四角锥形结构化合物BeB4X4(X=H,F,Cl),HBB4H4与BB4H4+中B4平面环的半芳香性
2012-11-13郝希云
郝希云
(吉林化工学院,化学与制药工程学院,化学系,吉林 132022)
四角锥形结构化合物BeB4X4(X=H,F,Cl),HBB4H4与BB4H4+中B4平面环的半芳香性
郝希云
(吉林化工学院,化学与制药工程学院,化学系,吉林 132022)
选用6-311++G(3d f,2p)基组,在二级微扰的理论下,对四角锥形结构化合物BeB4X4(X=H,F,Cl),HBB4H4与BB4H4+的分子振动频率,及原子间的相互作用进行了计算,作用能的计算使用了CCSD(T)方法。结果显示HBB4H4与BB4H4+是违反韦德规则的另两个特例,它们表现稳定的原因与芳香性有关。
韦德规则;核独立化学位移;硼氢化合物;芳香性;离域电子
0 Introduction
The study of boron hydrides has been a classic field in inorganic chemistry since their first preparation by Stock[1]. Because of the electron deficiency of the boron hydrides,the description of their bonding was a problem in theoretical chemistry formany years.In the early 1960s,Lipscomb applied the concept of the three-center bond to the higher boron hydrides and developed a simple method for describing and predicting the topology of such compounds[2-3]. Further progress in studying the relationship between structure and electron count have led,from Lipscombs styx-rules,to the generalizedelectron-count schemes known as Wade′s rules[4-6], today a lot of works are still focus on the electroncounting rules and tried tomake itmore perfect[7-9].
In general,Wade′s rules divide clusters into several groups with respect to the number of electron pairsassociated with cage bonding.A famous exception toWade′s rules is B4H4[2-10]In the tetrahedral geometry it has an open-shell triplet ground state.Oxidation of the tetrahedral dianion leads to the neutral cluster B4H4which is known for a tetrahedral shape with a closed-shell singlet ground state[11-12].Recently,some other structurally stable B4H4isomers were pointed out[13-14].Among them a D2d-symmetric B4H4isomer caused usmuch attention.In the following works,the dianion entity B4H42-which also have a D2d-symmetry wasgot[15].Although the B4H42-was calculated unstable, the derivatives B4(CN)42-and B4(BO)42-were predicted to be observable in suitable experiments.The similar researches about this kind of four member ring[16-19]provides the idea that the four pzatomic orbitals from four B atoms formed aπ-molecular orbital which is occupied with an excess electron pair.
Since some B4X42-cluster with a D2d-symmetric shape can exist stable,the netural B4ring should have a strong willing to get an excess electron pair.A Be atom has an electron pair located on its outside 2s orbital.If a Be atom close to a netural cluster B4X4with B4ring.There should have a strong interaction between them.Recently the similar study aboutmetalpolyboron compounds[20]MB6(M=Be,Mg,Ca,and Sr) species have shown some special interaction between metal and B6planar ring.The B62-which is proved to be antiaromatic[21-22]could be transformed into an aromatic one under the influence of themetal ions[20]. In this present paper,the interaction between the well known boron hydride B4H4(and its derivatives)and the metal Be was studied,the effects of the terminal atoms which is connected to the B atoms was discussed.And the aromaticity of the clusters BeB4X4(X=H,F,Cl)were studied by the nucleus-independent chemical shifts(NICS)[23-26]method.
At last,if the Be atom was a successful electron pair donor,the similar electron pair donor BH and B+should also be test.And the clusters HBB4H4and BB4H4+may be another twoexceptions toWade′s rules.
1 Com putation details
The geometric optimizations of the clusters BeB4X4(X=H,F,Cl),HBB4H4and BB4H4+were performed at the second-order Mller-Plesset perturbation theory (MP2)level.With the 6-311++G(3d f,2p) basis set the stationary points were got.The vibratinal frequencies calculated are all real for these clusters. The similar cluster MgB4H4was also calculated.
