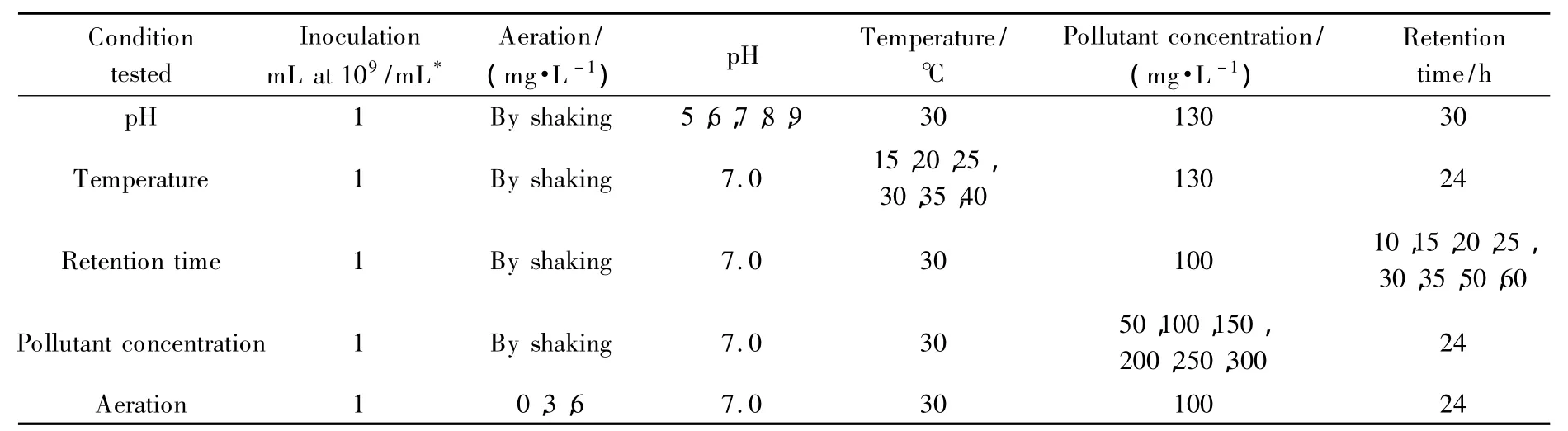
Table 2 Experimental factors used to test ideal degradation conditions
The pH levels tested were 5,6,7,8 and 9,and these levels were adjusted using NaOH(2 mol/L)and HCl(2 mol/L).The temperature levels tested were 15,20,25,30,35 or 40 ℃.The retention times tested were 10,15,20,25,30,35,50 and 60 h.Concentrations 50,100,150,200,250 and 300 mg/L were tested for pentyl amine,aniline or their 1∶1 mixture.The aeration levels tested were 0,3 and 6 mg/L of dissolved oxygen,where the dissolved oxygen corresponded simply to aeration by the flowmeter control valves at 0,2.3 and 4.7 mL/min.
1.4 Comparative Degradation Efficiency of PN1001 for Activated Sludge
This proceduretested thecapability of PN1001 in degrading petrochemicalactivated sludge rich in pentyl anime and aniline.A large sample of activated sludge was obtained from an aerated tank of an oil refinery,allowed to sediment for 30 min and then dewatered by removing its supernatant liquid.Using sterile washing water,the activated sludge sample was split into smaller samples and inoculated with 1.0 mL of PN1001 soliquoid.The sludge was enriched to 100 mg/L of pentyl amine,aniline and their 1∶1 mixture and then incubated at 30℃,pH 7 and reciprocal shaking 120 r/min for 24 h.Non inoculated samples were also incubated as control.The level of pentyl amine and aniline degradation was measured after the fermentation period.
1.5 Impact Experiment
A test was conducted to evaluate the growth of PN1001 when exposed to various levels of pentyl amine and aniline.Duplicate peptone media enriched to 1 000,2 000,3 000,4 000 and 5 000 mg/L of pentyl amine,aniline or their 1∶1 mixture were prepared.These media were inoculated with 1.0 mL of PN1001 and then plates and inoculated at 30℃and pH 7 for 24 h.Plates without the inoculation were also prepared as control group.The level of degradation of pentyl amine and aniline was again measured at 30℃,pH 7 and reciprocal shaking 120 r/min after 24 h.after sharking test,the practical wastewater which COD was 500 mg/L from the refinery was treated by the mixture of PN1001 and activated sludge(AS).
