人类真核翻译延长因子1A2过表达对胰腺癌SW1990细胞体外侵袭及体内转移的影响
2013-10-19许朝胡端敏章永平诸琦
许朝 胡端敏 章永平 诸琦
·论著·
人类真核翻译延长因子1A2过表达对胰腺癌SW1990细胞体外侵袭及体内转移的影响
许朝 胡端敏 章永平 诸琦
目的探讨人类真核翻译延长因子1A2(EEF1A2)过表达对人胰腺癌SW1990细胞体外侵袭和肺转移潜能的影响。方法通过携带EEF1A2的慢病毒感染人胰腺癌SW1990细胞建立EEF1A2稳定过表达的SW1990/EEF1A2细胞株及空质粒对照的SW1990/GFP细胞株,并以亲本SW1990细胞作为空白对照。采用实时PCR和蛋白质印迹法检测各组细胞EEF1A2 mRAN和蛋白的表达。应用Transwell小室检测细胞的体外侵袭能力。通过裸鼠尾静脉注射癌细胞方法构建肺转移模型,8周后观察并计数肺表面的转移结节。结果SW1990/EEF1A2细胞EEF1A2 mRNA相对表达量为3.252±0.344,显著高于SW1990/GFP细胞的1.000±0.060及亲本细胞的0.944±0.041(t值分别为2.255、2.305,P值均<0.01);EEF1A2蛋白表达量为0.833±0.050,显著高于SW1990/GFP细胞的0.247±0.035及亲本细胞的0.273±0.041(t值分别为0.572、0.559,P值均<0.01);穿膜细胞数为(60±4)个,显著多于SW1990/GFP细胞的(33±4)个和亲本细胞的(26±3)个(t值分别为31.33、34.78,P值均<0.01);裸鼠肺转移结节显著多于SW1990/GFP细胞及亲本细胞组(5/5比1/5、1/5,P值均<0.05)。结论EEF1A2过表达可以明显增强胰腺癌SW1990细胞的体外侵袭和肺转移能力。
胰腺肿瘤; 人类真核翻译延长因子1A2; 慢病毒感染; 肿瘤侵袭; 肿瘤转移
人类真核翻译延长因子1A2(eukaryotic translation elongation factor l alpha 2,EEF1A2)位于20q13.3,其编码蛋白通过结合氨酰tRNA指导核糖体与mRNA密码子的结合在蛋白延伸过程中起重要作用,被认为是一种管家基因[1]。近来研究发现,EEF1A2在多种人类肿瘤中表达异常增高,如乳腺癌、肝癌、肺癌、前列腺癌等[2-5],被认为是一种癌基因。本课题组前期研究发现,EEF1A2在正常胰腺组织中不表达,而在胰腺癌组织中高表达,过表达的EEF1A2可增强胰腺癌细胞的增殖能力和裸鼠体内移植瘤的生长[6]。本研究进一步探讨外源性EEF1A2过表达对细胞体外侵袭和体内转移的影响。
材料与方法
一、稳定过表达EEF1A2的胰腺癌SW1990细胞株的构建
EEF1A2全长基因(约1.4 kb)由上海吉玛制药技术有限公司合成,基因上、下游分别加上NotⅠ和NsiⅠ酶切序列及保护碱基。应用NotⅠ和NsiⅠ双酶切合成的EEF1A2片段及pGLV5-EF1a-EGFP/Puro质粒(上海吉玛制药技术有限公司),经电泳分离、回收、纯化,应用T4DNA连接酶进行连接,然后转化感受态DH5a,扩增,提取质粒。重组质粒经测序鉴定正确后与包膜蛋白质粒、核心蛋白/逆转录酶质粒和载体质粒应用脂质体方法共转染293T细胞,72 h后收集细胞培养上清,用0.45 μm过滤器过滤,滤液经4℃ 20 000 r/min离心2 h,置-80℃冰箱保存备用。将慢病毒原液(10~20 μl)用10% FBS的DMEM培养液10倍稀释4个梯度分别感染293T细胞,通过荧光显微镜计数荧光细胞,结合稀释倍数计算病毒滴度,约为1×109TU/ml。按1.2×106细胞/孔的密度接种于96孔板,混匀后于37℃ 5% CO2培养24 h。将重组慢病毒颗粒用10% FBS的DMEM细胞培养液稀释至1×108TU/ml,吸去96孔板中的培养液,每孔加入上述200 μl稀释的病毒液。同时将空病毒载体设为对照组,于37℃、5% CO2条件下培养,24 h后更换为DMEM完全培养液培养,48 h后更换为含嘌呤霉素(终浓度为0.35 μg/ml)的DMEM完全培养液培养,期间每2 d换液。14 d后得到稳定过表达EEF1A2的人胰腺癌SW1990细胞(SW1990/ EEF1A2细胞)和空病毒载体稳转的对照SW1990细胞(SW1990/GFP细胞)。