For the BeB4X4(X=H,F,Cl)clusters,the natural bond orbitals (NBO)[27]methods were used to analyze their populations and atomic charges.Just from the charges of the Be atom,the message about the electrons transfer can be got.
The interaction energy between B4X4ring and metal Be were calculated at the CCSD(T)/6-311++G (3d f,2p)level and the counterpoise procedure[28]was employed to remove basis-set superposition error (BSSE).
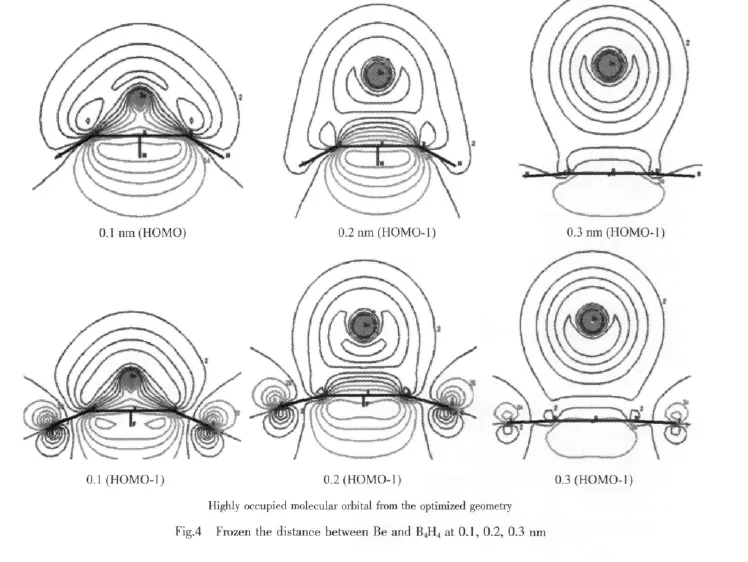
The distance between the geometric center of the B4plane and the Be atom are frozen at 0.1,0.2,0.3 nm.The four B atoms are forced on one plane.Then the structure of the BeB4H4are optimized to study the interactions between Be and B4H4.In another optimization the Be atoms were frozen at its optimized position for BeB4H4and BeB4F4,but the electron pairs were removed to study the effects of the terminal atoms.
NICS values for the BeB4X4(X=H,F,Cl)clusters were calculated at the GIAO-HF//MP2 method with the 6-311++G (3d f,2p)basis set.Five points were selected to calculate the absolute shielding.They are the center of the B4plane (defined as BC),0.05 nm out of the plane(BC-0.05 is the point close to the Be atom,BC+0.05 is the point far to the Be atom),0.1 nm out of the plane (BC-0.1 is the point close to the Be atom,BC+0.1 is the point far to the Be atom).
For all the clusters,their two-or three-dimensional plots ofmolecular orbitals were generated with the Molden program[29].All the calculations were performed with the GAUSSIAN 03 program package[30].