1.6 Statistical Analysis
All results were conducted to test the effect of specific conditions(pH,temperature,pollutant load,retention time and aeration)on the degradation capability of PN1001 for pentyl amine and aniline.Using Origin and Excel[5],ANOVA was used to express quantitatively the relationship between them,and the degree of correlation.Using Origin and Excel,regression analysis was also conducted and goodness of fit was verified using the method of least square curve fitting.Using Chem 3D,the molecular structure was obtained to determine the significance of degradation.
2 Results and Discussion
2.1 Microbial Identification
The organism PN1001,isolated from the acclimatization and culture of activated sludge,was recognized based on the characteristics of its colonies,its morphology,its biochemical and physiological properties and through differential staining.Its colonies demonstrated that the formation of pink-yellow oval colonies within(5±1)mm diameter in the agar medium plate with beef extract paste,0.05 % yeastextractpaste,0.05 %K2HPO4after overnight incubation at 30℃.Its morphology was motile curve rods with mono polar flagellum under observation of microscope(1 000×),and the cell size was(0.4±0.2)×(1.5±0.8)micron meters.The Gram straining gave negative.It chemical and physiological properties were such that the bacterial strain capably utilized sole aniline and pentyl amine as source of carbon and energy by oxidation at pH 6.5~7.5 for its growth and it was positive for nitrate and H2S reduction.Its hydrolysis was positive for starch and amide.The PN1001 of cytochrome c oxidase active also was positive after 24 h incubation.The strain was identified as belonging to the family of Pseudomonas sp.
2.2 Influence of Fermentation Conditions
The degradation efficiency ofthe strain PN1001 for pentyl amine,aniline and a mixture of both pollutants was tested under several ranges of pH,temperature,retention time,pollutant concentration and aeration level.
Tested under pHs varying from 5 to 9,the strain PN1001 degraded the most pentyl amine,aniline and their mixture under neutral conditions(Fig.1).After 24 h of degradation at 30℃and a pH of 7.0,the strain PN1001 degraded 82%,80%and 92%of pentyl amine,aniline and their 1∶1 mixture,respectively,when initially present at a concentration of 130 mg/L.Because significantly more(Pr<0.01)pollutants were degraded when the contaminant solution was a 1∶1 mixture,it can be concluded that the presence of pentyl amine enhances thedegradation ofanilineby PN1001.Thus,PN1001 must be able to cleave pentyl amine from catabolic results analysis,which the degradation ratio is 92%for the mixture pollutants,and the aniline degradation ratio also degraded effectively at 80%.The pollutants structures could affect the mechanisms.The Fig.2 shows the 3D structures of aniline and the isomers of pentyl amine in the wastewater.The black,green blue,navy blue and red ball describe the carbon,hydrogen,nitrogen and oxygen atom,respective in the molecular structures in the Fig.2.The isomer structures of pentyl amine are single bond which is in the status of σ electronic cloud;hence the attractive force is smaller than that of π electronic atmosphere of aniline for the function group of—NH2.From the analysis of 3D molecular structure,the special structure resistant of neopentyl amine is least,that of sec-pentyl amine and tert-pentyl amine is second and third smaller,respectively,but that of aniline presented π electronic atmosphere is largest.However the multienzyme system presence in PN1001 could help to crush the special resistant to degrade aniline in the wastewater[8].
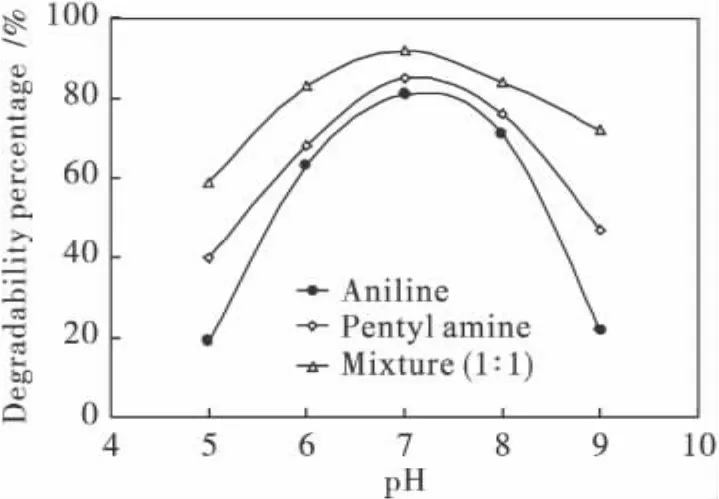
Fig.1 Effect of pH on degradation of pollutants
Table 3 illustrates that effect of aeration rate on pollutant degradation by PN1001.An aeration level of DO 6 mg/L produced the highest pentyl amine and aniline degradation levels of 93%and 80%,respectively,after 24 h of fermentation at 30 ℃.Thus,the strain PN1001 is an obligate aerobe and the level of dissolved oxygen significantly affects its biosynthesis and degradation efficiency.Aeration is therefore needed for PN1001 to degrade petrochemical wastewaters rich in penty1 amine and aniline.