二、EEF1A2 mRNA表达检测
收集各组细胞,Trizol试剂提取细胞总RNA。按反转录试剂盒说明书提供的方法合成cDNA第一链;以cDNA为模板,应用实时荧光定量PCR 检测试剂盒(日本TaKaRa公司)行实时PCR扩增,以GAPDH为内参。EEF1A2的上游引物5′-ATGCGG-AGGTATTGACAAAAGGAC-3′,下游引物5′-AGCACC-CCAGGCATACTTGAAGG-3′;扩增产物90 bp;内参GAPDH的上游引物5′-CATGAGAAGTATGACAAC-AGCCT-3′,下游引物5′-AGTCCTTCCACGATA-CCAAAGT-3′,扩增产物113 bp。引物由上海生工生物工程有限公司合成。PCR反应条件:95℃ 3 min,95℃ 12 s、62℃ 40 s, 40个循环。通过仪器自带软件获取Ct值,根据公式2-△△Ct计算EEF1A2 mRNA的相对表达量,△△Ct=SW1990/EEF1A2[CtEEF1A2-CtGAPDH]-SW1990/GFP[CtEEF1A2-CtGAPDH]。
三、EEF1A2蛋白表达检测
提取各组细胞总蛋白,应用BCA法测定蛋白浓度。取50 μg蛋白常规行蛋白质印迹法检测EEF1A2蛋白的表达。兔抗人EEF1A2多抗(美国Abcam公司) 1∶1000 稀释,内参GAPDH单抗(上海康成公司)1∶5000 稀释,HRP标记二抗1∶5000 稀释。应用ECL发光,显影,Bio-Rad公司数码成像分析系统软件进行图片扫描,以目的条带与内参条带的灰度比值表示目的蛋白的相对表达量。
四、Transwell小室侵袭实验
胰酶消化并收集各组细胞,用无血清DMEM培养液调整细胞密度为1×106个/ml,取100 μl加入 Matrigel Transwell小室(Chemicon公司)的上室,下室加入含10%胎牛血清的DMEM培养液500 μl,小室放置于24孔板中,常规培养24 h。弃上室液体,用棉签轻轻拭去滤膜上表面未穿透的细胞,经甲醇固定15 min,常规结晶紫染色,倒置显微镜下选择10个高倍镜视野(×200),计数滤膜下表面的穿膜细胞数。每组设3个复孔,实验重复3次。
五、裸鼠肺转移模型实验
4~6周龄BALB/C裸鼠购自中国科学院上海实验动物中心,饲养于SPF条件下。8周龄时,将裸鼠按数字表法随机分为2组,每组5只,每只裸鼠经尾静脉注射2×106个SW1990/EEF1A2细胞或SW1990/GFP细胞。8周后脱颈处死裸鼠,分离肺,观察计数肺表面的转移结节。
六、统计学处理
结 果
一、稳定过表达EEF1A2的胰腺癌SW1990细胞株的建立
SW1990/EEF1A2、SW1990/GFP和亲本SW1990细胞的EEF1A2 mRNA相对表达量分别为3.252±0.344、1.000±0.060和0.944±0.041;EEF1A2 蛋白相对表达量分别为0.833±0.050、0.247±0.035和0.273±0.041。SW1990/EEF1A2细胞的表达显著高于SW1990/GFP细胞(t值分别为2.255、0.572,P<0.01),也显著高于亲本SW1990细胞(t值分别为2.305、0.559,P<0.01,图1)。

图1SW1990/GFP、SW1990/EEF1A2细胞EEF1A2蛋白的表达