2 Results and discussion
2.1 Geometrical characteristics
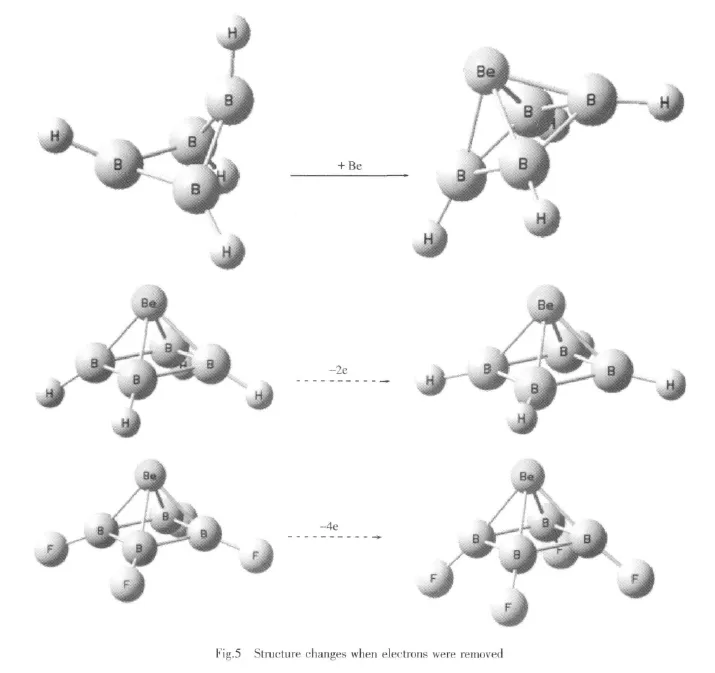
The optimized structures of BeB4X4(X=H,F,Cl) are shown in Fig.1 and Fig.2.The corresponding geometric parameters are listed in Table 1.It shows that the four B atoms located just on one plane and formed a square.The Be atom is on one side and the four X atoms are on the other side of the B4plane. This kind of cluster gives a square pyramidal shape with C4v-symmetriy.While just for the B4X4or B4X42-clusters without the Be atom the four B atoms were not on one plane.Here the Be atom locate very close to the B4ring.The distance between the geometric center of the B4plane and the Be atom are 0.127 nm for BeB4H4,0.135 nm for BeB4F4and 0.133 nm for BeB4Cl4.It shows that the terminal X atoms are repulsed by the Be atom and they are not located on the same plane of the B4ring.The optimized structure of the Mg-B4H4cluster is also given in Fig.2.It just shows a C4v-symmetriy,and don′t have the square pyramidal shape.The Mg atom is a bit far from the B4H4ring.
The highest occupied molecular orbital(HOMO) of the BeB4H4in Fig.3.shows that the B4H4part of the cluster act just as a bowl which accommodate the electrons transferred from the Be atom.For the three clusters B4X4(X=H,F,Cl)the shapes of the B4X4bowls have some difference.Theθangle shown in Fig.1.is 147.6°for B4H4,the same angle for B4F4is 11.3°larger.Then the B4H4bowl looks smaller than the B4F4one.
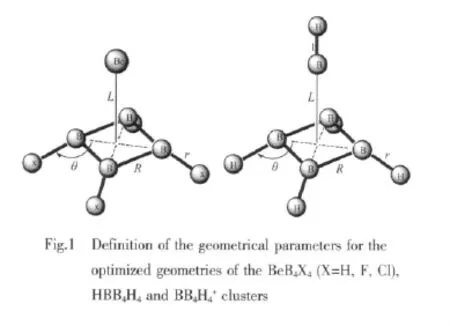

Table 1 Geometrical parameters for BeB4X4(X=H, F,Cl)cluster at MP2 levelw ith the 6-311++G(3d f,2p)
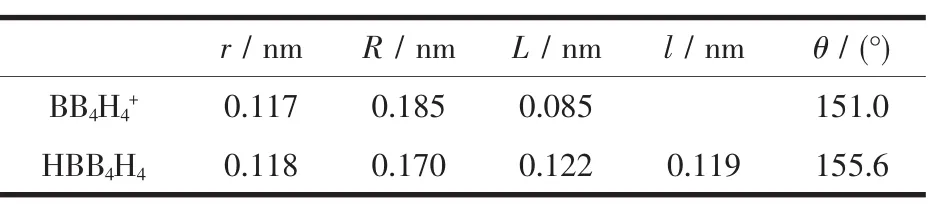
Table 2 Geometrical parameters for HBB4H4 and BB4H4+cluster w ith square pyram idal shape at M P2/6-311++G(3df,2p)level

When the Be atom were replaced by BH or B+, the stable clusters HBB4H4and BB4H4+were also got in the square pyramidal shape.They are also shown in Fig.2.The geometric parameters are listed in Table 2.According to the Wade magic electron count rule, they are short of four and six electrons respectively. But they all act stable here.

