尖晶石型NiCr2O4的燃烧法合成及表征
2012-11-13耿庆芬高祥虎杨生荣
耿庆芬 赵 鑫 高祥虎 杨生荣 刘 刚*,
(1中科院兰州化学物理研究所固体润滑国家重点实验室,兰州 730000) (2中国科学院研究生院,北京 100049) (3中科院兰州化学物理研究所环境材料与生态化学发展中心,兰州 730000)
尖晶石型NiCr2O4的燃烧法合成及表征
耿庆芬1,2赵 鑫3高祥虎3杨生荣1刘 刚*,3
(1中科院兰州化学物理研究所固体润滑国家重点实验室,兰州 730000) (2中国科学院研究生院,北京 100049) (3中科院兰州化学物理研究所环境材料与生态化学发展中心,兰州 730000)
通过溶胶-凝胶燃烧法制备了具有立方相的尖晶石型NiCr2O4颜料,并采用TG/DSC、XRD、FTIR和SEM等手段对其进行表征和研究。结果表明:将燃烧后的粉末于900℃下煅烧2 h可以得到单一相的NiCr2O4,温度达不到900℃时所得粉末(包括燃烧后粉末和500和700℃煅烧得到的粉末)为NiO和Cr2O3组成的混合物。利用紫外/可见/近红外分光光度计仔细研究了燃烧后粉末和煅烧后粉末的颜色参数。尽管具有相同的成分,500和700℃下煅烧得到的粉末与燃烧后粉末却具有明显不同的颜色,这可从一氧化镍为非整比化合物的角度加以解释。此外,900和1 000℃下煅烧得到的NiCr2O4粉末呈现出随温度变化的绿色。
燃烧合成法;尖晶石型NiCr2O4;颜色;晶体生长
0 Introduction
Nickel chromite (NiCr2O4),a transition-metal oxide with spinel-like structure, has attracted increasing interests as pigment[1],catalyst[2],magnetic material[3-4]and semiconductor[5].In this contribution, NiCr2O4was studied as ceramic pigment.NiCr2O4has been conventionally synthesized by solid-state reaction.However,conventional solid-state reaction for preparing spinel powders requires high temperature and usually produces agglomerated particles,which hinders their application in various fields.Other techniques have also been applied to prepare NiCr2O4powders such as co-precipitation[2,6], flux decomposition[7]and combustion synthesis[1].Structures and properties of NiCr2O4are strongly influenced by the synthetic method.The sol-gel combustion synthesis shows promising potential for the synthesis of spinel powders for the following advantages: (i)use of inexpensive precursors(metal nitrates and citric acid);(ii)relatively less synthesis steps;(iii)high yield;(iv)efficiency of scale-up;(v) energy-saving.
In this contribution,we report the synthesis of spinel NiCr2O4powder by a nitrate-citrate sol-gel selfcombustion process.The as-burnt powder is calcined at a wide range of elevated temperatures and subsequently the XRD and FTIR spectra techniques are used to find out the calcination temperature needed to obtain spinel NiCr2O4.As color is an important characteristic for a pigment,the diffuse reflectance and color parameters of powders obtained during the synthesis process and the as-prepared NiCr2O4powders were studied in detail using PerkinElmer Lambda 950 UV-Vis-NIR spectrometer.The results of color parameter characterization can help us to prepare suitable NiCr2O4with certain color.
