白藜芦醇对血管紧张素Ⅱ诱导的血管平滑肌细胞增殖的抑制作用及其机制观察
2013-08-02郜攀司良毅徐强王笑梅
郜攀,司良毅,徐强,王笑梅
白藜芦醇对血管紧张素Ⅱ诱导的血管平滑肌细胞增殖的抑制作用及其机制观察
郜攀,司良毅,徐强,王笑梅
目的探讨白藜芦醇对血管紧张素Ⅱ(AngⅡ)诱导的血管平滑肌细胞(VSMCs)增殖的影响及其可能机制。方法体外培养大鼠胸主动脉血管平滑肌细胞(VSMCs)。在检测白藜芦醇影响AngⅡ诱导VSMCs增殖和活力的实验中,将细胞分为对照组、AngⅡ组(1μmol/L)、白藜芦醇浓度梯度(10、30、100μmol/L)组及AngⅡ+白藜芦醇浓度梯度组,各组细胞均反应0、6、12、24h,采用细胞计数法检测VSMCs增殖情况,MTT法检测VSMCs活力。在检测腺苷酸活化蛋白激酶(AMPK)抑制剂复合物C对VSMCs生物活性影响的实验中,将细胞分为对照组、AngⅡ组、白藜芦醇+AngⅡ组及复合物C组+白藜芦醇+AngⅡ组,各组细胞反应24h后采用Western blotting检测增殖细胞核抗原(PCNA)蛋白表达。结果与对照组比较,6、12、24h后AngⅡ组细胞活力和细胞数目均显著增高(P<0.05);与AngⅡ组比较,不同浓度白藜芦醇+AngⅡ组细胞活力和细胞数目均显著降低(P<0.05)。另一方面,与对照组比较,AngⅡ组PCNA蛋白表达显著增高(P<0.05);与AngⅡ组比较,白藜芦醇+AngⅡ组PCNA蛋白表达显著降低(P<0.05);而与白藜芦醇+AngⅡ组比较,复合物C+白藜芦醇+AngⅡ组细胞数目、细胞活力及PCNA蛋白表达显著增高(P<0.05)。结论白藜芦醇可抑制AngⅡ诱导的VSMCs增殖,其机制可能与AMPK的激活有关。
白藜芦醇;肌细胞,平滑肌;血管紧张素Ⅱ;腺苷酸活化蛋白激酶
动脉粥样硬化是众多心脑血管疾病发展的基础。研究提示,炎症因子刺激血管平滑肌细胞(vascular smooth muscle cells,VSMCs)表型转换和异常增殖进而导致血管重构是动脉粥样硬化发生的重要病理基础[1-2]。近年来,红酒中的天然提取物白藜芦醇(resveratrol)在心血管疾病中的保护作用逐渐受到关注,相关研究表明白藜芦醇能够抑制VSMCs增殖,但其作用机制尚未阐明[3-4]。本课题组前期研究发现,白藜芦醇能激活内皮细胞腺苷酸活化蛋白激酶(adenosine monophosphate-activated protein kinase,AMPK),诱导一氧化氮释放、改善内皮功能,提示AMPK对细胞功能具有重要影响[5]。血管紧张素Ⅱ(angiotensin Ⅱ,AngⅡ)是肾素-血管紧张素-醛固酮系统(RAAS)中最重要的一种生物活性肽,除有收缩血管、促醛固酮分泌及心肌肥大[6]等作用外,还具有很强的促VSMCs增殖作用,同时AngⅡ是VSMCs分泌的一种重要炎症介质,除诱导血管平滑肌细胞增殖之外,还可促进炎症介质释放,导致动脉粥样硬化发生[7]。因此观察白藜芦醇与VSMCs内AngⅡ的相互作用,进而探讨其相互作用机制对理解白藜芦醇在心血管系统中的保护作用具有重要意义。