2.2 Effects of the term inal atoms
From the NBO calculations,the natural charges of the Be atomswere got for the clusters BeB4X4(X= H,F,Cl).They are 1.39 for BeB4H4,1.21 for BeB4F4and 1.35 for BeB4Cl4.That means for the BeB4H4cluster there are 1.39 electrons transferred from the Be atom to the B4ring,that is the largest number in these three cluster.While for the BeB4F4cluster which have the strong electron pulling terminal atoms F,the transferred electrons from the Be atom to the B4ring are only 1.21,which is the smallest one in the three cluster.Since there are electrons in the bowls, the X (H,F,Cl)atoms should have a direction interaction with the electrons.The corresponding positive charged H atoms should close to the electron cloud and the corresponding negative charged F atoms will leave away.Then the shape of the bowl can be in control.And the largest electrons transferred from the Be atom to the B4ring for BeB4H4is obviously owes to the direct interaction between the electrons in B4H4bowl and the four H atoms nearby.And because of this H-e interaction the Be atom locate more close to the B4plane in the BeB4H4cluster than the other two.
The interaction energies between Be and B4X4(X=H,F,Cl)calculated are listed in Table 3.At the CCSD(T)level.It is 6.235 eV for BeB4H4,3.643 eV for BeB4F4and 4.196 eV for BeB4Cl4.The BeB4H4cluster which has the shortest Be and B4plane distance(0.127 nm)get the largest interaction energy. And in the BeB4H4cluster the Be atom also has the largest natural charge.On the contrary,the BeB4F4cluster which has the smallest charge on its Be atom get the smallest interaction energy,and the distance between its B4plane and the Be atom is the longest one(0.135 nm).There is a strong correlation between the electrons transfer and the direct X-e interactions, the electrons transfer brings the X-e interaction,and for the BeB4H4cluster the H-e interaction promote the electrons transfer effect.
When the distance between the geometric center of the B4plane and the Be atom are frozen at 0.1,0.2, 0.3 nm.The optimized structures of the BeB4H4cluster are shown in Fig.3.It is clear that the electrons transfer is so sensitive to the distance.When the distance is frozen at 0.3 nm,there are few electrons transferred from the Be atom to the B4ring. And the bowl shape B4H4cluster turns to a plane shapewithout the special H-e interactions.
In another test the Be atoms were frozen at its optimized position for BeB4H4and BeB4F4,but the electron pairswere removed to study the effects of the terminal atoms.When two electrons were removed from the BeB4H4cluster,theθangle of the cluster changes from 147.6°to 168.1°.it increased about 20.5°and becomes larger than that of the BeB4F4cluster.For the BeB4F4itsθangle changes little when one electron pair was removed.Since its HOMO-1 is our interested orbital,we removed two electron pairs, and theθangle of the BeB4F4changes from 158.9°to 139.0°.it decreased 19.9°.Now the B4H4bowl turns larger than the B4F4one without the special X-e interactions.

Table 3 Interaction energies between Be and B4X4(X=H,F,Cl)
2.3 Aromaticity
NICS is a simple and efficient aromaticity criterion in a wide range ofmolecules.It is based on the negative value of themagnetic shielding computed at or above the geometrical centers of rings or clusters.Aromaticity is characterized by the negative NICS values(given in ppm),antiaromaticity is shown by positive NICS values,and nonaromatic compounds have NICSvalues close to zero[23-26].Themore negative the NICS,themore aromatic themolecule is.
In this study,The NICS values were calculated at five different points on and above the B4plane (described in the computation details part).The results were listed in Table 4.For the three BeB4X4clusters,The NICS values are different on the two sides of the B4plane.On the Be atom side the NICS values calculated are all positive,while on the four terminal atoms side the NICS values calculated are negative.At the center of the B4ring,the NICS value is-19.887 1 ppm for BeB4H4and-7.209 0 ppm for BeB4Cl4which is suggesting the existence of delocalization and aromaticity.And for BeB4F4the NICS values is 0.375 3 ppm,which indicate that the BeB4F4is a nonaromatic compound.