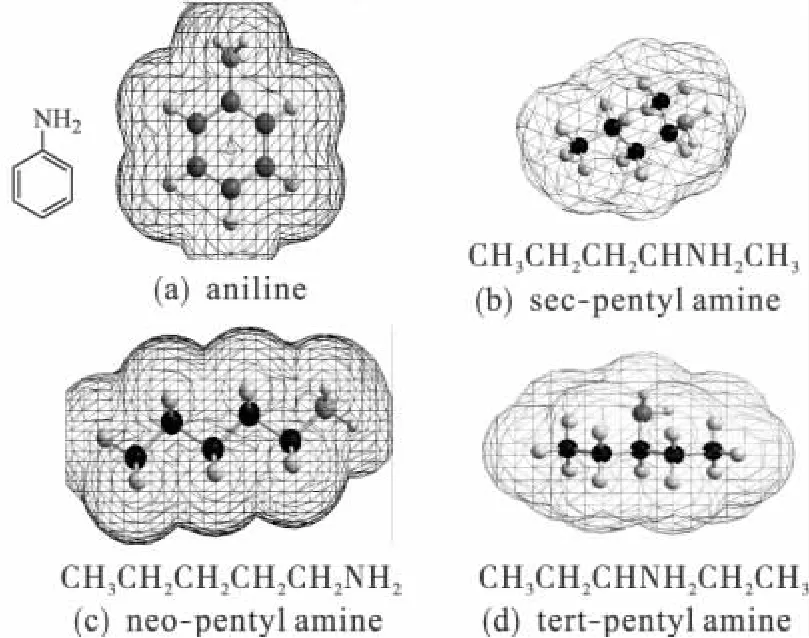
Fig.2 Three dimensional models of aniline and pentyl amine in the wastewater

Table 3 The remaining contaminants(RC)of aniline degradation
Temperature also had a major impact on pollutant degradation(Fig.3).The degradation of pentyl anime and aniline both peaked at a temperature of 30℃.At 15℃,40%and 19%of pentyl anime and aniline,while were degraded at 30℃,degradation reached 82%and 79%.For the 1∶1 mixture,90%degradation was reached at 30℃.At temperatures above 30℃,degradation level dropped indicating that the chemical and enzymatic reactions of the strain PN1001 were affected.
Degradation of the pentyl anime and aniline with fermentation or retention time is illustrated in Fig.4.Maximum degradation rate occurred between 0 and 20 h,while peak degradation was reached after 30 h.Thus,a 24 h fermentation period was found to be the most efficient in terms of rate of pollutant degradation.
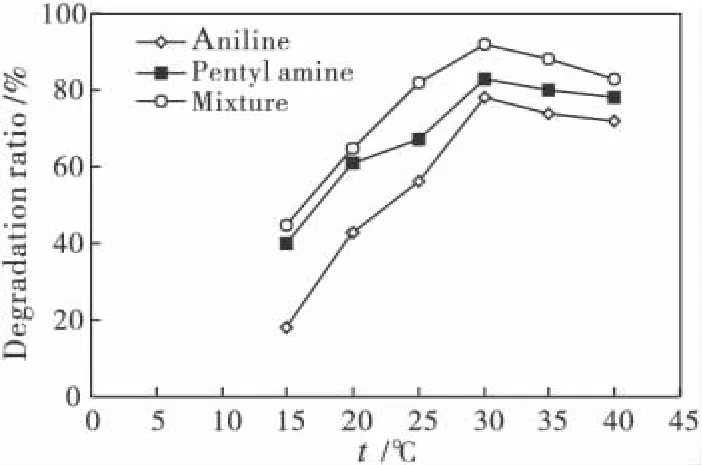
Fig.3 The relationship between degradation and temperature

Fig.4 Relationship between HRT and degradation ratio
The effect of pentyl amine and aniline concentration on their degradation by PN1001 is presented in Fig.5.Degradation level of 86% and 82%were obtained for penty1 amine and aniline at 150 mg/L and 200 mg/L,while with 300 mg/L of pollutants,these levels were 28%and 16%.The degradation ratio of mixture was 93%and 89%at 150 and 200 mg/L,and the results were better than that of other pollutants performed after over 24 h incubation at pH 7 and 30℃.The concentration pollutants that were tested covered the typical pollutant levels in wastewater treatment facilities.Two stages,then,would be required for the safe removal of pentyl amine and aniline at over 99%ratio of degradation in the wastewater.The equation(1)was obtained after the regression analysis was also conducted by using the method of least square curve fitting at pH 7,30 ℃ and concentration is not larger than 300 mg/L,as shown at below.