1 材料与方法
1.1 动物及试剂 清洁级SD大鼠购自第三军医大学动物实验中心。白藜芦醇、血管紧张素Ⅱ、复合物C(AMPK抑制剂)均购自Sigma公司(美国);细胞计数试剂盒及MTT试剂盒购买自北京鼎国重庆分公司;SM-actin蛋白、增殖细胞核抗原(proliferating cell nuclear antigen,PCNA)蛋白、β-actin蛋白一抗购自Santa Cruz公司(美国);兔抗山羊二抗购自北京中杉金桥生物公司。
1.2 原代VSMCs的培养和鉴定[8]组织贴块法培养大鼠胸主动脉VSMCs。倒置相差显微镜下观察细胞形态及生长状况。采用SM-actin免疫荧光法对VSMCs进行细胞鉴定,阳性表达为胞质呈细颗粒状绿色沉淀反应。
1.3 细胞分组 选择第3~5代VSMCs进行实验。胰蛋白酶消化制备细胞悬液,调整细胞密度为5.0×107/L,接种于培养板备用。在37℃、5%CO2条件下经10%胎牛血清预培养24h和0.5%胎牛血清预处理12h后进行分组。在检测白藜芦醇对AngⅡ诱导的VSMCs增殖和细胞活力的影响作用实验中,将细胞随机分为对照组(不加特殊处理因素)、AngⅡ组(1μmol/L AngⅡ处理)、不同浓度白藜芦醇组(10、30、100μmol/L白藜芦醇处理)、不同浓度白藜芦醇+AngⅡ组(1μmol/L AngⅡ+10、30、100μmol/L白藜芦醇处理),各组细胞反应0、6、12、24h后进行检测。在检测复合物C对VSMCs生物活性影响的实验中,将细胞随机分为对照组、AngⅡ组(1μmol/ L AngⅡ处理)、白藜芦醇+AngⅡ组(1μmol/L AngⅡ和100μmol/L白藜芦醇处理)和复合物C+白藜芦醇+AngⅡ组(以1μmol/L复合物C预处理细胞24h后,再加入100μmol/L白藜芦醇+1μmol/L AngⅡ),各组细胞反应24h后进行检测。
1.4 细胞计数[9]VSMCs培养24h后换用含0.5%胎牛血清的DMEM培养液同步化24h。按实验分组换用含不同干扰因素及10%胎牛血清的DMEM培养液继续培养。分别在给予干扰因素后0、6、12、24h计数各组细胞。实验重复3次,每次计数3个复孔。
