三缺位Keggin结构磷钨酸甲基苯基硅衍生物[(C4H9)4N]3[α-A-PW9O34(C6H5SiCH3)3]的合成和表征
2012-09-18张东娣王科燕庆霞马鹏涛王敬平
张东娣王 科燕庆霞马鹏涛王敬平*,
(1河南大学药学院,开封 475004)
(2河南大学化学化工学院分子与晶体工程研究所,开封 475004)
三缺位Keggin结构磷钨酸甲基苯基硅衍生物[(C4H9)4N]3[α-A-PW9O34(C6H5SiCH3)3]的合成和表征
张东娣1,2王 科2燕庆霞2马鹏涛2王敬平*,2
(1河南大学药学院,开封 475004)
(2河南大学化学化工学院分子与晶体工程研究所,开封 475004)
通过三缺位Keggin结构杂多阴离子[α-A-PW9O34]9-和二氯甲基苯基硅烷在乙腈溶液中反应,合成了一例结构新颖的甲基苯基硅衍生物[(C4H9)4N]3[α-A-PW9O34(C6H5SiCH3)3](1),并对其进行元素分析,红外光谱,紫外光谱,热分析和X-射线单晶衍射等表征。该配合物属于三方晶系,空间群为 R3m,晶胞参数:a=2.261 3(2)nm,b=2.261 3(2)nm,c=1.797 6(4)nm,V=7.960 2(18)nm3,Z=3。在配合物中,阴离子[α-A-PW9O34(C6H5SiCH3)3]3-呈C3v对称,3个甲基苯基硅基团连接在三缺位的阴离子[α-A-PW9O34]9-表面,整个阴离子显示“开放结构”。
有机硅;三缺位磷钨酸盐;晶体结构;合成
The design and synthesis of derivatized polyoxometalates (POMs)have attracted considerable attention in recent years originating from the fundamental interest of modeling catalysis by metal oxides as well as potential applications in different fields,including bifunctional catalysis, antiviral and antitumoral chemotherapy[1-2].It is known that covalent attachment of organic or organometallic groups to POMs can be a strategy for increasing the structural diversity and improving their properties[3-7].This approach has beenvery successful for the synthesis of organic-inorganic hybrid POMs and a large number of such POMs have been synthesized and characterized in solution or in solid state since the pioneering work of Klemperer et al.[8].
To date,there are three main types available in the literature regarding organometallic groups,namely organostannyl derivatives[9-15],organophosphoryl[16-20]and organosilyl derivatives[21-32],also,there are a few of organonoblemetallic derivatives[33-35]reported in the latest years.Among them,organosilyl derivatives are of particular interest and have been investigated for a long time probably because the isolated organosilyl groups can be easily incorporated into mono-,di-,or trivacant POMs.In 1979,the group of Knoth obtained the first organosilyl derivative[α-SiW11O39{O(SiR)2}]4-(R=C2H5,C6H5,NC (CH2)3,C3H5)by reaction of trichloro organosilanes with lacunary precursor[α-SiW11O39]8-[24].In particular,Thouvenot and co-workers studied this system deeply,and have reported a series of orgnosilylderivatives,such as[α2-P2W17O61(RSi)2O]6-[25],[(γ-SiW10O36)(RSi)2O]4-[27],[(γ-SiW10O36)(RSiO)4]4-[27],α-A-[PW9O34(tBuSiO)3(RSi)]3-[28],α-B-[AsW9O33(tBuSiO)3(HSi)]3-[28],[(α-PW10O36)-(tBuSiOH)2]3-[29],and α-A-[PW9O34(tBuSiO)3(SiR)]3-[32].Several years ago we reported on two new monoorganosilyl group-substituted organosilyl derivatives, α-A-[NnBu4]3[PW9O34(RSiO)3(RSi)](R=C2H5,CH3)[30].However,in marked contrast to the extensive reports of monoorganosilyl derivatives aforementioned,few diorganosilylgroup-substituted organosilyl derivatives areknown.Therefore,we decided to investigate the interaction of diorganosilyl groups with trivacant heteropolytungstates in some detail.Herein we report on the synthesis,single-crystal X-ray structure of[(C4H9)4N]3[α-A-PW9O34(C6H5SiCH3)3](1),which represents the first polyoxoanion-based diorganosilyl group-substituted organosilyl derivative.