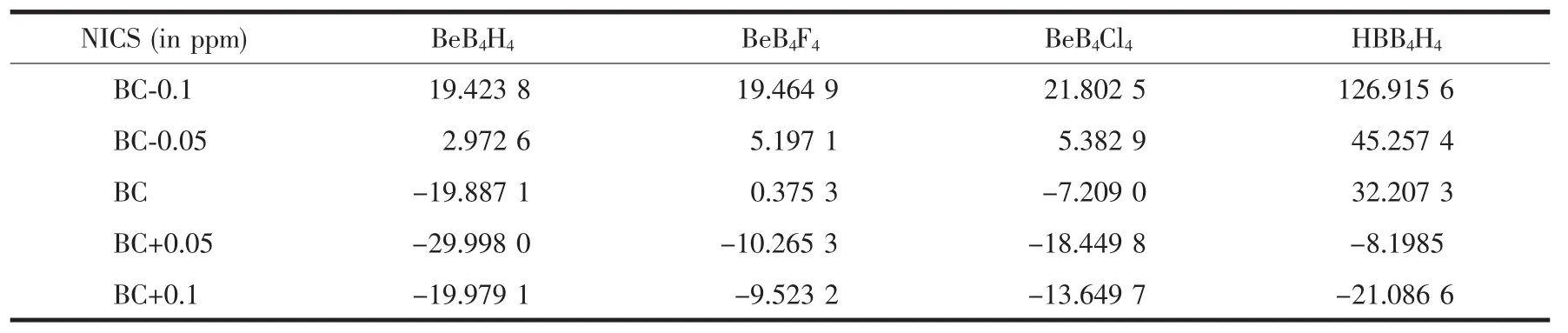
Table 4 Calculated NICS values(ppm)w ith GIAO-HF//MP2/6-311++G(3df,2p)method for the BeB4X4(X=H,F,Cl)clusters
Some delocalized higher occupied molecular orbitals of the BeB4X4(X=H,F,Cl)and HBB4H4were shown in Fig.2.For the BeB4H4cluster the highest occupied molecular orbital (HOMO,1a1)is rather special.It is a combination of the in-plane 4-centerσ bond(σp1)and the out-of-planeπbond.The HOMO-1 (1b1)is another in-plane 4-centerσbond(σp2). These two kind of 4-centerσ bonds have been discussed by Zhan et al[31].(σp1 was shown in Fig.3 andσp2 was shown in Fig.2 in the reference 31)in their article.Zhan et al.first proposed the orbital analysis approach of the multiple-fold aromaticity for the square-planar Al42-structure which can be determined by three independent delocalized (σandπ) bonding systems.While in this BeB4H4cluster the multi-centerσ and π orbital which should be independent are combined together in HOMO.The HOMO-2 (1e1)includes two degenerated orbitals formed from the out-of-plane p orbitals.The HOMO-4 (2a1)includes some parts of the in-plane 4-centerσ bond(σp1)as that type in HOMO.The HOMO-6(3a1) is another 4-centerσbond which wasmade from the s orbitals of each B atoms.
Theσ-Aromaticity initially introduced in hydrocarbons[32-34]has been extended tometal,nonmetal, andmetal-nonmetalclusters[31,35-37].In theBeB4H4cluster, the HOMO-1 and HOMO-6 are two independent delocalizedσ-bonding orbitals.Each of the twoσ delocalized bonding systems containing two σ electrons that rendersσaromaticity.The delocalized σ-bonding component in HOMO-4 takes only a small part.For the delocalized π-bonding systems the HOMO-2 with two degenerated orbitals contains four πelectrons.If the HOMO was regard as aπbonding orbital,theπ electrons will be six and satisfy the famous 4n+2 electron counting rule.From the HOMO picture it shows clear that themain electron cloud are localized down the B4plane on the four legends side. Then in a simple word,there are fourπ electrons active above the B4plane on the Be atom side,and there are sixπelectrons active down the B4plane on the other side.Thatmaybe explains the different sign NICS values on the two sides of the B4plane.And the B4ring here has both the aromaticity and antiaromaticity.This is called half Aromaticity in brief here.