1.5 MTT法检测细胞活力[10]按每孔1×103个细胞接种于96孔板,用含10%胎牛血清的DMEM培养液培养24h后换用含0.5%胎牛血清的DMEM培养液同步化24h。按实验分组用含不同干扰因素及10%胎牛血清的DMEM培养液继续培养。分别在给予干扰因素后6、12、24h后用MTT法检测,于490nm波长处测定各孔A值。实验重复3次,每次6个复孔。
1.6 Western blotting检测PCNA蛋白的表达[11]提取细胞总蛋白,以SDS-PAGE凝胶行蛋白电泳,上样量为50μg。电泳完毕将蛋白转到聚偏氟乙烯膜(PVDF)上。转膜后在含5%脱脂奶的TBST中封闭1h,TBST洗涤3次,与1:250稀释的PCNA及β-actin抗体反应,4℃过夜。洗膜3次,加1:2000稀释的二抗,室温孵育1h,ECL化学发光法显色,X线胶片曝光、显影、定影,采用Quantity One软件行灰度分析,以兔抗鼠β-actin作为内参照。
1.7 统计学处理 采SPSS 13.0统计软件进行分析。计量资料结果以±s表示,组间比较采用单因素方差分析,进一步两两比较采用Tukey多重比较法,P<0.05为差异有统计学意义。
2 结 果
2.1 VSMCs的体外培养及鉴定 倒置相差显微镜下观察,细胞为梭形或长梭形,排列成放射状、旋涡状,呈典型“谷”与“峰”生长现象(图1A)。SM-actin单克隆抗体免疫组化染色可见胞质肌丝呈典型棕黄色细颗粒状沉淀,阳性率达95%以上,表明VSMCs纯度高(图1B)。
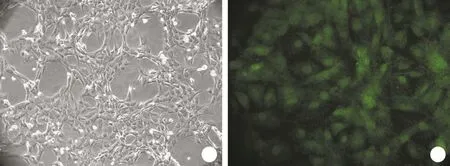
图1 体外培养的VSMCs形态及鉴定Fig. 1 Morphology and identification of VSMCs cultured in vitro
2.2 白藜芦醇对AngⅡ诱导VSMCs增殖的影响在6、12、24h时间点,AngⅡ组细胞数均明显高于对照组(P<0.05),而10、30、100μmol/L白藜芦醇组细胞数在各时间点与对照组比较差异均无统计学意义(P>0.05)。AngⅡ+30μmol/L白藜芦醇组和AngⅡ+100μmol/L白藜芦醇组各时间点细胞数均低于AngⅡ组(P<0.05),而AngⅡ+10μmol/L白藜芦醇组细胞数在各时间点与AngⅡ组比较差异无统计学意义(P>0.05,表1),表明30μmol/L和100μmol/L白藜芦醇均可阻断AngⅡ诱导的VSMCs增殖,而10μmol/L白藜芦醇无此作用。