1 Experimental
1.1 Synthesis of[(C4H9)4N]3[α-A-PW9O34(C6H5SiCH3)3]
Compound 1 can be synthesized as follows:Na9[α-A-PW9O34]·nH2O (1.95 g,0.80 mmol)[36]and NnBu4Br (0.78 g,2.42 mmol)were suspended in 50 mL of CH3CN and then C6H5SiCH3Cl2(0.62 g,3.25 mmol)was added dropwise under vigorous stirring.This solution was refluxed for 24 h and filtered.And then the resulting solution was allowed to evaporate slowly at room temperature.Colorless block crystals of 1 suitable for X-ray crystallography were obtained after several days.Yield:ca.30% (Based on Na9[APW9O34]·nH2O).Anal.Calcd.for C69H132N3O34PSi3W9(%):C,24.98;H,4.01;N,1.27;P,0.93;Si,2.54;W,49.87.Found(%):C,24.88;H,4.00;N,1.10;P,1.01;Si,2.57;W,49.97.
Similar to [α-A-PW9O34(tBuSiO)3(RSi)]3-[28],the formation for 1 can be written as follow:
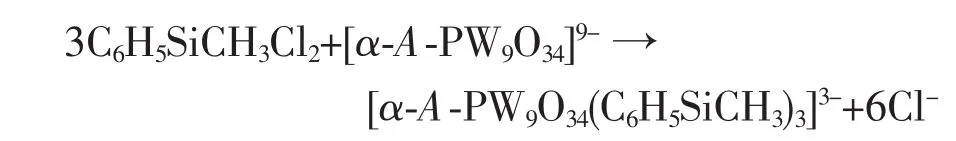
1.2 X-ray crystallography
Intensity data for 1 were collected at 296 K on a Bruker ApexⅡdiffractometer using the graphitemonochromated Mo Kα radiation (λ=0.071 073 nm).The structure was solved by combination of SHELXS-97 (direct methods)and SHELXH-97 (Fourier and least-squares renement)[37].Lorentz polarization and Muti-scan absorption corrections were applied.All non hydrogen atoms were refined anisotropically.Hydrogen atoms attached to carbon atoms were geometrically placed.All hydrogen atoms were refined isotropically asaridingmodeusingthedefaultSHELXTL parameters.Crystallographic data and structure refinements for 1 are summarized in Table 1.
CCDC:830334.
1.3 Characterization
Elemental analyses(C,H,and N)were performed on a Perkin-Elmer 240C elemental analyzer.ICP analyses were performed on a Perkin-Elmer Optima 2000 ICP-OES spectrometer.IR spectra were obtained on a Nicolet 170 SXFT-IR spectrometer using the technique of pressed KBr pellets in the range 400~4 000 cm-1.XRPD were recorded on a Philips X′Pert-MPD instrument withCu Kα radiation (λ=0.154 056 nm)in the range 2θ=10°~40°at 293 K.TG analyses were carried out under N2atmosphere on a Mettler-Toledo TGA/SDTA 851einstrument with theheating rate of 10℃·min-1from 25 to 800℃.UV-Vis absorption spectra were obtained with a U-4100 spectrometer(distilled water as solvent)at 300 K.