二、EEF1A2过表达对SW1990细胞体外侵袭力的影响
SW1990/EEF1A2、SW1990/GFP和SW1990的穿膜细胞数分别为(60±4)、(33±4)和(26±3)个,SW1990/EEF1A2的穿膜细胞数显著高于SW1990/GFP细胞(t=31.33,P<0.01),也显著高于亲本SW1990细胞(t=34.78,P<0.01,图2)。
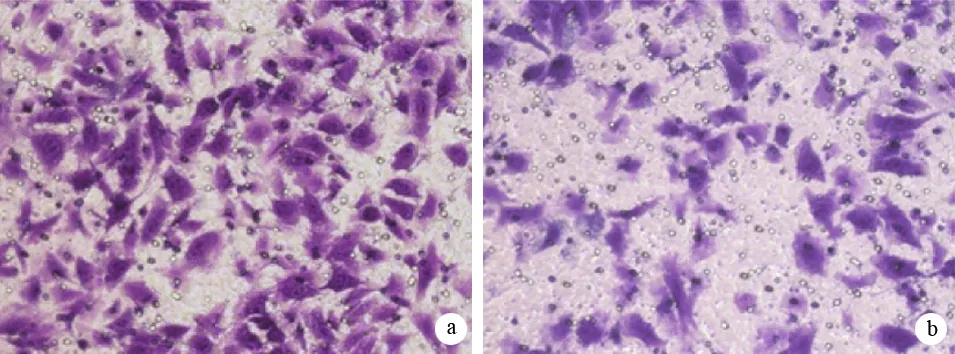
图2SW1990/EEF1A2(a)、SW1990/GFP(b)的穿膜细胞(结晶紫染色 ×200)
三、EEF1A2过表达对SW1990细胞肺转移的影响
SW1990/GFP和SW1990细胞组裸鼠中各有1只可见肺表面转移结节(1/5),而SW1990/EEF1A2组裸鼠均见肺表面转移结节(5/5),两者间差异具有统计学意义(P<0.05)。

图3 裸鼠肺表面的转移结节
讨 论
恶性肿瘤组织EEF1A2的异常表达不仅可导致肿瘤细胞增殖及凋亡的失控,在肿瘤侵袭、转移过程中也发挥重要作用。Edmonds等[7]报道,EEF1A2在具有转移潜能的大鼠乳腺癌细胞中表达水平明显高于无转移能力的细胞,过表达EEF1A2的乳腺癌BT549细胞可明显增强肿瘤细胞的迁移和侵袭能力;相反,沉默乳腺癌HCC1937细胞EEF1A2表达则可抑制细胞的侵袭能力[8-9]。本课题组前期研究也发现,利用特异性siRNA靶向沉默EEF1A2可抑制胰腺癌细胞BxPC3体外迁移和侵袭力[10]。
为进一步研究EEF1A2对胰腺癌细胞体内转移潜能的影响,我们构建了携带EEF1A2基因的慢病毒表达载体,并成功筛选出稳定过表达外源EEF1A2的SW1990细胞株。结果显示EEF1A2过表达可增强SW1990细胞的体外侵袭能力及体内的肺表面转移。但其分子机制目前仍不清楚。Amiri等[8]的研究显示,EEF1A2可激活Akt信号通路从而促进瘤细胞骨架重构和侵袭转移。具体机制尚有待进一步探讨。
[1] Lukash TO, Turkivska HV, Negrutskii BS, et al. Chaperone-like activity of mammalian elongation factor eEF1A: renaturation of aminoacyl-tRNA synthetases. Int J Biochem Cell Biol, 2004, 36: 1341-1347.
[2] Tomlinson VA, Newbery HJ, Wray NR, et al. Translation elongation factor eEFlA2 is a potential oncoprotein that is overexpressed in two-thirds of breast tumours. BMC Cancer, 2005, 5: 113.
[3] Schlaeger C, Longerich T, Schiller C, et al. Etiology-dependent molecular mechanisms in human hepatocarcinogenesis. Hepatology, 2008, 47: 511-520.
[4] Zhu H, Lam DC, Han KC, et al. High resolution analysis of genomic aberrations by memphase and array comparative genomic hybridization identifies candidate tumour genes in lung cancer cell lines. Cancer Lett, 2007, 245: 303-314.