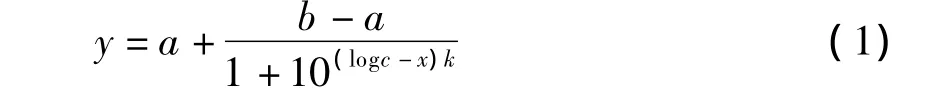
Where y is removal ratio(%);x is concentration of pollutants(mg/L);k is slope coefficient;a,b and c are constants.The degradation parameters of three group pollutants were described in Table 4 and matrix as following.

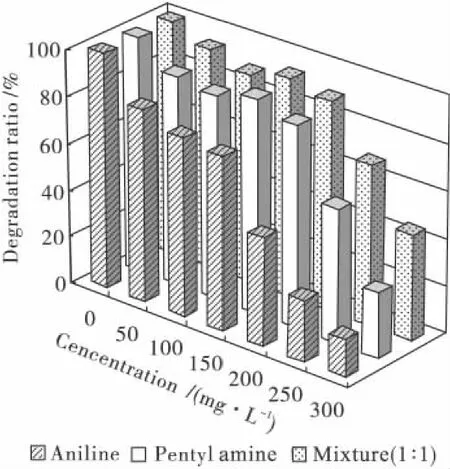
Fig.5 Effect of concentration gradient
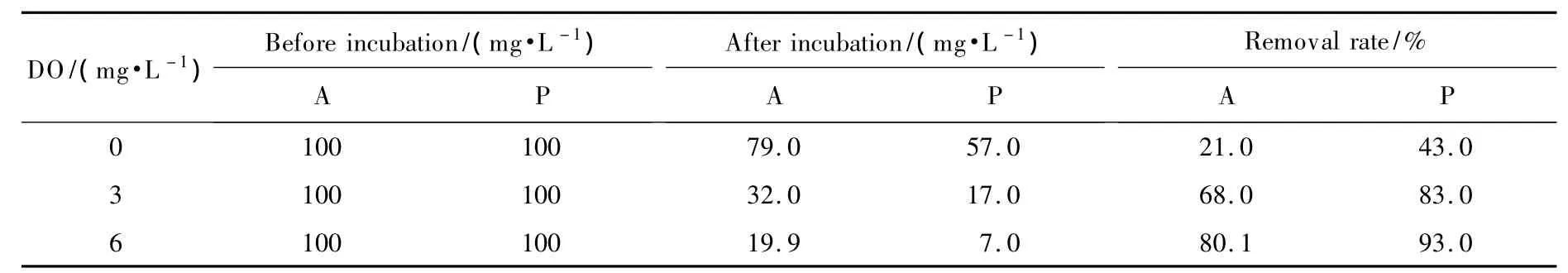
Table 4 Effect of aeration on pollutant degradation
During this pH test,the effluent was periodically sampled and analyzed for the degradation of aniline and the appearance of its derivatives(Table 2).Furthermore,most test conducted to optimize fermentation conditions indicated at degradation was enhanced when a mixture of pollutants was present,as opposed to individual pollutants.Thus,the pentyl amine degradation was completed by simply denitrification and lipid metabolism to CO2and nitrate,hence here the details of pathway were unnecessary[9].
