β-连环蛋白在不同胰腺病变中的表达
2012-11-06满晓华王伟郑建明李兆申高军龚燕芳
满晓华 王伟 郑建明 李兆申 高军 龚燕芳
·论著·
β-连环蛋白在不同胰腺病变中的表达
满晓华 王伟 郑建明 李兆申 高军 龚燕芳
目的检测β-连环蛋白(β-catenin)在胰腺正常导管(NP)、胰腺上皮内瘤变(PanINs)、胰腺导管内乳头状黏液性肿瘤(IPMNs)及胰腺导管腺癌(PDAC)中的表达水平,分析其在胰腺癌发生过程中的作用。方法收集正常胰腺组织12例、IPMN 52例(IPMA 20例,IPMB 13例,IPMC 19例)、PanINs 118灶(PanIN-1 73灶,PanIN-2 29灶,PanIN-3 16灶)、PDAC 50例,采用免疫组化SP法检测β-catenin在上述组织中的表达,并分析其在胰腺癌的表达与临床病理特征及患者术后生存期的关系。结果NP中β-catenin主要表达于细胞膜,表达量为(4.38±2.11)分;PanINs、PDAC和IPMN的β-catenin 膜表达明显降低或缺失,PanIN-1、PanIN-2、PanIN-3、PDAC、 IPMA 、IPMB 、IPMC的膜表达量分别为(5.22±2.21)、(2.24±2.31)、(1.44±1.37)、(2.71±2.08)、(4.85±2.28)、(4.15±2.51)、(2.68±2.75)分。PanIN-1的胞质表达率为12.3%(9/73),胞核表达率为0; PanIN-2为34.5%(10/29)和3.4%(1/29);PanIN-3为43.8%(7/16)和12.5%(2/16);PDAC为44.0%(22/50)和10.0%(5/50)。在由PanINs至PDAC的进展过程中β-catenin膜表达逐渐降低,而胞质、胞核异位表达率逐渐升高。β-catenin膜表达与肿瘤大小、神经浸润、淋巴转移有关(P<0.05);胞质异位表达率与肿瘤大小有关(P<0.05);胞核异位表达率与肿瘤的分化程度有关(P<0.01);胞膜、胞质表达均与患者的术后生存期显著相关(P<0.05)。结论Wnt/β-catenin通路异常活化导致的β-catenin膜表达减弱及异位表达率增加是PDAC发生、发展的重要机制,并促进PDAC的恶性生长与转移,β-catenin的表达与PDAC患者的预后相关。
胰腺导管腺癌; 胰腺上皮内瘤变; 导管内乳头状黏液性肿瘤; β-连环蛋白
