藏波罗花化学成分的研究
2012-12-22蒋思萍朱华结
沈 岚,蒋思萍,朱华结
1中国科学院昆明植物研究所植物化学与西部植物资源持续利用国家重点实验室,昆明650204; 2西藏高原生物研究所,拉萨850001;3中国科学院研究生院,北京100049
藏波罗花(Incarvillea younghusbandii Sprague)为紫葳科Bignoniaceae角蒿属(Incarvillea Juss.)矮小宿根草本植物。别称:乌确码子布(藏语),角蒿(西藏)。主要生长于青海、西藏海拔4000~5000 m的高山沙质草甸及山坡砾石垫状灌丛中,在尼泊尔也有分布[1]。该植物干燥的花、种子和根均可入药。6~7月采花,8~9月采种子和根,就近以流水洗净,去根外皮,晾干或晒干。该药性平味苦,有消食,聪耳,调经,利肺,降血压,排黄水,消气滞的功用;对于治疗胃病、黄疸、消化不良、膨胀、耳流脓、耳聋、月经不调、高血压、肺出血等都有效。四川德格藏医用于食道疼痛的呕吐、食道癌。风湿痛用花外敷[2]。到目前为止,有关藏波罗花的化学成分研究较少,最近才有少数相关报道。除挥发油的GC-MS分析[3]外,仅报道了2个具有抗氧化和抗衰老活性的化合物[4,5]及以呋喃香豆素为主的15个化合物[6]。因此我们对藏波罗花的化学成分进行研究,从其全草95%乙醇提取物中分离得到11个化合物,所得化合物均首次从该植物中分离得到。
1 仪器和材料
质谱(MS)用VGA Autospec-3000型质谱仪测定;核磁共振谱用Bruker AM-400和DRX-500超导核磁共振仪测定,TMS为内标,δ单位为ppm,J为Hz;旋光经OA AA-55型数字旋光仪测定;柱层析用碱性氧化铝(200~300目)产自上海五四化学试剂有限公司,硅胶(200~300目),拌样用硅胶(80~100目),硅胶H和制备、分析薄层色谱(GF-254)产自青岛海洋化工和青岛美高公司;Sephadex LH-20为Pharmacia公司生产;RP-18柱层析用材料为YMC和Merck公司生产;MCI CHP 20P材料购自日本三菱化工公司。
植物样品于2009年采样自西藏,经西藏高原生物研究所蒋思萍研究员鉴定为紫葳科角蒿属植物藏波罗花(Incarvillea younghusbandii Sprague),标本保存于中国科学院昆明植物研究所植物化学与西部植物资源持续利用国家重点实验室。
2 提取分离
20 kg藏波罗花干燥全株,粉碎后用工业乙醇提取过滤(重复三次),浓缩得到粗提浸膏约2 kg。石油醚脱脂后用HCl将其调至pH=2,用乙酸乙酯萃取,得到约5 g浸膏,再用NaOH将其调至pH=11,用氯仿萃取7次浓缩获得浸膏287 g,将氯仿萃取得到的浸膏用氯仿、甲醇溶解后吸附于约1 kg氧化铝,室温挥发干后,经8 kg氧化铝柱层析,经氯仿,CHCl3-MeOH(100∶1,50∶1,20∶1,10∶1,1∶1)梯度洗脱,TLC检测,合并相同部分,纯氯仿,100∶1和50∶1为第一部分,20∶1为第二部分,10∶1为第三部分,1∶1为第四部分。各部分经 ODS柱甲醇-水(20-80%)梯度洗脱后,经乙酸乙酯-丙酮系统(10∶1~1∶1)梯度洗脱得到化合物1(4 mg)、4(54 mg)、5(48 mg)和7(90 mg),经氯仿-甲醇系统(30∶1~5∶1)梯度洗脱得到化合物2(109 mg)、3(400 mg)、6(36 mg)、8(100 mg)、9(43mg)、10(255 mg)和11(140 mg)。
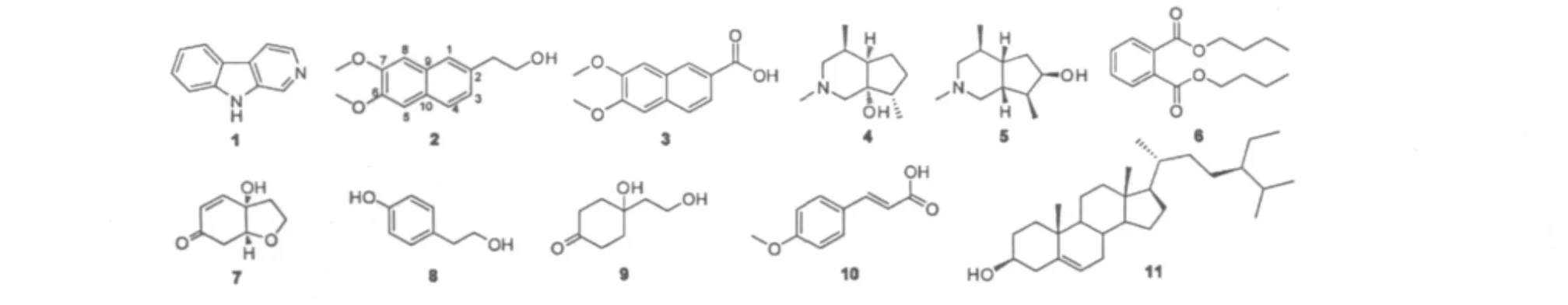
图1 化合物1~11的化学结构Fig.1 Structures of compounds 1-11
3 结构鉴定