1 Experimental
1.1 Combustion synthesis of NiCr2O4
Ni(NO3)2·6H2O,Cr(NO3)3·9H2O,Polyethylene Glycol 200 (PEG 200)and citric acid were used as raw materials to prepare NiCr2O4powder.The ideal redox reaction between citric acid and metal nitrates for NiCr2O4synthesis is presented in Eq.(1):
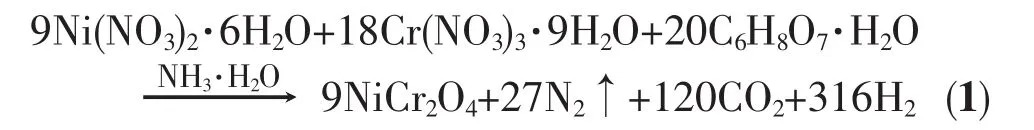
In a typical experimental procedure,Ni(NO3)2· 6H2O(20 mmol),Cr(NO3)3·9H2O(40 mmol)and PEG 200 (12 mmol)were dissolved into 40 mL ultrapure water.Then a stoichiometric amount of citric acid was added.After the mixing is completed,a homogenous transparent solution was achieved within a few seconds.Ammonia solution then was slowly added to adjust the pH value to 7.The resulting mixture was continuously stirred and kept at 70℃ for a sufficient period of time to allow the formation of Ni-Cr-citric acid gel.Then the gelwas placed in an oven and kept at 135℃ for about 3 h.Subsequently,the obtained xerogel was ignited in air using a small amount of ethanol as initiating agent.The xreogel burnt in a selfpropagating combustion manner until it was completely burnt out to form loose black powder.Finally,the as-burnt powder was calcined at various elevated temperatures(500~1 000℃)for 2 h.
1.2 Characterization techniques
Synchronous thermogravimetry/differential scanning calorimetry(TG/DSC)analysis of the xerogel were carried out using STA 449C simultaneous thermal analyzer(NETSCH,Germany)at a heating rate of 10℃·min-1.Air at 20 mL·min-1was used as purge gas.FTIR spectra in the range of 400~4 000 cm-1for the obtained powders pressed with KBr were recorded on a TENSOR27 FTIR spectrometer (Bruker Optics, Germany).The phase analysis for the prepared powders was carried out with X-ray diffractometer (XRD,Rigaku D/max 2400/PC,Japan)under the following conditions:graphite monochromatic copper radiation (Cu Kα,λ=0.154 18 nm);sodium iodide scintillation detector;scintillalion counter;40 kV as accelerating voltage;100mA as emission current;and the 2θrange of 15°~95°for all powder samples at a scan rate of 10°min-1.Scanning electron microscope (SEM)images were taken with a JEOL JSM-6701F field emission scanning electronmicroscope at5.0 kV.Diffuse reflectance of the as-burnt and calcined powders were measured by the LAMBDA 950 UVVis-NIR spectrometer(Perkin Elmer,USA)equipped with an integration sphere (module 150 mm),from 380~780 nm range with a data interval of 10.00 nm.L*a*b*color parameterswas obtained with the assistant of the color software from the PerkinElmer Lambda 950 UV-Vis-NIR spectrometer.The CIE-L*a*b*colorimetricmethod was used,as recommended by the CIE (Commission Internationale de IEclairage)[8].In thismethod,L*is the lightness axis[black(0)→white (100)],a*the green(-)→red(+)axis,and b*the blue (-)→yellow(+)axis.
2 Results and discussion
TG/DSC results of the xerogel precursor are shown in Fig.1.The DSC curve shows two sharp exothermic peaks.The first exothermic peak at around 220℃,accompanied by 77%weight loss at the same temperature,is caused by the autocatalytic anionic oxidation-reduction reaction between the nitrates and citric acid.The weight loss corresponds to the liberation of a large amount of gases during the combustion process.The second exothermic peak at about 310℃ is caused by the combustion of PEG 200.The corresponding 7%weight loss coincideswith the mass fraction of PEG 200 in the xerogel system.There is no evidence of weight loss in the TG curve above 350℃,so the phase transition,if it takes place,it should be observed by XRD technique.