2.3 白藜芦醇对AngⅡ调节VSMCs细胞活力的影响 MTT法检测显示,AngⅡ组A值在6、12、24h均明显高于对照组(P<0.05),而10、30、100μmol/L白藜芦醇组A值在各时间点与对照组比较差异均无统计学意义(P>0.05)。与AngⅡ组比较,AngⅡ+30μmol/L白藜芦醇组和AngⅡ+100μmol/L白藜芦醇组各时间点A值均明显降低,差异有统计学意义(P<0.05),而AngⅡ+10μmol/L白藜芦醇组A值在各时间点与AngⅡ组比较无明显差异(P>0.05,表2),表明30、100μmol/L白藜芦醇均可阻断AngⅡ增强VSMCs细胞活力的作用,而10μmol/L白藜芦醇则无此作用。
表1 各组血管平滑肌细胞计数结果(×105/ml,±s,n=3)Tab. 1 VSMCs count in different groups (×105/ml,±s, n=3)
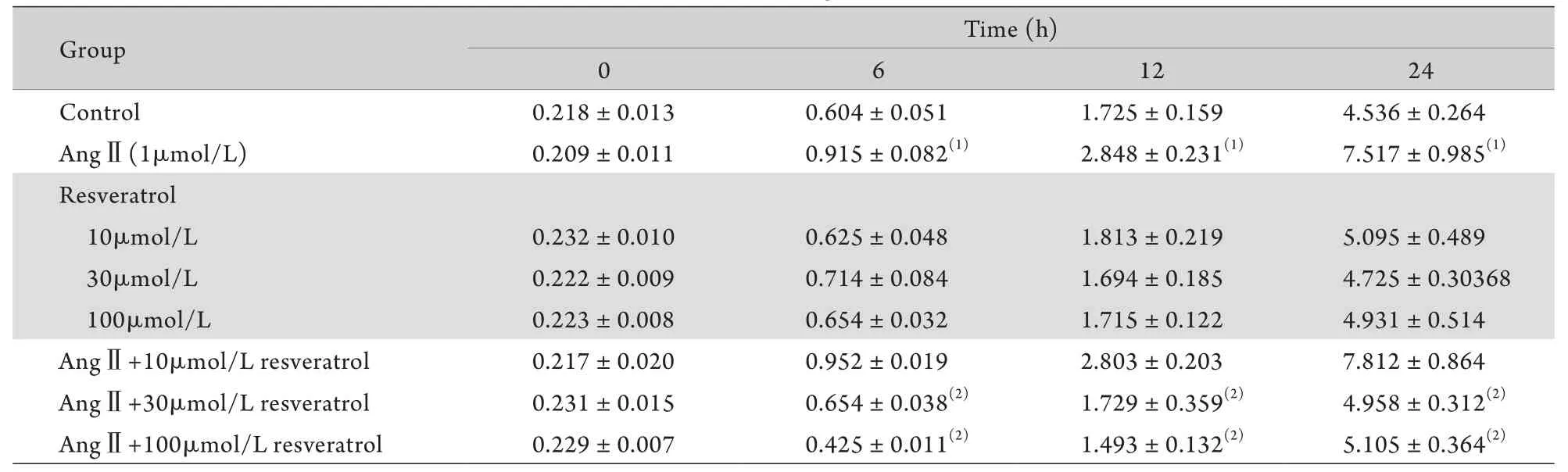
表1 各组血管平滑肌细胞计数结果(×105/ml,±s,n=3)Tab. 1 VSMCs count in different groups (×105/ml,±s, n=3)
(1)P<0.05 compared with control group; (2)P<0.05 compared with AngⅡgroup
2.4 复合物C对白藜芦醇抑制AngⅡ诱导VSMCs细胞增殖及PCNA蛋白表达的影响 采用1μmol/L复合物C预处理后,与AngⅡ+白藜芦醇组比较,复合物C+AngⅡ+白藜芦醇组VSMCs细胞数增加38.0%,细胞活力增加22.1%,差异有统计学意义(P<0.05)。Western blotting检测显示:与对照组比较,AngⅡ组VSMCs内PCNA蛋白表达显著升高,差异有统计学意义(P<0.05)。与AngⅡ组比较,白藜芦醇+AngⅡ组PCNA蛋白表达显著降低(P<0.05)。采用复合物C预处理后,复合物C+AngⅡ+白藜芦醇组VSMCs细胞内PCNA蛋白表达显著高于白藜芦醇+AngⅡ组,差异有统计学意义(P<0.05,图2)。
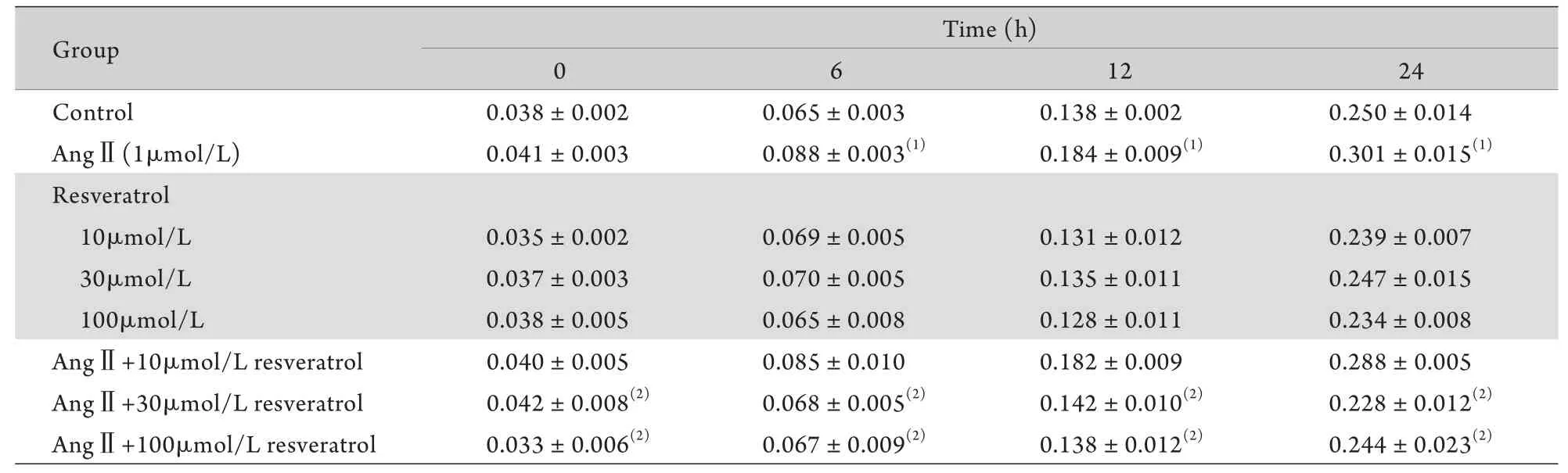
表2 MTT法检测细胞活力(A值,n=3)Tab. 2 Activity of VSMCs measured by MTT assay (A value, n=3)

图2 不同处理对VSMCs内PCNA蛋白表达的影响Fig. 2 Effects of resveratrol and AngⅡ on the protein expression of PCNA in VSMCs
3 讨 论
动脉粥样硬化是由多种病因造成的始发于动脉壁内膜的一系列复杂的分子和细胞改变的总结果,也是诱发冠心病、心肌梗死、脑梗死等心脑血管疾病的主要原因[12]。流行病学调查发现,有饮用适量葡萄酒习惯的人其心血管疾病的发病率和病死率相对较低,进一步研究发现这一现象可能与葡萄酒中含有较高含量的白藜芦醇有关[13]。现代药理学研究证实,白藜芦醇具有多种药理学作用,如抗炎、抑制血小板聚集、调节脂质代谢、保护心血管缺血性损伤和抗肿瘤等[14]。