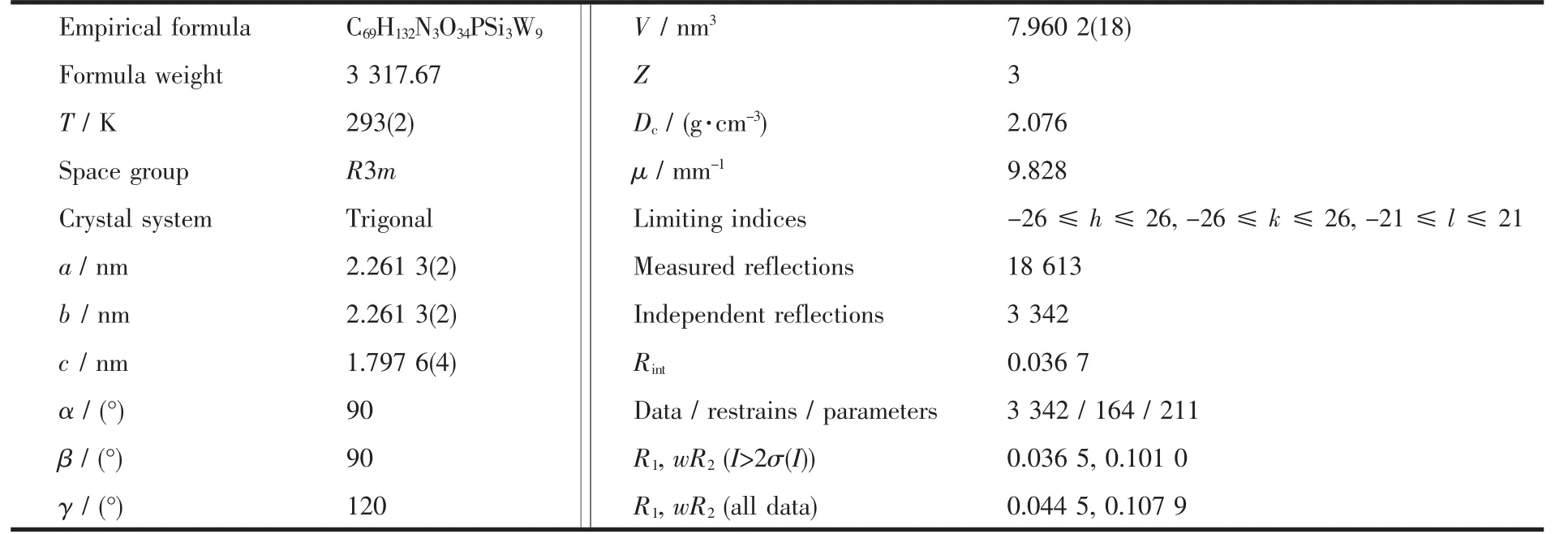
Table 1 Crystallographic data and structural refinements for 1
2 Results and discussion
2.1 Crystal structure
Single crystal X-ray diffraction reveals that 1 crystallizes in the trigonal space group R3m.The molecular structure of 1 is composed of one[α-A-PW9O34(C6H5SiCH3)3]3-polyoxoanions and three [(C4H9)4N]+cations(Fig.1).As shwon in Fig.1a,the polyoxoanion[α-A-PW9O34(C6H5SiCH3)3]3-consists of a[α-A-PW9O34]9-framework with three equivalent C6H5SiCH3groups,and each C6H5SiCH3group isgrafted onto this polyoxoanion by two Si-O-W bridges.Different from the close cage structure of α-A-[NnBu4]3[PW9O34(RSiO)3(RSi)](R=C2H5,CH3)[30],the polyoxoanion[α-A-PW9O34(C6H5SiCH3)3]3-displays an open structure while keeping the geometry of the parent trivacant polyoxoanion[α-A-PW9O34]9-.As we know,the POM-based organosilyl derivatives previously reported are limited and mainly confined to monoorganosilyl group-substituted species.There is still no report about diorganosilyl analogue.Consequently,the most remarkable structural feature of 1 is that it is the first trivacant tungstophosphatebased example ofdiorganosilylgroup-substituted organosilylderivative in which the Siatom is connected to two organic groups.There are minor disorder in the ligand with C2,C3,C4/C4,C5,C6,C7/C7 in 1 which lie about an inversion centre.