From such observation,the gradual degradation of aniline was observed to produce p-aminophenol,which peaked at 11 mg/L,after 10 h,but undetectable levels of m-aminophenol and o-aminophenol were observed during the full 30 h fermentation period.Some short chain carboxylic acids also were detected,such as oxalic acid,malonic acid and formic acid,and these peaked at 2.8,0.5 and 1.3 mg/L,respectively,after 30 h of fermentation.Levels ofandwere found to increase from 1.4 and 0.8 mg/L after 10 h to 7.4 and 3.5 mg/L after 30 h.
Based on the intermediate derivatives produced,the degradation pathway of aniline is presented in Fig.3.The aniline degradation was finished via catalyzed p-deamination into p-aminophenol,which was cleaved into oxalic acid,malonic acid,formic acid,and then to CO2.After deaminationwas oxidated intoandin the wastewater treatment system.
2.3 The Efficiency Analysis of PN1001 and Activated Sludge
Significantly better pentyl amine and aniline degradation rates were obtained with the activated sludge of a wastewater treatment facility,once inoculated with PN1001,as compared to the activated sludge or PN1001 alone(Table 5).Thus,the strain PN1001 that isolated had a positive effect on the degradation efficiency of the activated sludge at pH 7.The removal rate was lowest,which was 72.0%and 81.7%,respectively,while the active sludge only used degraded aniline and pentyl amine.The active sludge properties were modified after PN1001 was incubated.The degradation efficiencies of the two pollutants were 88.0%and 93.0%.The significance of such findings was that PN1001 could be to help the improving efficiency of active sludge treatment system as an enhancing bio-added agent of active sludge.
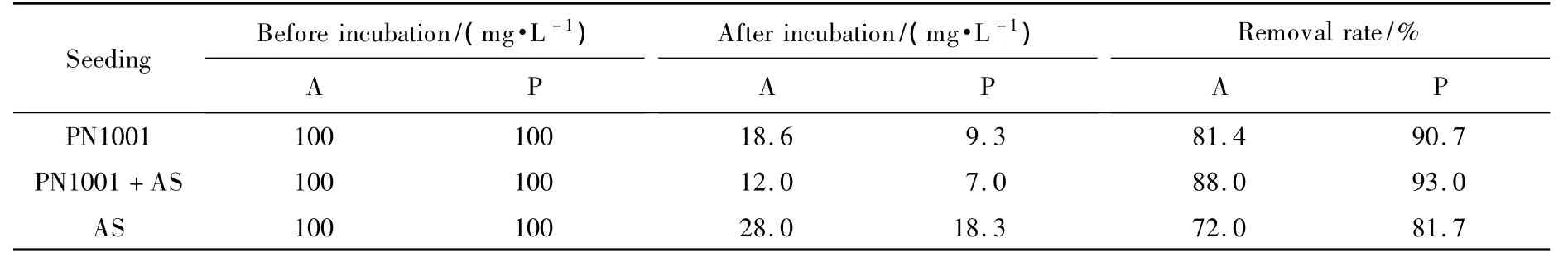
Table 5 Degradation efficiency of PN1001 in activated sludge
2.4 Impact of Exposure to Pentyl Amine and Aniline
The impact of exposing the strain PN1001 to high levels of penty1 amine and aniline is illustrated in Fig.6 and Fig.7.Higher degradation levels were obtained with the 1∶1 mixture rather than with the individual pollutants at the same concentrations,and with pentyl amine as compared to aniline,thus indicating that toxic was least with the 1∶1 mixture and highest with aniline.The π electron atmosphere of aniline could cause more the toxic than that of others,but the π and σ electron atmosphere coordination function of the two pollutants could be able to reduce their toxic.The application showed that when PN1001 had strong ability of degradation for the wastewater.Fig.7 also shows that when PN1001 was mixed with activated sludge the ability of degradation was stronger.The results are:COD,before treatment,is 500 mg/L;COD,after treatment by PN1001 and AS,is 75 mg/L and 97 mg/L by PN1001.