VSMCs异常增殖是高血压、冠状动脉粥样硬化和冠状动脉介入治疗术后再狭窄等疾病的主要病理生理机制[15]。AngⅡ是一种重要的血管活性物质,在体内和体外均可诱导VSMCs过度增殖,而抑制此作用可阻止多种心血管疾病的发生及发展[16]。本研究发现AngⅡ能够显著增加VSMCs细胞的增殖能力、细胞活性及增殖标志物PCNA蛋白的表达,且30、100μmol/L白藜芦醇能够抑制AngⅡ诱导的VSMCs增殖,而10μmol/L白藜芦醇则无此作用,提示白藜芦醇对VSMCs增殖的影响呈浓度依耐性。Schreiner等[17]研究发现白藜芦醇可抑制AngⅡ诱导的VSMCs信号转导分子激活,进而发挥相应的生物学作用,且这一作用与白藜芦醇抗氧化作用有关,但其具体机制尚未阐明。
本课题组前期研究发现,100μmol/L白藜芦醇可显著减轻高糖对血管舒张活动的抑制作用,进一步研究发现白藜芦醇能够激活AMPK,进而诱导内皮细胞内一氧化氮等生物活性物质释放,起到抗氧化和抗炎症反应的作用[5]。AMPK是广泛存在于真核生物细胞中的一种信号通路分子,介导多种细胞生物学功能,本研究结果显示,给予复合物C抑制细胞内AMPK活性后,白藜芦醇抑制细胞增殖、活力和PCNA蛋白表达的作用被逆转。该结果提示AMPK是白藜芦醇发挥细胞保护作用的重要信号分子,激活AMPK后抑制氧化应激可能是白藜芦醇抑制平滑肌细胞增殖的重要作用途径。
综上所述,白藜芦醇能够抑制血管平滑肌细胞的异常增殖,进而起到预防高血压、冠心病等心脑血管疾病的作用,而AMPK信号激活在白藜芦醇细胞保护过程中起到重要作用。
[1] Little PJ, Getachew R, Rezaei HB, et al. Genistein inhibits PDGF-stimulated proteoglycan synthesis in vascular smooth muscle without blocking PDGFbeta receptor phosphorylation[J]. Arch Biochem Biophys, 2012, 525(1): 25-31.
[2] Badimon L, Vilahur G. LDL-cholesterol versus HDL-cholesterol in the atherosclerotic plaque: inflammatory resolution versus thrombotic chaos[J]. Ann N Y Acad Sci, 2012, 1254(2): 18-32.