化合物1 白色粉末,C11H8N2;1H NMR(DMSO-d6,400 MHz)δ:11.63(1H,br.s,-NH),8.88 (1H,s,H-1),8.31(1H,d,J=5.1 Hz,H-3),8.22 (1H,d,J=7.9 Hz,H-5),8.09(1H,d,5.1 Hz,H-4),7.58(1H,d,J=8.1 Hz,H-8),7.52(1H,m,H-7),7.23(1H,m,H-6);13C NMR(DMSO-d6,100 MHz)δ:140.6(C-8a),138.1(C-3),136.9(C-9a),134.0(C-1),128.2(C-7),127.5(C-4a),121.8(C-5),120.6(C-4b),119.3(C-6),114.7 (C-4),112.0(C-8)。上述光谱数据与文献报导[7]一致,可鉴定该化合物为β-咔啉。
化合物 2 白色粉末,C14H16O3;1H NMR (CDCl3,400 MHz)δ:6.84(1H,s,H-1),6.80(1H,d,J=8.0 Hz,H-4),6.71(1H,d,J=8.0 Hz,H-3),6.33(1H,s,H-8),6.02(1H,s,H-5),3.80(6H,s,-OCH3),3.66(2H,t,J=6.4 Hz,H-1'),3.0(1H,br s,-OH),2.98(1H,s,-OH),2.38(2H,m,H-2');13C NMR(CDCl3,100 MHz)δ:148.7(C-7),148.2 (C-6),132.0(C-2),131.9(C-4),130.3(C-9, 10),124.3(C-1),118.9(C-4),110.9(C-8),108.3(C-5).上述光谱数据与文献报导[8]一致,可鉴定该化合物为6,7-二甲氧基-2-萘乙醇。
化合物 3 白色晶体(CHCl3),C13H12O4;1H NMR(CDCl3,400 MHz)δ:7.53(1H,s,H-8),6.95 (1H,s,H-1),6.94(1H,d,J=6.8 Hz,H-3),6.82 (1H,d,J=6.8 Hz,H-4),5.98(1H,br s,-OH),3.73(6H,s,-OCH3);13C NMR(CDCl3,100 MHz)δ: 167.8(-COOH),148.0(C-6),146.8(C-7),144.9 (C-1),131.5(C-10),126.3(C-9),122.6(C-8),115.0(C-4),114.7(C-3),114.3(C-2),109.4(C-5),55.4(-OCH3),51.3(-OCH3)。上述光谱数据与文献报导[9]一致,可鉴定该化合物为6,7-二甲氧基-2-萘乙酸。
化合物5 无色针状晶体(CHCl3);[α-69.7 (c 0.25,CHCl3);C11H21NO;1H NMR(CDCl3,400 MHz)δ:3.74(1H,m,H-7),3.26(1H,m,H-1α),3.14(1H,m,H-3α),2.63(3H,s,N-CH3),2.21 (1H,m,H-3β),2.18(1H,m,H-4),2.15(1H,m,H-6β),2.11(1H,m,H-1β),1.98(1H,m,H-8),1.50 (1H,m,H-6α),1.39(2H,m,H-5,H-9),1.07(3H,d,J=5.2,4-CH3),0.92(3H,d,J=4.6,8-CH3);13C NMR(CDCl3,100 MHz)δ:73.1(C-7),58.1(C-1,C-3),46.3(N-CH3),45.9(C-5),42.2(C-9),37.4(C-8),32.5(C-6),30.5(C-4),17.4(4-CH3),14.1(8-CH3)。上述光谱数据与文献报导[11]一致,可鉴定该化合物为异角蒿素。
化合物 6 白色粉末,C14H18O4;1H NMR (CDCl3,400 MHz)δ:7.71(2H,m,H-3),7.52 (2H,m,H-4),4,30(4H,d,J=6.7Hz,H-5),1.72 (4H,m,H-6),1.43(4H,m,H-7),0.95(6H,t,J= 7.4Hz,H-8);13C NMR(CDCl3,100 MHz)δ:167.7 (C-1),132.2(C-2),130.9(C-3),128.8(C-4),65.5(C-5),30.5(C-6),19.1(C-7),13.7(C-8)。上述光谱数据与文献报导[12]一致,可鉴定该化合物为邻苯二甲酸二丁酯。