β-连环蛋白(β-catenin)作为一种重要的细胞信号转导分子和细胞间黏附分子,已经成为近年来肿瘤研究中的热点,它既可介导Wnt信号通路参与调节细胞的增殖分化,也可通过构成E-cad/cat复合体参与维持细胞间黏附连接及细胞极性[1-5]。本研究检测胰腺正常导管(normal pancreatic duct, NP)、胰腺上皮内瘤变(pancreatic intraepithelial neoplasias, PanINs)、胰腺导管内乳头状黏液性肿瘤(intraductal papillary mucinous neoplasms, IPMNs)及胰腺导管腺癌(pancreatic ductal adenocarcinoma, PDAC)中β-catenin蛋白的表达,分析其变化规律,探讨其在PDAC发病机制中的作用。
材料和方法
一、胰腺组织标本
收集2001年至2010年我院胰腺组织标本及临床资料,包括正常胰腺12例、IPMN 52例。另从医院病理科获赠包含PDAC 50例的胰腺癌组织芯片1片和PanINs芯片3片,其中PanIN-1 73灶,PanIN-2 29灶,PanIN-3 16灶。
二、免疫组化染色(IHC)及评定标准
采用鼠抗人β-catenin单抗(MAB13291, R&D)行SP法免疫组化染色。根据细胞定位(胞膜、胞质、胞核)、染色强度和分布判断。PanINs及IPMNs计数导管内细胞,胰腺癌观察5~10个高倍视野(HPF)。结果判定:阳性细胞数<25%计1分,25%~50% 2分,50%~75% 3分,>75% 4分;无染色计0分,弱阳性1分,中等阳性2分,强阳性3分。两分乘积为免疫组化评分(IHCS)。正常胰腺导管上皮细胞膜的强阳性和个别细胞的胞质弱阳性视为正常表达,其余均视为异常表达[6]。
三、统计学处理
采用SPSS13.0统计软件。各病变组之间IHCS比较采用单因素方差分析;胞质、胞核异位表达率比较及其与临床病理特征的相关性分析采用χ2检验;与患者生存期的关系采用Kaplan-Meier行longrank检验。P<0.05为差异具有统计学意义。
结 果
一、NP、PanINs、IPMNs和PDAC的β-catenin表达
β-catenin在正常胰腺腺泡、导管上皮和胰岛细胞膜上呈均质的强阳性表达,个别细胞为胞质弱阳性染色,胰腺间质细胞不染色。PanINs、IPMNs和PDAC的β-catenin膜表达明显减少或缺失,并可出现胞质和(或)胞核的异位染色(图1)。各组β-catenin膜表达的IHCS分别为:NP(4.38±2.11)分,PanIN-1(5.22±2.21)分,PanIN-2(2.24±2.31)分,PanIN-3(1.44±1.37)分,PDAC(2.71±2.08)分,IPMA(4.85±2.28)分,IPMB(4.15±2.51)分,IPMC (2.68±2.75)分;胞质异位表达率为:NP 0,PanIN-112.3%(9/73),PanIN-2 34.5%(10/29),PanIN-3 43.8%(7/16),PDCA 44.0%(22/50),IPMA 25%(5/20),IPMB 30.8%(4/13),IPMC36.8%(7/19);胞核异位表达率为:NP 0,PanIN-1 0,PanIN-2 3.4%(1/29)、PanIN-3 12.5%(2/16),PDCA 10.0%(5/50),IPMA 5%(1/20),IPMB 15.4%(2/13),IPMC 15.8(3/19)。