The molecular orbital picture of the BeB4F4cluster is similar to the BeB4H4cluster except the consequence of the HOMO and HOMO-1.The corresponding orbital picture of the BeB4Cl4cluster is similar to the BeB4F4cluster,but the (HOMO-7,2a1) for BeB4Cl4which has little components of the multicenter bond was not list.And for the HBB4H4 cluster,itsmolecular orbital picture is adjacent to the BeB4H4cluster.
3 Summary
Here the BeB4X4(X=H,F,Cl),HBB4H4and BB4H4+clusters were calculated to be stable and all have the square pyramidal shape.The vertexes of these clusters Be,BH and B act as electron pair donors.And the B4X4(X=H,F,Cl)part act as electron pair accepters.The B4X4part looked just like a bowl to accommodate the electron pair.
For the BeB4X4(X=H,F,Cl)clusters the interaction energies between Be and B4X4(X=H,F, Cl)part decrease in the sequence of BeB4H4>BeB4Cl4> BeB4F4,the charges of the Be atoms decrease in the same sequence.A direct interaction between the terminal atoms X(H,F,Cl)and the electrons in bowls maybe owns to this phenomenon.The shape of the B4X4bowls are also affected through these X-e interactions.With the positive charged Be atom and the electron cloud in the B4X4bowl,the stable BeB4X4(X=H,F,Cl)clusters should have some special characterwhen actwith other reagents.
From the NICS values and the phenomenon that two delocalized electrons were forced on one side of the B4plane in B4X4bowl,The B4plane here was proved to have the half Aromaticity.
The stable clusters HBB4H4and BB4H4+are another two exceptions to theWade′s rules.
[1]Stock A.Hydrides of Boron and Silicon.Ithaca,New York: Cornell University Press,1933.
[2]LipscombW N.Boron Hydrides.New York:Benjamin,1963.
[3]Lipscomb W N.Science,1977,196:1047-1055
[4]O′Neill M E,Wade K.J.Mol.Struct.:Theochem,1983,103: 259-268
[5]O′NeillM E,Wade K.Polyhedron,1984,3:199-202
[6]Mingos DM P,Wales D J.Introduction to Cluster Chemistry; Englewood Cliffs,NJ:Prentice Hall,1990.
[7]Eluvathingal D J,Musiri M B,Pattath D P.J.Am.Chem. Soc.,2001,123:4313-4323
[8]Eluvathingal D J,MusiriM B.J.Am.Chem.Soc.,2001,123: 4324-4330
[9]MusiriM B,Eluvathingal D J.J.Am.Chem.Soc.,2000,122: 4516-4517
[10]Porterfield W W,Jones M E,GillW R,et al.Inorg.Chem., 1990,29:2914-2919
[11]Kalvoda S,Paulus B,Dolg M,et al.J.Phys.Chem.Chem. Phys.,2001,3:514-522
[12]Lin C S,Li J,Liu CW.Chin.J.Chem.,1994,12:305-313
[13]McKee M L.Inorg.Chem.,1999,38:321-330
[14]Mach P,Hubacˇ I,Mavridis A.Chem.Phys.Lett.,1994,226: 469-474
[15]Dreuw A,Zint N,Cederbaum L S.J.Am.Chem.Soc.,2002, 124:10903-10910
[16]PrEsang C,Hofmann M,Geiseler G,et al.Angew.Chem. Int.Ed.,2002,41:1526-1529
[17]Maier A,Hofmann M,Pritzkow H,et al.Angew.Chem.Int. Ed.,2002,41:1529-1532