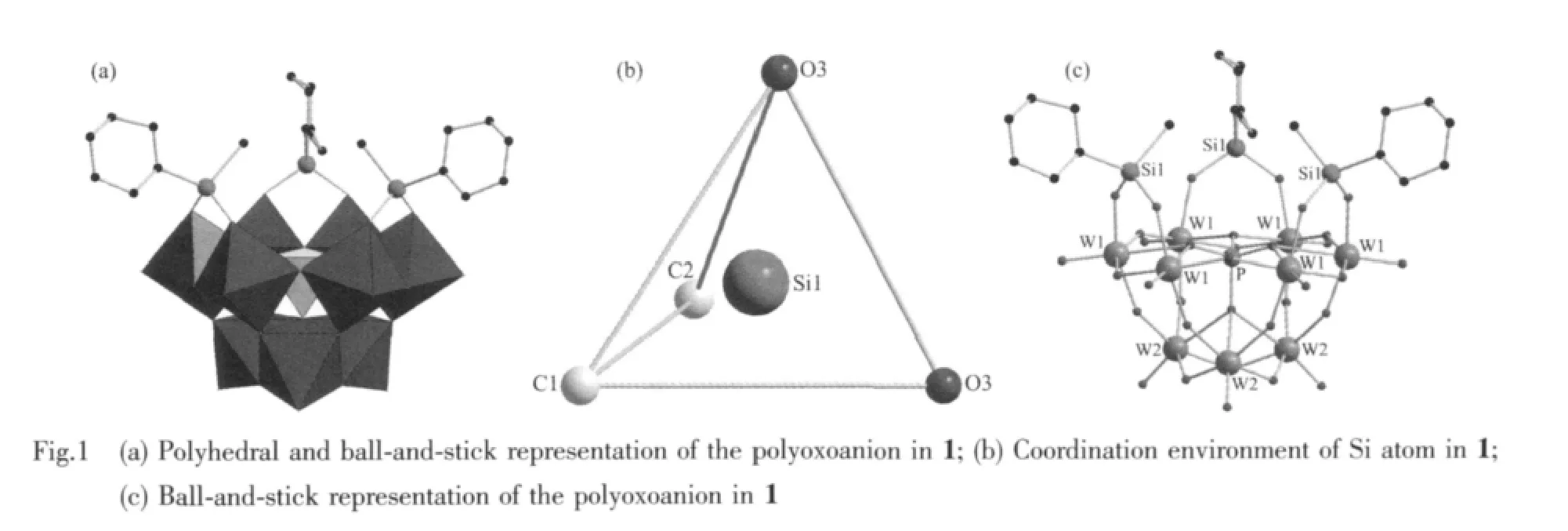
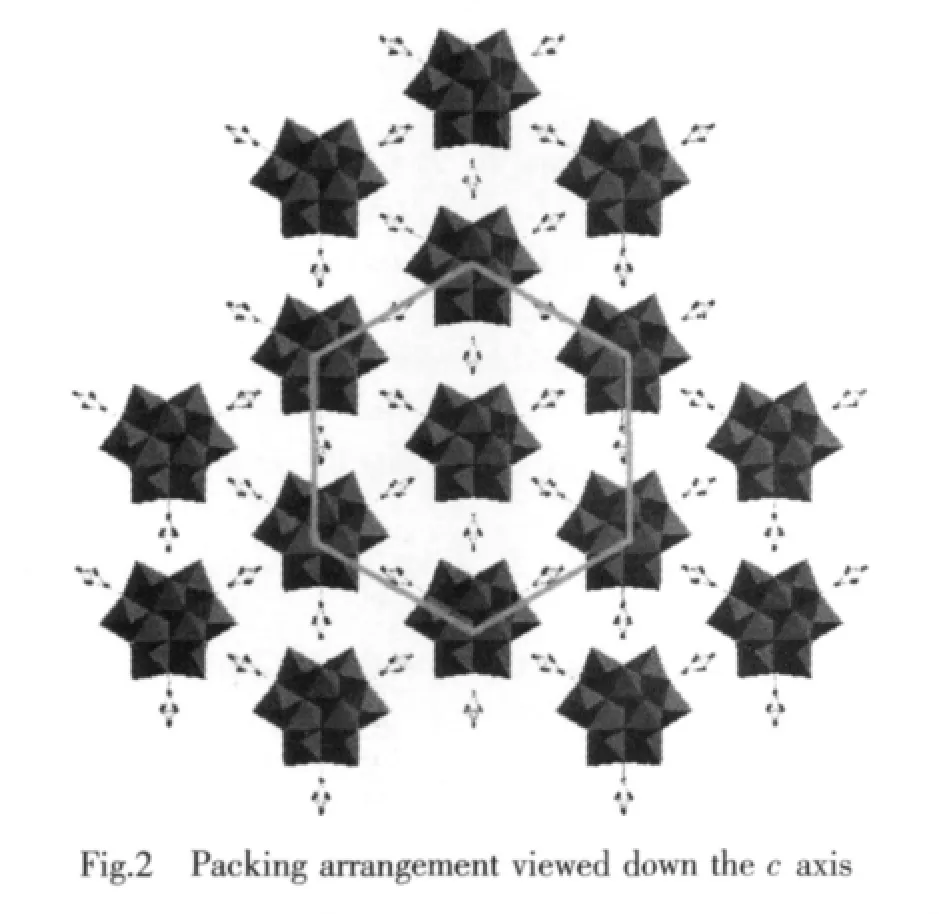
Compared to the saturated Keggin structure,the P heteroatom adopts a slightly distorted tetrahedral geometry coordinated by oxygen atomswith an average P-O bond length of 0.153 3(12)nm,which is ascribed to the removal of three corner-shared WO6octahedra and the incorporation of three C6H5SiCH3groups.Fig.1b shows that the incorporated silicon atoms are defined by two O atoms from[α-A-PW9O34]9-moieties with average bond lengths 0.162 6(9)nm and twocarbon atomsfrom themethyland phenyl,respectively.This coordination mode is similar to Sn atoms of[{Sn(CH3)2}4(H2P4W24O92)2]28-[15],Si atoms aswell as Sn atoms are bound to two organic groups.Although there has been such organotin