PanIN-2、PanIN-3、PDAC及IPMC的β-catenin膜表达均较NP组明显降低(P值均<0.05);异位表达率均较NP组增加。而PanIN-2、PanIN-3与PDAC间及IPMNs各组间差异均无统计学意义。
二、胰腺癌β-catenin的表达与临床病理特征及患者术后生存期的关系
PDAC的β-catenin膜表达与肿瘤大小、神经浸润、淋巴转移有关;胞质异位表达率与肿瘤大小有关;胞核异位表达率与肿瘤的分化程度有关(表1)。
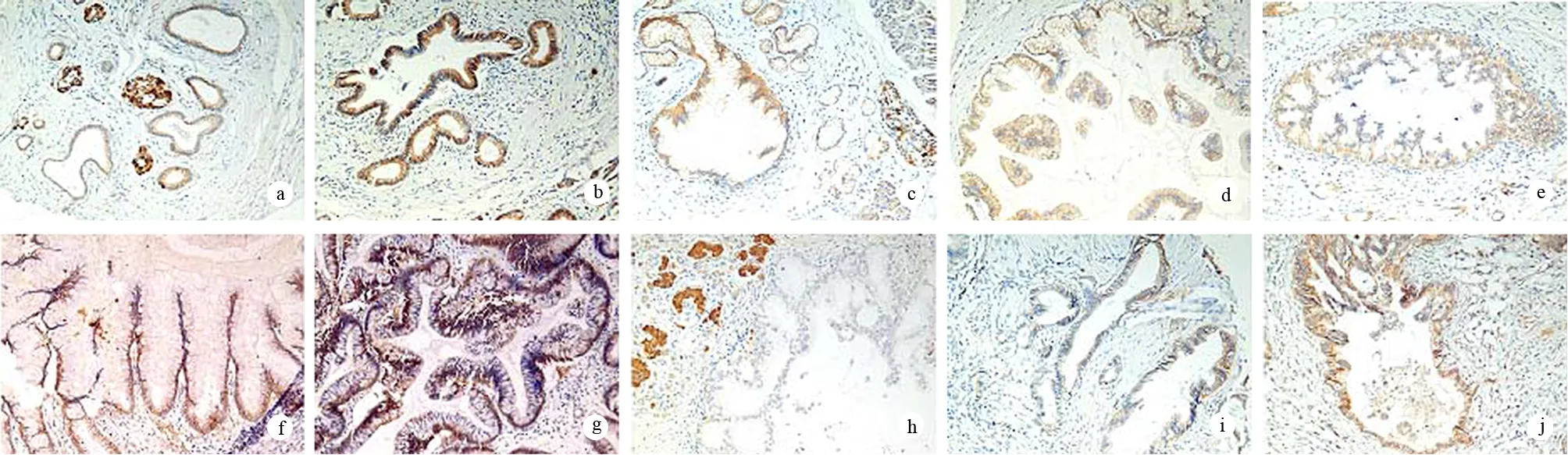
图1β-catenin在胰腺组织中的表达 a:NP;b:PanIN-1A;c:PanIN-1B;d:PanIN-2;e:PanIN-3;f:IPMA;g:IMPB;h:IPMC;i:PDAC;j:PDAC(HE a:×400,b~i:×200)
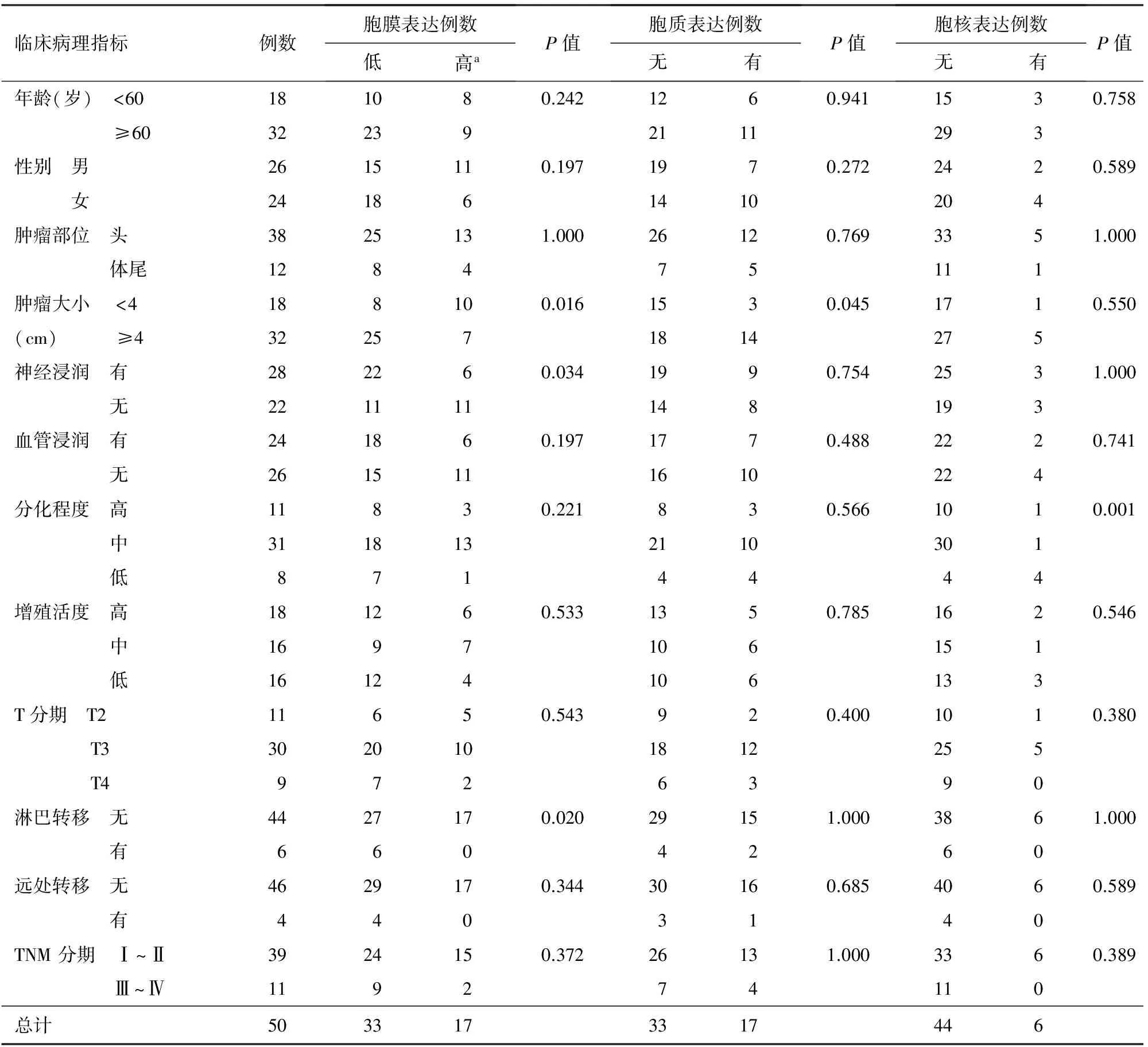
表1 胰腺癌β-catenin的表达与临床病理特征的相关性分析
注:a:IHCS≥4分
β-catenin胞膜、胞质表达与患者的术后生存期显著相关(图2),β-catenin膜高表达患者的中位生存期为25个月(95% CI 18.371~31.629),显著低于低表达患者18个月的中位生存期(95% CI14.624~21.376,P=0.018);胞质异位表达患者的中位生存期为14个月(95% CI 10.975~17.025),显著低于无异位表达患者21个月的中位生存期(95% CI 17.624~24.376,P=0.039)。
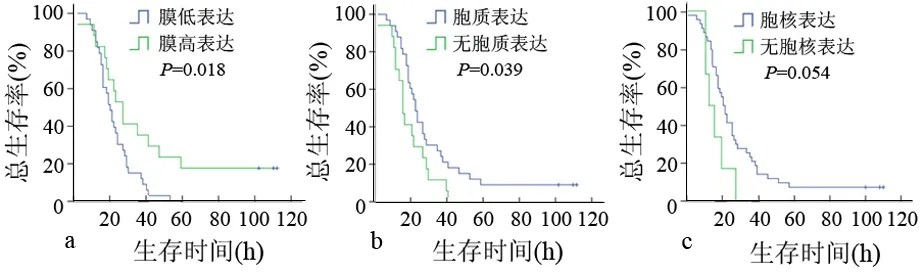
图2β-catenin膜表达(a)、胞质表达(b)、胞核表达(c)与患者术后生存期的关系
讨 论
目前,关于β-catenin在胰腺癌发病机制中作用的报道并不完全一致。Al-Aynati等[6]报道,PanINs和PDAC均异常表达β-catenin。Qiao等[7]报道,β-catenin参与胰腺癌的发生,其胞膜表达减弱、胞质的异位表达均与胰腺癌的预后紧密相关。Lowy等[8]发现,β-catenin在胰腺癌细胞中表达下调而不是上调,β-catenin表达减少导致细胞间的连接被削弱进而促进肿瘤侵袭、转移。而Heiser等[9]发现,上调β-catenin的表达可以促进胰腺肿瘤的形成,但肿瘤表型类似于实性假乳头状瘤(SPN);在诱导K-ras突变的基础上,激活β-catenin反而抑制PanINs的形成。