In order to remove the residual organic components and study the phase transformation,the as-burnt powder was calcined at various elevated temperatures.Fig.2 shows the XRD patterns of the xerogel,the as-burnt and calcined powders.The xerogel precursor shows citrate crystals structure in amorphous substances.XRD patterns for the as-burnt powder and powders calcined at 500 and 700℃reveal that all of them aremixed phases of Cr2O3(PDF No.84-0312)[9]and NiO (PDF No.44-1159)[10].Therefore,the heat released in the combustion process of xerogel is sufficient for complete conversion of the metal compounds to metal oxides,but insufficient for the formation of single-phase spinel NiCr2O4.Elevating the calcined temperature to 900℃,the XRD patterns of the powder are in accordance with the data of PDF No.89-6615[11],which reveals that cubic spinel NiCr2O4is eventually generated.The 1 000 ℃calcined powder has the same XRD pattern but different in half peak width with that of the 900°C calcined powder,whichmanifests the crystal growth of NiCr2O4with the temperature.


To further explore themicroscopic changes of the xerogel in the combustion progress and the subsequent calcination process,the TG/DSC and XRD analysis was supplemented by FTIR spectra analysis.Fig.3 shows the FTIR spectra of xerogel,as-burnt powder and calciend powders.The spectrum of xerogel indicates the characteristic bands of the-CO2ˉanti-symmetrical and symmetrical stretching vibration of citric acid at about 1 614 cm-1[12],while the N-O stretching vibration and bending vibration related to NO3ˉare located at1 380 and 825 cm-1,respectively[13]. The as-burnt powder only shows two bands at about 620 and 550 cm-1which are characteristic bands of Cr-O bonds in Cr2O3[14].The disappearance of the characteristic bands of the-COO-group and NO3-indicate that all of the citric acid and NO3-ions take part in the reaction during combustion.Spectra for 500 and 700℃ are the same as that of the as-burnt powder.When calcined at 900 and 1 000℃,powders only reveal absorption bands at 620 and 500 cm-1corresponding to the octahedral sublattice infrared vibrationsν1andν2in spinel structure,respectively[15], confirming that these two powders may have a chemical composition as NiCr2O4.
Fig.4 presents the SEM morphology of the asburnt powder and powders calciend at 500,700,and 900℃ for 2 h.The as-burnt powder exhibits a loose and porous structure and the formation of which may be caused by the liberation of gases during the combustion process.The 700°C calcined powders shown in Fig.4(b),consisted of Cr2O3and NiO crystals, are highly agglomerated particles.Elevating the calcination temperature to 900 ℃ ,crystals of polyhedral shape appears in the powder(as shown in Fig.4(c)),which isNiCr2O4crystalsasconfirmed by the XRD analysis.The 1 000℃calcined powder possesses a nearly regular octahedron-shaped morphology with well crystallization,suggesting the perfection of the NiCr2O4crystals with elevating the calcination temperature.


Fig.5 shows the spectra of diffuse reflectance as a function of wavelength in the range of 380 to 780 nm for the as-burnt and calcined powders.The as-burnt powder,mixture of NiO and Cr2O3,displays week reflectance bands at around 410 nm and 540 nm,which corresponds to the human eyes visible region to stimulate the green color.As the reflectance in the wavelength range of 380~780 nm is low,the asburnt powder manifests a dark green color.The 500℃and 700℃calcined powder,also amixture of NiO and Cr2O3,shows nearly no reflectance band in the wavelength analyzed,so these two powders have a black hue.The brightness of the 700℃ calcined powder is higher than that of 500℃calcined powder, which can be interpreted by the difference in reflectance shown in Fig.5.However,the black color of these two powders seems unreasonable because the intrinsic color of NiO is green.This phenomenon could possibly be interpreted as follows.Like many other binary metal oxides,NiO is often nonstoichiometric,meaning that the Ni∶O ratio deviates from 1∶1.In nickel oxide this non-stoichiometry is accompanied by a color change, with the stoichiometrically correct NiO being green and the non-stoichiometric NiO being black[16-17].In our case, the as-burnt powder was calcined at 500℃ and 700℃,the oxygen atom excess in the NiO crystalline leads to Ni∶O ratio deviates from 1∶1.Therefore,a black hue is displayed by these two powders.The 900 ℃ and 1 000 ℃ calcined powders,spinel CuCr2O4,show intense reflectance bands at 540 nm and display green color.Compared with 900 ℃calcined powder,1 000℃calcined powder has higher reflectance,so it has a higher brightness.