[3] Chu LM, Lassaletta AD, Robich MP, et al. Resveratrol in the prevention and treatment of coronary artery disease[J]. Curr Atheroscler Rep, 2011, 13(6): 439-446.
[4] Paffett ML, Lucas SN, Campen MJ. Resveratrol reverses monocrotaline-induced pulmonary vascular and cardiac dysfunction: a potential role for atrogin-1 in smooth muscle[J]. Vascul Pharmacol, 2012, 56(1-2): 64-73.
[5] Xu Q, Hao X, Yang Q, et al. Resveratrol prevents hyperglycemiainduced endothelial dysfunction via activation of adenosine monophosphate-activated protein kinase[J]. Biochem Biophys Res Commun, 2009, 388(2): 389-394.
[6] Li L, Xu YF. Effect of Angiotensin Ⅱ on the expression of Kv4.2 potassium channel and its underlying mechanism in cardiomyocytes of neonatal rat[J]. Acta Acad Med CPAPF, 2010, 19(1): 26-28. [李亮, 许彦芳. Ang Ⅱ对心肌细胞Kv4.2钾通道蛋白表达的影响及机制[J]. 武警医学院学报, 2010, 19(1): 26-28.]
[7] Putnam K, Shoemaker R, Yiannikouris F, et al. The reninangiotensin system: a target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome[J]. Am J Physiol Heart Circ Physiol, 2012, 302(6): H1219-H1230.
[8] Guo RW, Wang H, Gao P, et al. An essential role for stromal interaction molecule 1 in neointima formation following arterial injury[J]. Cardiovasc Res, 2009, 81(4): 660-668.
[9] Li CY, Jiang S, Yue YJ, et al. Effect and pathway of Id1 on the cell growth of nasopharyngeal carcinoma[J]. Med J Chin PLA, 2012, 37(6): 569-572. [李春燕, 江山, 岳渝娟, 等. 分化抑制因子1对鼻咽癌细胞增殖的影响及其途径[J]. 解放军医学杂志, 2012, 37(6): 569-572.]
[10] Feng F, Gao XD, Lu YY, et al. Regulatory effect of LINE-1 ORF-1p on hepatocellular carcinoma cells and proliferation of immortalized hepatocellular cells[J]. Med J Chin PLA, 2012,37(3): 213-216. [冯帆, 高旭东, 陆荫英, 等. LINE-1 ORF-1p对肝脏肿瘤细胞系和肝源永生化细胞系增殖的调控作用[J].解放军医学杂志, 2012, 37(3): 213-216.]
[11] Jia QH, Yang XP, Hui L, et al. Effect of lead acetate on apoptosis of human renal mesangial cells and caspase-3 expression[J]. Med J Chin PLA, 2012, 37(6): 628-631. [贾庆华, 杨霄鹏, 惠玲, 等.醋酸铅对人肾小球系膜细胞凋亡及Caspase-3表达的影响[J]. 解放军医学杂志, 2012, 37(6): 628-631.]
[12] Campbell KA, Lipinski MJ, Doran AC, et al. Lymphocytes and the adventitial immune response in atherosclerosis[J]. Circ Res, 2012, 110(6): 889-900.
[13] Kroon PA, Iyer A, Chunduri P, et al. The cardiovascular nutrapharmacology of resveratrol: pharmacokinetics, molecular mechanisms and therapeutic potential[J]. Curr Med Chem, 2010, 17(23): 2442-2455.
[14] Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging[J]. Cell, 2011, 146(5): 682-695.
[15] Jing QM, Huang GQ, Han YL, et al. Comparative study on percutaneous coronary intervention of unprotected left main coronary artery disease: transradial versus transfemoral approach[J]. Med J Chin PLA, 2011, 36(11): 1149-1153. [荆全民, 黄贵奇, 韩雅玲, 等. 经桡、股动脉途径行无保护左主干病变介入治疗的对比研究[J]. 解放军医学杂志, 2011, 36(11): 1149-1153.]