本结果显示,β-catenin蛋白膜表达在PanINs和IPMNs中随病变级别升高而逐渐减弱,在PDAC中显著降低,部分PDAC为阴性表达。同时,在PanIN-1、IPMA的导管上皮细胞开始出现β-catenin的胞质表达,表达率随病变级别升高而升高;β-catenin的胞核表达最早出现于PanIN-2和IPMA,在高级别病变的表达率显著升高,提示β-catenin膜表达缺失及胞质、胞核的异位表达在PDAC的发生中发挥了重要作用,这与在肝癌、结肠癌等恶性肿瘤中的研究结果相一致[10-12]。
本结果还显示,胰腺癌组织β-catenin膜表达在瘤体积较大、发生神经浸润与淋巴转移肿瘤中明显减少,表明β-catenin膜表达减少是导致PDAC恶性生长与转移的重要因素。此外,β-catenin的胞质异位表达与肿瘤大小呈正相关;胞核表达与肿瘤的分化程度呈负相关,表明β-catenin的异位表达亦是肿瘤的恶性生长的重要因素。β-catenin的膜表达与胞质表达还与患者的术后生存期显著相关,与Qiao等[7]的报道一致,提示β-catenin在PDAC预后判断中具有一定价值。
[1] Clevers H. Wnt/beta-catenin signaling in development and disease. Cell, 2006, 127:469-480.
[2] Easwaran V, Pishvaian M, Byers S, et al. Cross-regulation of beta-catenin-LEF/TCF and retinoid signaling pathways. Curr Biol, 1999, 9: 1415-1419.
[3] Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science, 1997, 275:1787-1790.
[4] Ilyas M, Tomlinson IP. The interactions of APC E-cadherin and beta-catenin in tumour development and progression. J Pathol, 1997, 182:128-137.
[5] Novak A, Hsu SC, Leung-Hagesteijn C, et al. Cell adhesion and the integrin-linked kinase regulate the LEF-1 and β-catenin signaling pathways. Proc Natl Acad Sci USA,1998, 95:4374-4379.