derivative,stillhasno any similarreportforPOM-based diorganosilyl group-substituted organosilyl derivative.To our knowledge,1 is the first example of POM-based diorganosilyl group-substituted organosilyl derivative.Packing arrangement viewed down c axis of the polyoxoanion in 1 is illustrated in Fig.2.The infinite hexa-number rings are formed via π-π and electrostatic interactions with one polyoxoanion locating in the center of the ring.
2.2 FT-IR spectra
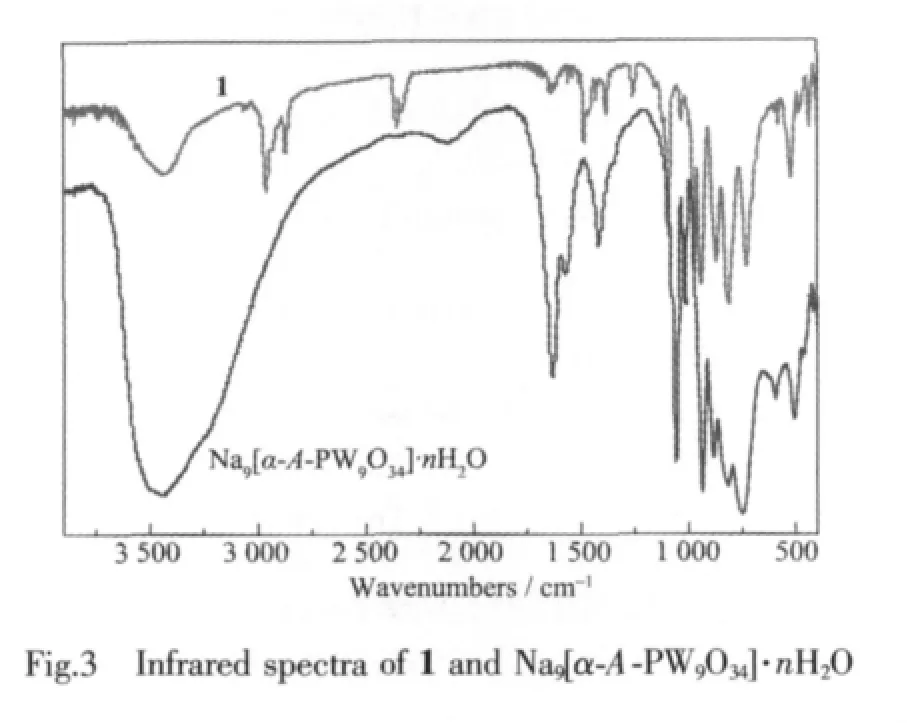
The IR spectrum of 1 (Fig.3)is very similar to that of Na9[α-A-PW9O34]·nH2O,which is indicative of the retention of the [α-A-PW9O34]9-framework.In the low-wavenumber region, characteristic vibration patterns derived from the Keggin frameworks are observed.Four