The color parameters (L*a*b*)of the powders analyzed above are shown in Table 1.From these data,it can be found that the color of spinel NiCr2O4(powders calcined at 900 and 1 000 ℃ )is significantly different from the mixture of NiO and Cr2O3(the as-burnt powder and powders calcined at 500 and 700℃).As the color parameters of NiCr2O4change with the calcination temperature and the spinel pigments always withstand high temperatures (≈2 000℃),we can choose suitable NiCr2O4with certain color according to our practical need.For example,in our laboratory we have used the 900°C calcined spinel NiCr2O4as a cooperative pigment in the colored solar spectrally selective paint coatings.Ideal color parameter accompanied by ideal spectrally selectivity of the coating has been obtained.

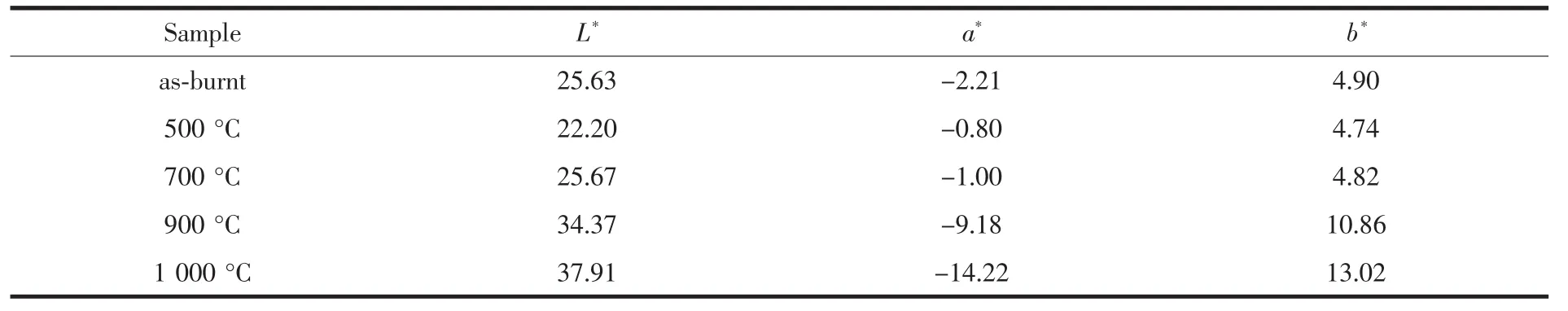
Table 1 Coordinated Lab of the as-burnt powder and powders calcined at various temperatures
3 Conclusions
In this contribution we prepared cubic spinel NiCr2O4through a sol-gel combustion method.The XRD and FTIR spectra results indicate that the calcination temperature up to 900°C is necessary for obtaining spinel NiCr2O4.The 1 000 ℃ calcined NiCr2O4powder possesses a perfect octahedron-shaped morphology with well crystallization as revealed by SEM.All of the as-burnt powder,500℃ and 700℃calcined powders aremixtures of NiO and Cr2O3.The difference in composition of the as-burnt powder and calcined powders leads to the color differences among these powders.The color for the as-burnt powder, powders calciend at 500 and 700℃ and powders calciend at 900 and 1 000℃ are black green,black, and green,respectively.The color difference between the as-burnt powder and powder calciend at 500 and 700℃ is explained in terms of non-stoichiometry in NiO crystals.The color of NiCr2O4changes with calcination temperatures and so we can prepare suitable NiCr2O4with certain color according to our practical need.