化合物 8 无色针状晶体(acetone);C8H10O2;1H NMR(acetone-d6,400 MHz)δ:8.2(1H,br s.1-OH),7.4(2H,d,J=8.3Hz,H-2),7.3(2H,d,J =8.3Hz,H-3),3.68(2H,t,J=7.2,H-6),3.11 (1H,br s,6-OH),2.69(2H,t,J=7.2,H-5);13C NMR(acetone-d6,100 MHz)δ:156.5(C-1),130.9 (C-4),130.6(C-2),115-8(C-3),64.2(C-6),39.4(C-5)。上述光谱数据与文献报导[14]一致,可鉴定该化合物为对羟基苯乙醇。
化合物 9 无色油状;C8H14O3;1H NMR (CDCl3,400 MHz)δ:3.88(2H,t,J=5.4Hz,H-6),2.71(2H,t,J=5.4Hz,H-5),2.12(2H,m,H-2),2.10(2H,m,H-2),1.78(2H,m,H-3),1.72(2H,m,H-3);13C NMR(CDCl3,100 MHz)δ:213.0(C-1),70.5(C-4),59.5(C-6),41.5(C-5),37.0(C-3),36.8(C-2)。上述光谱数据与文献报导[15]一致,可鉴定该化合物为Cleroindicin B。
化合物 10 白色针状晶体(CHCl3);C10H10O3;1H NMR(CDCl3,500 MHz)δ:7.57(1H,d,J= 16 Hz,H-7),7.40(2H,d,J=8 Hz,H-2,6),6.77 (2H,d,J=8 Hz,H-3,5),6.27(1H,d,J=16 Hz,H-8),3.72(3H,s,-OCH3);13C NMR(CDCl3,100 MHz)δ:169.8(C-9),161.2(C-4),146.6(C-7),131.2(C-2,6),127.1(C-1),116.8(C-3,5),114.9(C-8),52.1(-OCH3)。上述光谱数据与文献报导[16]一致,可鉴定该化合物为4-甲氧基肉桂酸。
化合物11 白色粉末;C29H50O,ESI-MS(pos.) m/z:415[M+1]+;1H NMR(CDCl3,400 MHz)δ: 5.28(1H,br d,J=4.8 Hz,H-6),3.46(1H,m,H-3),0.94(3H,s,H-19),0.86(3H,d,J=8.0 Hz,H-21),0.84(3H,m,H-29),0.78(3H,m,H-26),0.75 (3H,m,H-27),0.61(3H,s,H-18);13C NMR (CDCl3,100 MHz)δ:140.7(s,C-5),121.7(d,C-6),71.8(d,C-3),56.7(d,C-14),56.0(d,C-17),50.1(d,C-9),45.7(d,C-24),42.3(s,C-13),42.2(d,C-4),39.7(t,C-12),37.2(t,C-1),36.6 (s,C-10),36.1(d,C-20),33.9(t,C-22),31.9(d,C-2).31.8(t,C-7),31.6(t,C-8),29.1(d,C-25),28.2(t,C-16),25.9(t,C-23),24.3(t,C-15),23.0 (t,C-28),21.0(t,C-11),19.8(q,C-26),19.4(q,C-27),19.0(q,C-19),18.7(q,C-21),12.0(q,C-29),11.8(q,C-18)。上述光谱数据与文献报导[17]一致,可鉴定该化合物为β-谷甾醇。
1 Delectis Florae Reipublicae Popularis Sinicae Agendae Aca-demiae Sinicae Edita.Florae Reipublicae Popularis Sinicae; Science Press:Beijing,1988.46-49.