[6] Al-Aynati MM,Radulovich N,Riddell RH,et al.Epithelial-cadherin and beta-catenin expression changes in pancreatic intraepithelial neoplasia.Clin Cancer Res,2004,15,10:1235-1240.
[7] Qiao Q, Ramadani M, Gansauge S, et al. Reduced membranous and ectopic cytoplasmic expression of beta-catenin correlate with cyclin D1 overexpression and poor prognosis in pancreatic cancer. Int J Cancer, 2001, 95:194-197.
[8] Lowy AM, Fenoglio-Preiser C, Kim OJ, et al. Dysregulation of beta-catenin expression correlates with tumor differentiation in pancreatic duct adenocarcinoma. Ann Surg Oncol, 2003, 10:284-290.
[9] Heiser PW, Cano DA, Landsman L, et al. Stabilization of beta-catenin induces pancreas tumor formation. Gastroenterology, 2008, 135:1288-1300.
[10] Nhieu JT,Renard CA,Wei Y,et al.Nuclear accumulation of mutated beta-catenin in hepatocellular carcinoma is associated with increased cell proliferation.Am J Pathol, 1999,155:703-710.
[11] Brabletz T, Jung A, Reu S, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA, 2001, 98:10356-10361.
[12] Kim YS, Kang YK, Kim JB, et al. beta-catenin expression and mutational analysis in renal cell carcinomas. Pathol Int, 2000, 50:725-730.
Expressionofβ-catenininPanINs,IPMNsandPDAC
MANXiao-hua,WANGWei,ZHENGJian-ming,LIZhao-shen,GAOJun,GONGYan-fang.
DepartmentofGastroenterology,ChanghaiHospital,SecondMilitaryMedicalUniversity,Shanghai200433,China
LIZhao-shen,Email:zhsli@81890.net
ObjectiveTo detect the expressions of β-catenin protein in different pancreatic tissues (NP, PanINs, IPMNs and PDAC) and evaluate its significance during the carcinogenesis of PDAC.MethodsThe expression of β-catenin protein in 12 samples of normal pancreatic tissues, 52 samples of IPMN (IPMA 20 foci, IPMB 13 foci, IPMC 19 foci), PanINs 118 foci (PanIN-1 73 foci, PanIN-2 29 foci, PanIN-3 16 foci), 50 cases of PDAC was determined by using immunohistochemistry. The correlation between β-catenin expression and clinicopathologic characteristics of PDAC was analyzed.Resultsβ-catenin was mainly expressed in cell membrane of NP, the quantity was 4.38±2.11; in PanINs, PDAC and IPMN, β-catenin membrane expression was significantly decreased or absent, the β-catenin membrane expressions of PanIN-1, PanIN-2, PanIN-3, PDAC, IPMA, IPMB, IPMC were 5.22±2.21, 2.24±2.31, 1.44±1.37, 2.71±2.08, 4.85±2.28, 4.15±2.51, 2.68±2.75. The cytoplasm expression of PanIN-1 was 12.3% (9/73), while the nuclear expression was 0; and the corresponding values were 34.5%(10/29) and 3.4%(1/29) in PanIN-2; 43.8%(7/16) and 12.5%(2/16) in PanIN-3; 44.0%(22/50) and 10.0%(5/50) in PDAC. The IHCS of β-catenin membrane expression decreased with the severe tissue atypia along the progressive multistage. The β-catenin membrane expression was significantly associated with tumor size, neural infiltration and lymphatic metastasis (P<0.05). Ectopic cytoplasm expression was significantly associated with tumor size (P<0.05). Ectopic nuclear expression was significantly associated with tumor differentiation (P<0.01). The membrane or ectopic cytoplasm expression of β-catenin was significantly associated with postoperative survival.ConclusionsAbnormal Wnt/β-catenin signal activation induces decreased β-catenin membrane expression and increased ectopic expression, which is an important mechanism of pathogenesis and development of PDAC, and promotes the growth and metastasis of PDAC. The expression of β-catenin was associated with postoperative survival.
共同第一作者:王伟
Pancreatic ductal adenocarcinoma; Pancreatic intraepithelial neoplasias; Intraductal papillary mucinous neoplasms; β-catenin
10.3760/cma.j.issn.1674-1935.2012.01.009
重大国际合作项目(30910103911)
200433 上海,第二军医大学长海医院消化内科(满晓华、王伟、李兆申、高军、龚燕芳),病理科(郑建明)
李兆申,Email:zhsli@81890.net
2011-10-21)
(本文编辑:屠振兴)