characteristic vibration bands assigned to Ⅴ(W-Ot), Ⅴ(P-Oa), Ⅴ(W-Ob)and Ⅴ(W-Oc)appear at 974 and 938,1 088 and 1 028,872,and 812 cm-1for 1,respectively.Additionally,the stretching bands of-CH3and-C6H5have been typically observed at 2 874~2 961 and 3 040~3 072 cm-1,respectively.The bands at wide 3 420 and strong 1 625 cm-1are attributed to the lattice water and ligand water molecules.As above mentioned,the resultsofIR spectrum arewell identical with those of X-ray diffraction strutural analysis.
2.3 UV-Vis spectrum
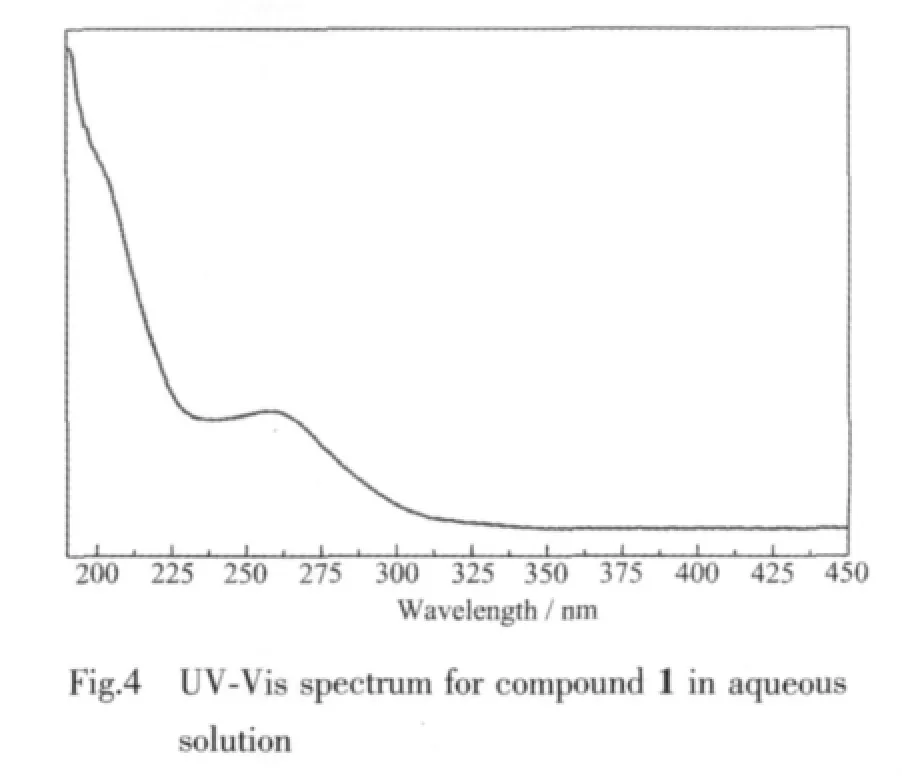
The UV-Vis spectrum(Fig.4)of 1 displays a strong absorption at 260 nm and a weak peak at near 191 nm,which are associated with the charge-transfer bands corresponding to Ot→W and Ob(c)→W,respectively.