[5] Scaggiante B, Dapas B, Bonin S, et al. Dissecting the expression of EEF1A1/2 genes in human prostate cancer cells: the potential of EEF1A2 as a hallmark for prostate transformation and progression. Br J Cancer, 2012, 106:166-173.
[6] Cao H, Zhu Q, Huang J, et al. Regulation and functional role of eEF1A2 in pancreatic carcinoma. Biochem Biophys Res Commun,2009, 380:11-16.
[7] Edmonds BT, Wyckoff J, Yeung YG, et al. Elongation factor-1 alpha is an overexpressed actin binding protein in metastatic rat mammary adenocarcinoma. J Cell Sci, 1996, 109:2705-2714.
[8] Amiri A, Noei F, Jeganatham S, et al. eEF1A2 activates Akt and stimulates Akt-dependent actin remodelling, invasion and migration. Oncogene, 2007, 26:3027-3040.
[9] Pecorari L, Marin O, Silvestri C, et al. Elongation factor 1 alpha interacts with phospho-Akt in breast cancer cells and regulates their proliferation, survival and motility. Mol Cancer, 2009, 8:58.
[10] 许朝,胡端敏,诸琦. 靶向沉默人类真核翻译延长因子1A2可抑制胰腺癌细胞BxPC-3体外迁移和侵袭. 肿瘤,2012,32:567-571.
(本文编辑:屠振兴)
Effectsofhomosapienseukaryotictranslationelongationfactor1alpha2overexpressiononinvitroinvasionandinvivometastasisofpancreatic
cancerSW1990cellsXUChao,HUDuan-min,ZHANGYong-ping,ZHUQi.
DepartmentofGastroenterology,RuijinHospital,ShanghaiJiaotongUniversitySchoolofMedicine,Shanghai200025,China
ZHUQi,Email:zhuqi@medmail.com.cn
ObjectiveTo investigate the effects of over-expression of homo sapiens eukaryotic translation elongation factor 1 alpha 2 (EEF1A2) on in vitro invasion and lung metastasis of human pancreatic cancer SW1990 cells.MethodsLetivirus-mediated delivery of EEF1A2 was used to enhance the expression of EEF1A2 gene in human pancreatic cancer SW1990 cells, the SW1990 cells stably over-expressing EEF1A2 protein (SW1990/EEF1A2 cells) were obtained, and the parent SW1990 cells and SW1990/GFP cells were used as the control, and the expressions of EEF1A2 mRNA and protein were determined by Real-time PCR and Western blotting. The invasion ability of cells was determined by Transwell assay. The lung metastasis model was established by injection of SW1990 cells into the tail vein. Whole lung tissues were harvested, and visible nodules on lung surface were counted macroscopically 8 weeks later.ResultsThe EEF1A2 mRNA expression of SW1990/EEF1A2 was 3.252±0.344, which was significantly higher than those in SW1990/GFP cells (1.000±0.060) and SW1990 cells (0.944±0.041,t=2.255, 2.305,P<0.01); the EEF1A2 protein expression was 0.833±0.050, which was significantly higher than those in SW1990/GFP cells (0.247±0.035) and SW1990 cells (0.273±0.041,t=0.572, 0.559,P<0.01). The ability of invasion of SW1990/EEF1A2 cells was (60±4) cells, which was significantly higher than (33±4) cells in SW1990/GFP
group and (26±3) cells in SW1990 group (t=31.33, 34.78,P<0.01). Furthermore, SW1990/EEF1A2 cells had a much higher incidence of lung metastasis in nude mice than SW1990/GFP cells and SW1990 cells in vivo (100%vs. 20%, 20%,P<0.05).ConclusionsEEF1A2 over-expression can obviously increase the in vitro invasion and lung metastasis of pancreatic cancer SW1990 cells.
Pancreatic neoplasms; Homo sapiens eukaryotic translation elongation factor 1 alpha 2; Lentivirus infections; Neoplasm invasiveness; Neoplasm metastasis
10.3760/cma.j.issn.1674-1935.2013.01.006
200025 上海,上海交通大学医学院附属瑞金医院消化内科
诸琦,Email: zhuqi@medmail.com.cn
2012-09-11)