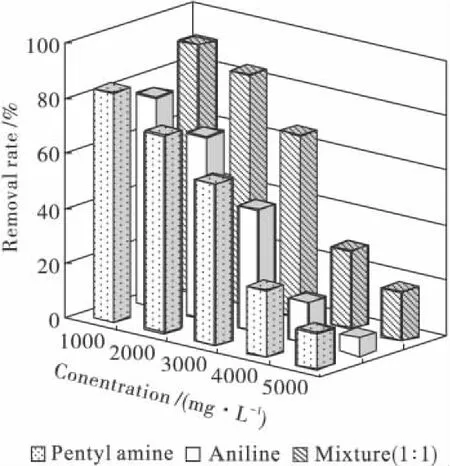
Fig.6 Relationship between impact load and removal rate for different samples
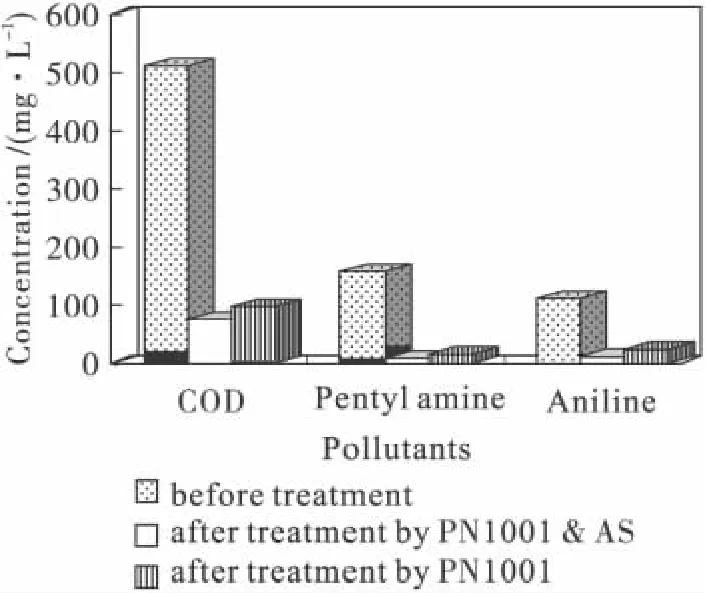
Fig.7 Treatment results of the petrochemical wastewater
3 Conclusion
Biodegradation rates of petrochemical wastewater that contained pentyl amine and aniline indicate that a mixed culture PN1001 with activated sludge can treat this wastewater.The PN1001 has been isolated from the activated sludge of CBDS when a petrochemical wastewater that contained pentyl amine and aniline was treated by CBDS.This strain had strong ability of degradation for the wastewater.After it was acclimatized and made enrichment culture in the media,PN1001 was identified as Pseudomonas sp,and PN1001 was symbiotic for degradation of the wastewater.When pH was neutral,aeration was full and temperature was about 30℃ ,the quality of the wastewater treated had met to the guideline of surface water quality standard.The reaction pathway of aniline biodegradation was proposed by the research,and the efficiency of the biodegradation of petrochemical pollutants could be contributed by using the target microbial recombinationtechnology.The results will lay a foundation for further engineering research of the wastewater treatment.
[1] Sambrook,Fritsh EF,Maniatis.Molecular Cloning:Laboratory Manual[M].2thed.New York:Cold Spring Press,1989:110-120.
[2] APHA.Standard Methods for The Examination of Water And Wastewater[M].18thed.Washington D.C:American Public Health Association,American Water Works Association and Water Environment Federation,1992:87-92.
[3] Fernandez-Lopez M,Olivares J,Bedmar E J.Two Differentially Regulated Nitrate Reductases Required for Nitratedependent,Microaerobic Growth of Bradyrhizobium Japonicum [J].Arch.Microbiol.,1994,162:310-315.
[4] Bell L S,Devlin J F,Gillham R W,et al.A Sequential Zero Valent Iron and Aerobic Biodegradation Treatment System for Nitrobenzene[J].Journal of Contaminant Hydrology,2003,66(3/4):201-217.
[5] Lars Toräng,Peter Reuschenbach,Britta Müller,et al.Laboratory Shake Flask Batch Tests can Predict Field Biodegradation of Aniline in the Rhine[J].Chemosphere,2002,49(10):1257-1265.
[6] Buchanan R E,Gibbons N E.Bergey’s Manual of Determinative Bacteriology[M].8thed.Baltimore:williamas & Wikins,1974:50-77.
[7] Billo E Joseph.Excel for Chemists:A Comprehensive Guide[M].Hoboken,New Jersoy:John Wiley& Sons,Inc.,2001:65-78.
[8] Kovac N.Identification of Pseudomonas Pyocyances by the Oxidase Reaction[J].Nature,1956,178(703):1403-1408.
[9] Wackette L P,Hershberger C D.Biocatalysis and Biodegradation:Microbial Transformation of Organic Compounds[M].New York:ASM Press,2001:120-145.