[18]Sahin Y,PrEsang C,Amseis P,et al.Angew.Chem.Int. Ed.,2003,42:669-671
[19]Mesbah W,PrEsang C,Hofmann M,et al.Angew.Chem. Int.Ed.,2003,42:1717-1719
[20]LiQ S,Qiao J.J.Phys.Chem.A,2003,107:7869-7873
[21]Alexandrova A N,Boldyrev A I,Zhai H J,et al.J.Phys. Chem.A,2003,107:1359-1369
[22]Ma J,Li Z H,Fan K N,et al.Chem.Phys.Lett.,2003,372: 708-716
[23]Schleyer P v R,Maerker C,Dransfekd A,et al.J.Am. Chem.Soc.,1996,118:6317-6318
[24]Schleyer P v R,Jiao H.Pure Appl.Chem.,1996,68:209-218
[25]Schleyer P v R,Jiao H,Hommes N V E,et al.J.Am. Chem.Soc.,1997,119:12669-12670
[26]Goldfuss B,Schleyer P v R,Hampel F.Organometallics, 1996,15:1755-1757
[27]Reed A E,Curtiss L A,Weinhold F.Chem.Rev.,1988,88: 899-926
[28]Boys SF,Bernardi F.Mol.Phys.,1970,19:553-566
[29]Schaftenaar G,Noordik JH.J.Comput.-Aided Mol.Design., 2000,14:123-134
[30]Frisch M J,Trucks GW,Schlegel H B,et al.Gaussian03,Revision A.01;Gaussian Inc.:Pittsburgh,PA,2003.
[31]Zhan C G,Zheng F,Dixon D A.J.Am.Chem.Soc.,2002, 124:14795-14803
[32]Minkin V I,Glukhovtsev M N,Simkin B Y.Aromaticity and Antiaromaticity.New York:Wiley,1994.
[33]Cremer D,Binkley JS,Pople JA,et al.J.Am.Chem.Soc., 1974,96:6900-6903
[34]Chandraekhar J,Jemmis E D,Schleyer P v R.Tetrahedron Lett.,1979,39:3707-3710
[35]Kuznetsov A E,Corbett JD,Wang LS,etal.Angew.Chem., Int.Ed.,2001,40:3369-3372
[36]Alexandrova A N,Boldyrev A I.J.Phys.Chem.A,2003, 107:554-560
[37]Ma J,Li Z H,Fan K N,et al.Chem.Phys.Lett.,2003,372: 708-716
One Kind of B4Plane Ring in the Square Pyram idal Clusters BeB4X4(X=H,F,Cl), HBB4H4and BB4H4+w ith Special Half Aromaticity
HAO Xi-Yun
(Departmentof Chemistry,Jilin Institute of Chemical Technology,Jilin,Jilin 132022,China)
Using the 6-311++G(3d f,2p)basis set,the clusters BeB4X4(X=H,F,Cl),HBB4H4and BB4H4+which all have a square pyramidal shape were got at the second-order Mller-Plesset perturbation (MP2)level with all frequencies real.The vertexes of these square pyramidal clusters are Be,BH and B separately.They all act as a two electron donor for B4X4,and the B4X4act as a bowl to accommodate the pair of electrons.The interaction energies between the vertexes and the B4X4bowlswere calculated at the CCSD(T)level.The interactions between bowls with different terminal atoms and transferred electrons were discussed.The character of the B4ring is affected deeply with the terminal atoms.For HBB4H4and BB4H4+,they are four and six electrons short of the Wademagic electron count,but they all have their strong bonding here.
Wade′s rules;NICS;boron hydride;aromaticity;delocalized electrons
O613.8+1
A
1001-4861(2012)09-1950-09
2012-02-16。收修改稿日期:2012-05-30。
吉林化工学院博士启动基金资助项目。
E-mail:cchxyh@163.com;会员登记号:S06N8129M1004。