[1]Barro B S,Costa A C F M,Kiminami R H G A,et al.J. Metast.Nano Mater.,2004,20-21:325-332
[2]Sloczyński J,Zilkowski J,Grzybowska B,et al.J.Catal., 1999,187:410-418
[3]Ishibashi H,Yasumi T.J.Magn.Magn.Mater.,2007,310: 610-612
[4]Goryaga A N,Antoshina LG,Kokorev A I,et al.Phys.SolidState,2002,44:759-762
[5]Hu ZQ,Qin Y,Liu X Q,et al.Adv.Mater.Res.,2011,415-417:1594-1598
[6]Whipple E,Wold A.J.Inorg.Nucl.Chem.,1962,24:23-27
[7]Crottaz O,Kubel F,Schmid H.J.Mater.Chem.,1997,7: 143-146
[8]CIE,Recommendations of Uniform Color Spaces,Color Difference Equations,Phychometrics Color Terms,supplement, No.2 of CIE.Publication No.15(El-1.31)1971,Bureau Central de 1a CIE,Paris,1978.
[9]Finger LW,Hazen R M.J.Appl.Phys.,1980,51:5362-5367
[10]Wies S,Eysel W,Mineral.-Petrograph.,Institut der Universitaet Heidelberg,Germany,ICDDGrant-in-Aid, (1992)
[11]Ueno G,Sato S,Kino Y,Acta Crystallog.,Sec.C,1999,55: 1963-1966
[12]Yue Z,Zhou J,Li L,et al.J.Magn.Magn.Mater., 2000,208:55-60
[13]Yue Z,Guo W,Zhou J,et al.J.Magn.Magn.Mater., 2004,270:216-223
[14]Geng Q F,Zhao X,Gao X H,et al.J.Sol-Gel Sci.Techn., 2012,61:281-288
[15]Mazen SA.Mater.Chem.Phys.,2000,62:131-138
[16]Greenwood N N,Norman N,Earnshaw A.Chemistry of the Elements.Oxford:Pergamon,1984:1336-1337
[17]Fiévet F,Germi P,de Bergevin F,et al.J.Appl.Cryst., 1979,12:387-394
Combustion Synthesis and Characterization of Spinel NiCr2O4
GENG Qing-Fen1,2ZHAO Xin3GAO Xiang-Hu3YANG Sheng-Rong1LIU Gang*,3
(1State Key Laboratory of Solid Lubrication,Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences,Lanzhou 730000,China) (2Graduate University of Chinese Academy of Sciences,Beijing 100049,China) (3Research&Development Center for Eco-material and Eco-chemistry,Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences,Lanzhou 730000,China)
Cubic spinel NiCr2O4,as a pigment,was synthesized via a sol-gel combustionmethod and characterized using TG/DSC,XRD,FTIR and SEM techniques.The results reveal that a single-phase NiCr2O4with the cubic structure isobtained after heat treatmentof the as-burntpowder at900℃for 2 h.The powders obtained below 900℃appear as amixture of NiO and Cr2O3.The color parameters for the as-burnt powder and the powder calcined at various temperatureswere studied using PerkinElmer Lambda 950 UV-Vis-NIR spectrometer.Although having the same compositions,the 500 and 700℃calcined powders show a different color(black)from the as-burnt powder (black green).This phenomenonmay be interpreted in terms of non-stoichiometry in NiO crystals.Moreover,the asprepared spinel NiCr2O4powders calcined at900 and 1 000℃show green colorwhich changeswith the calcination temperature.
combustion synthesis;spinel-type NiCr2O4;color;crystal growth
TQ622.2+1
A
1001-4861(2012)09-1979-06
2012-02-22。收修改稿日期:2012-04-11。
中科院“西部之光”人才培养计划,中科院太阳能行动计划(1731012394),国家自然科学基金(No.51003111)资助项目。
*通讯联系人。E-mail:gangliu@licp.cas.cn;会员登记号:S291001692S。