2 Northwest Institute of Plateau Biology.Tibetan flora.Xining: Qinghai People's Publishing House,1991,464.
3 Fu Y(傅予),Li PJ(李普济),Bai Y(白央),et al.Chemical analysis of essential oil from Incarvillea younghusbandii Sprague by GC-MS.J Instrum Anal(分析测试学报),2008,27(增刊):70-71.
4 Pan WG(潘为高),Jiang SP(蒋思萍),Luo P(罗彭),et al.Isolation,purification and structure identification of two phenolic glycosides from the roots of Incarvillea younghusbandii Sprague and their antioxidant activities.Acta Pharmaceutica Sinica(药学学报),2011,46:422-427.
5 Pan WG,Jiang SP,Luo P,et al.Isolation,purification and structure identification of antioxidant compound from the roots of Incarvillea younghusbandii Sprague and its life span prolonging effect in Drosophila melanogaster.Nat Prod Res,2008,22:719-725.
6 Fu Y(傅予),Bai Y(白央),Dawa ZM(达娃卓玛),et al.Chemical constituents of Incarvillea younghusbandii.Chin J Chin Mater Med(中国中药杂志),2010,35:58-62.
7 Corbally RP,Mehta LK,Parrick J,et al.Experimental and calculated13C chemical shifts for α-,β-,γ-and δ-carbolines.Magn Reson Chem,2000,38:1034-1036.
8 Bailey, DM.Heterocyclic aminoalkylnaphthols.US1979-63343 4327022,1979-8-1.
9 Goeksu S,Kazaz C,Suetbeyaz Y,et al.A concise synthesis of 2-amino-1,2,3,4-tetra-hydronaphthalene-6,7-diol("6,7-ADTN")from naphthalene-2,3-diol.Helv Chim Act,2003, 86:3310-3313.
10 Kam TS,Choo YM,Chen W,et al.Indole and monoterpene alkaloids from the leaves of Kopsia dasyrachis.Phytochem,1999,52:959-963.
11 Su YQ,Shen YH,Lin S,et al.Two new alkaloids from Incarvillea mairei var.grandiflora.Helv Chim Act,2009,92:165-170.
12 Jiang PP(姜佩佩),Wang JH(王吉鸿),Ji MH(纪明慧),et al.Chemical constituents of Crotalaria mucronata.Chin Tradit Herb Drugs(中草药),2011,10:1925-1928.
13 Busque F,Canto M,de March P,et al.From p-benzoquinone to cyclohexane chirons:first asymmetric synthesis of(+)-rengyolone and(+)-and(-)-menisdaurilide.Tetrahedron: Asym,2003,14:2021-2032.
14 Wu SX(吴少雄),Guo YD(郭亚东),Guo SY(郭祀远),et al.Study of the Chemical Constituents of Ethanol Extracts of Rhodiola Crenulata.H.Modern Food Sci Tech(现代食品科技),2008,24:322-326.
15 Huang ZS,Pei YH,Shen YH,et al.Cyclohexyl-ethanol derivatives from the roots of Incarvillea mairei.J Asian Nat Prod Res,2009,11:523-528.
16 Chen JW(陈建伟),Duan ZF(段志富),Li X(李祥),et al.Study on water-soluble active constituents of Radix.Nat Prod Res Dev(天然产物研究与开发),2010,22:232-234,247.
17 Tang JS(唐京生),Chen J(陈谨),Tian J(田军),et al.Study of Chemical Constituents of Pittosporum Omeiense.J Sichuan Univ(Nat Sci Edit)(四川大学学报,自科版),2002,39:538-541.