2.4 TG analyses
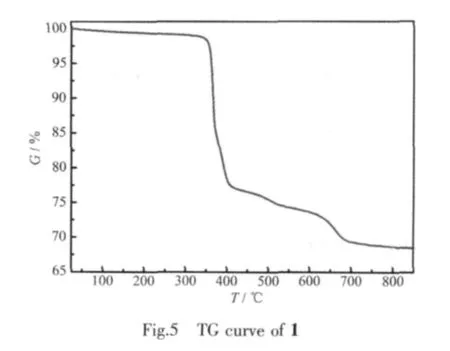
The thermalgravimetric curve of1 (Fig.5)indicates two steps of weight loss,giving a total weight loss of 30.63%in the range of 25~850 ℃ ,accordant with the calculated loss of 29.28% .Thefirst stage from 25 to 412℃is attributed to the removal of three organic ammonium molecules,and the observed weight loss 22.40%is consistent with the calculated value 21.93%.The second stage with the weight loss of 8.23%occurs between 412 and 800℃,which may be assigned to the loss of three methyl and three phenyl(calcd.7.35%).
2.5 XRPD patterns
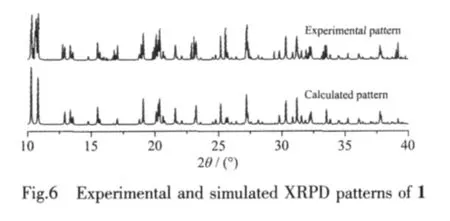
The experimental XRPD pattern of the bulk product of 1 is in good agreement with the simulated one that are based on the results from single-crystal XRD,which indicates the phase purity of the sample(Fig.6).The different intensities of the experimental and simulated XRPD patterns are due to the variation in the preferred orientation of the powder sample during data collection.
3 Conclusions
In summary,we have successfully incorporated diorganosilylgroupsintoa trivacantKeggin-type tungstophosphate.The title compound is the first example of POM-based diorganosilyl group-substituted organosilyl derivative.Further,the successful synthesis of 1 may provide possibilities for designing new diorganosilyl group-substituted organosilyl derivatives.
[1]Pope M T.Heteropoly and Isopoly Oxometalates.Berlin:Springer-Verlag,1983.
[2]Pope M T,Müller A.Angew.Chem.Int.Ed.Engl.,1991,30:34-38
[3]Proust A,Thouvenot R,Gouzerh P.Chem.Commun.,2008,6:1837-1852
[4]Yamase T.Chem.Rev.,1998,98:307-326
[5]CoronadoE,Gómez-GarciaCJ.Chem.Rev.,1998,98:273-296
[6]Rhule J T,Hill C L,Judd D A.Chem.Rev.,1998,98:327-358
[7]Gouzerh P,Proust A.Chem.Rev.,1998,98:77-111
[8]Ho R K C,Klemperer W G.J.Am.Chem.Soc.,1978,100:6772-6774
[9]Chorghade G S,Pope M T.J.Am.Chem.Soc.,1987,109:5134-5138
[10]Xin F,Pope M T.Organometallics,1994,13:4881-4886
[11]Xin F,Pope M T.Inorg.Chem.,1996,35:5693-5695
[12]Xin F,Pope M T,Long G J,et al.Inorg.Chem.,1996,35:1207-1213
[13]Sazani G,Pope M T.Dalton Trans.,2004,13:1989-1994
[14]Bareyt S,Piligkos S,Hasenknopf B,et al.J.Am.Chem.Soc.,2005,127:6788-6794
[15]Hussain F,Kortz U,Keita B,et al.Inorg.Chem.,2006,45:761-766
[16]Sun Z G,Liu Q,Liu J F.Inorg.Chem.Commun.,2000,3:328-330
[17]Mayer C R,Hervé M,Lavanant H,et al.Eur.J.Inorg.Chem.,2004,5:973-977
[18]Mayer C R,Thouvenot R.J.Chem.Soc.Dalton Trans.,1998:7-13
[19]Kim G S,Hagen K S,Hill C L.Inorg.Chem.,1992,31:5316-5324
[20]Mayer C R,Herson P,Thouvenot R.Inorg.Chem.,1999,38:6152-6158