[16] Wang ZM, Wu X. Influence of atorvastatin on left ventricular hypertrophy in spontaneous hypertension rats[J]. J Zhengzhou Univ (Med Sci), 2012, 47(4): 554-556. [王智敏, 吴瑕. 阿托伐他汀对自发性高血压大鼠左心室肥厚的影响[J]. 郑州大学学报(医学版), 2012, 47(4): 554-556.]
[17] Schreiner CE, Kumerz M, Gesslbauer J, et al. Resveratrol blocks Akt activation in angiotensin Ⅱ- or EGF-stimulated vascular smooth muscle cells in a redox-independent manner[J]. Cardiovasc Res, 2011, 90(1): 140-147.
Inhibitory effect of resveratrol on proliferation of vascular smooth muscle cells induced by angiotensin Ⅱ and its underlying mechanism
GAO Pan, SI Liang-yi*, XU Qiang, WANG Xiao-mei
Department of Geriatrics, Southwest Hospital, Third Military Medical University, Chongqing 400038, China
*
, E-mail: doctorsly@126.com
This work was supported by the National Natural Science Foundation of China (81000132), and the Special Fund of Chongqing Key Laboratory (CSTC) from Institute of Cardiovascular Science in Xinqiao Hospital (CQZDSYS201202)
ObjectiveTo explore the effect of resveratrol on proliferation of vascular smooth muscle cells (VSMCs) as induced by angiotensin Ⅱ (Ang Ⅱ) and its underlying mechanism.MethodsVSMCs of rat′s thoracic aorta were primarily cultured in vitro. For detecting the effects of resveratrol on Ang Ⅱ-induced VSMC proliferation, the cultured cells were divided into a control group, Ang Ⅱ group (1μmol/L), gradient concentrations of resveratrol groups, and Ang Ⅱ + gradient concentration of resveratrol groups. The gradient concentrations of resveratrol were set as 10, 30 and 100μmol/L. Cells in each group were treated for 0, 6, 12 and 24h, respectively. VSMC proliferation was detected by cell count assay, and the viability of the VMSC was measured by MTT assay. For detecting the effects of compound C [adenine monophosphate-activated protein kinase (AMPK) inhibitor] on biological effects of VSMCs, the cultured cells were divided into a control group, Ang Ⅱ group, resveratrol + Ang Ⅱ group and compound C + resveratrol + Ang Ⅱ group. The protein expression of proliferated cell nuclear antigen (PCNA) in each group was detected by Western blotting after being treated for 24 hours.ResultsCompared with control group, the cell viability and cell number in Ang Ⅱ group were significantly increased (P<0.05) after Ang Ⅱ stimulation for 6, 12 and 24 hours. Compared with AngⅡ group, the cell vitality and cell number were significantly decreased in different concentrations of resveratrol + Ang Ⅱ groups (P<0.05). On the other hand, compared with the control group, the expression of PCNA protein in Ang Ⅱ group was significantly increased (P<0.05); as compared with Ang Ⅱ group, the expression of PCNA protein in resveratrol + Ang Ⅱ group was significantlydecreased (P<0.05); as compared with resveratrol + Ang Ⅱ, the cell number, cell viability, and PCNA protein expression in compound C + resveratrol + Ang Ⅱ group was significantly increased (P<0.05).ConclusionResveratrol can inhibit Ang Ⅱstimulated cell proliferation through activation of AMPK.
resveratrol; myocytes, smooth muscle; angiotensin Ⅱ; adenosine monophosphate-activated protein kinase
R541.4
A
0577-7402(2013)04-0269-05
2012-10-03;
2013-01-09)
(责任编辑:张小利)
国家自然科学基金(81000132);重庆市新桥医院全军心血管研究所重点实验室专项经费资助项目(CQZDSYS201202)
郜攀,医学博士,主治医师。主要从事冠心病及其发病机制研究
400038 重庆 第三军医大学西南医院老年病科(郜攀、司良毅、徐强、王笑梅)
司良毅,E-mail:doctorsly@126.com