[21]Schroden R C,Blanford C F,Melde B J,et al.Chem.Mater.,2001,13:1074-1081
[22]Mayer C R,Thouvenot R,Lalot T.Macromolecules,2000,33:4433-4437
[23]Judeinstein P,Deprun C,Nadjo L.J.Chem.Soc.Dalton.Trans.,1991,8:1991-1997
[24]Knoth W H.J.Am.Chem.Soc.,1979,101:759-760
[25]Mayer C R,Roch-Marchal C,Lavanant H,et al.Chem.Eur.J.,2004,10:5517-5523
[26]Mayer C R,Neveu S,Cabuil V.Angew.Chem.,Int.Ed.Engl.,2002,41:501-503
[27]Mayer C R,Fournier I.Chem.Eur.J.,2000,6:105-110
[28]Mazeaud A,Ammari N,Robert F,et al.Angew.Chem.,Int.Ed.Engl.,1996,35:1961-1964
[29]Mazeaud A,Dromzee Y,Thouvenot R.Inorg.Chem.,2000,39:4735-4740
[30]Niu J Y,Li M X,Wang J P.J.Organomet.Chem.,2003,675:84-90
[31]Hasegawa T,Shimizu K,Seki H,et al.Inorg.Chem.Commun.,2007,10:1140-1144
[32]Agustin D,Coelho C,Mazeaud A,et al.Z.Anorg.Allg,Chem.,2004,630:2049-2053
[33]Sakai Y,Shinohara A,Hayashi K,et al.Eur.J.Inorg.Chem.,2006,1:163-171
[34]Nomiya K,Hayashi K,Kasahara Y,et al.Bull.Chem.Soc.Jpn.,2007,80:724-731
[35]Bi L H,Al-Kadamany G,Chubarova E V,et al.Inorg.Chem.,2009,48:10068-10077
[36]Klemperer W G,Ginsberg A P.Inorganic Syntheses,1990,27:74-85
[37]Sheldrick G M.SHELXTL97,Program for Crystal Structure Solution,University of Göttingen,Göttingen,Germany,1997.
A Methylphenylsilyl Group-Substituted Derivative Based on the Trivacant Keggin Structure Tungstophosphate[(C4H9)4N]3[α-A-PW9O34(C6H5SiCH3)3]:Synthesis and Structural Characterization
ZHANG Dong-Di1,2WANG Ke2YAN Qing-Xia2MA Peng-Tao2WANG Jing-Ping*,2
(1Pharmaceutical College,Henan University,Kaifeng,Henan 475004,China)
(2Institute of Molecular and Crystal Engineering,College of Chemistry and Chemical Engineering,Henan University,Kaifeng,Henan 475004,China)
A methylphenylsilyl group-substituted derivative[(C4H9)4N]3[α-A-PW9O34(C6H5SiCH3)3](1)has been obtained by reaction of the trivacant [α-A-PW9O34]9-anion with dichloromethylphenylsilane C6H5SiCH3Cl2in acetonitrile.The new complex was characterized by elemental analysis,IR spectra,UV spectra,thermogravimetric analysis and X-ray crystallography.The compound 1 crystallizes in the trigonal space group R3m,with lattice constants a=2.261 3(2)nm,b=2.261 3(2)nm,c=1.797 6(4)nm,V=7.960 2(18)nm3,and Z=3.The polyoxoanion[α-A-PW9O34(C6H5SiCH3)3]3-has a structure of virtual C3vsymmetry with three C6H5SiCH3groups grafted on the surface of the trivacant tungstophosphate and displays an “open-structure”.CCDC:830334.
organosilyl;trivacant tungstophosphate;crystal structure;synthesis
O614.61+3
A
1001-4861(2012)10-2236-05
2012-03-26。收修改稿日期:2012-09-06。
国家自然科学基金(No.21071042);河南省基础与前沿技术研究课题(No.122300410126)资助项目。
*通讯联系人。E-mail:jpwang@henu.edu.cn
