Cloning and Sequence Analysis of Ribosomal Protein L21 Gene from the Ailuropoda melanoleuca
2012-08-06XiangDing
Xiang Ding
(Key Laboratory of Southwest China Wildlife Resources Conservation,China West Normal University College of Life Science,China West Normal University,Nanchong 637009,China)
1.INTRODUCTION
Ribosomes are the universal ribonucleoprotein particles that translate the genetic code into proteins.They are built of two subunits that associate upon initiation of protein synthesis.Ribosomal proteins exhibits various secondary functions in DNA repair, apoptosis, drug resistance and proliferation,which are the components of ribosome[1].With the continuous advancement of science and technology,the researchers are gradually finding the physiological functions of ribosomal proteins which play an important role in human disease and its development[2].
Ribosomal protein L21,encoded by rpL21gene,a component of the 60s large subunit,belongs to the L21E family of ribosomal proteins.rpL21 consists of two structural domains,the N-terminal(L21-NTD)and C-terminal(L21-CTD).It is located in the cytoplasm.As is typical for genes encoding ribosomal proteins,there are multiple processed pseudo genes of this gene dispersed through the genome.Some study examined the precise expression pattern of rpL21 mRNA in the mouse mandibular first molar by in situ hybridization.rpL21 mRNA was expressed in the presumptive dental epithelium and the underlying mesenchyme at E10.5,and in the thickened dental epithelium at E12.0[3-4].
The Giant Panda(Ailuropoda melanoleuca)is one of the oldest and rarest species in the world,known as“National treasure of China”,belonging to national level of endangered animal.In addition,the Giant Panda is renowned as living fossil,which has important scientific value in exploring environment change of the earth,and the researching and protecting of biodiversity.For years,the studies about Giant Panda have fo-cused on macrograph.However,recent researches on functional genes of giant panda are becoming a hot issue.Especially,the studies on ribosomal phosphoprotein P1 gene(rpLP1),rpS15,rpS19 etc.has made much progress[5-16].Nonetheless,there are few reports about rpL21 gene of the Giant Panda.
In this study,RT-PCR technique was used for amplifying the cDNA of rpL21 gene from the total RNA.Touchdown-PCR technique was employed to amplify the genomic sequence of the rpL21 from the total DNA from the Ailuropoda melanoleuca.At the same time,its characteristics were identified by homologous analysis according to the related sequences in GenBank.The study provides scientific data for inquiring into the hereditary traits of the gene from the Ailuropoda melanoleuca and formulating the protective strategy for the Ailuropoda melanoleuca.
2.MATERIALS AND METHODS
2.1 Materials
Skeletal muscle was collected from a dead Ailuropoda melanoleuca at the Wolong Conservation Center,Sichuan,China.The collected skeletal muscle was frozen in liquid nitrogen and then used for DNA and RNA isolation.
2.2 DNA and RNA isolation
The genomic DNA of the Ailuropoda melanoleuca was isolated from muscle tissue.The DNA obtained was dissolved in sterile water and kept at-20℃.
Total RNAs were isolated from about 400mg of muscle tissue using the Total Tissue/Cell RNA Extraction Kits(Waton Inc.,Shanghai,China)according to the manufacturer’s instructionsand,and dissolved in DEPC(diethypyrocarbonate)water,and kept at -70℃.
DNA and RNA sample quality was checked using Experion(Bio-Rad)and quantification was performed spectrophotometrically.
2.3 RT-PCR,Cloning of cDNA sequence
The PCR primers were designed by Primer Premier 5.0,according to the mRNA sequence of rpL21 from H.sapiens(NM_000975),B.Taurus(NM_001075581),P.abelii(NM_001131913),S.scrofa(NM_001001638),M.musculus(NM_025919),R.norvegicus(NM_001025739).The specific primers of cDNA sequence are as follows:
rpL21-F:5’—ATCTTCCAGTAATTCGCCAAA—3’;
rpL21-R:5’—AGTACGGTACTTAAGTATCCC—3’.
Total RNAs were synthesized into the first-stranded cDNAs using a reverse transcription kit with Oligo dT as the primers according to the manufacturer’s instructions(Promega).Reverse transcription reactions were perfomed in duplicate.Lack of genomic DNA contamination was confirmed by PCR amplification of RNA samples in the absence of cDNA synthesis.After amplification,PCR products were separated by electrophoresis in 1.5%agarose gel with 1 × TAE(Tris-acetate-EDTA)buffer,stained with ethidium bromide and visualized under UV light.The expected fragments of PCR products were harvested and purified from gel using a DNA harvesting kit(O-mega,China),and then ligated into a pET28a vector at 22℃for 12 hours.The recombinant molecules were transformed into E.coli complete cells(JM109),and then spread on the LB-plate containing 50μg/mL ampicillin,200mg/mL IPTG(isopropyl-beta-Dthiogalactopyranoside), and 20mg/mL X-gal.Plasmid DNA was isolated and digested by PstI and ScaII to verify the insert size.Plasmid DNA was sequenced by Huada Zhongsheng Scientific Corporation(Beijing,China).Repeat the procedure above three times.
2.4 Cloning the Genomic sequence of rpL21
The PCR primers were the same as the rpL21-F and rpL21-R presented above.The genomic sequence of the rpL21 gene was amplified using Touchdown-PCR with the following conditions:94℃ for 30s,62℃ for 45s,72℃ for 4 minutes in the first cycle and the anneal temperature deceased 1℃ per cycle;after 20 cycles conditions changed to 94℃ for 30s,52℃ for 45s,72℃ for 4 minutes for another 15 cycles.The fragment amplified was also purified,ligated into the clone vector and tansformed into the E.coli competent cells.Finally,the recombinant fragment was sequenced by Sangon(Shanghai,China).Repeat the procedure above three times.
2.5 Data analysis
The sequence data were analyzed by GenScan software.Homology research of the Ailuropoda melanoleuca rpL21 compared with the gene sequences of other species were performed using Blast 2.1.ORF of the DNA sequence was searched using ORF finder software.Protein structure of the rpL21 sequence cloned was deduced using Predict Protein software(http://cubic.Bioc.Columbia.edu/predictprotein/).Multiple sequence alignment was performed by DNAMAN 6.0.The prediction of protein functional sites and biochemical characteristics depend on the software ExPASy Proteomics Server.
3.RESULTS
3.1 Analysis of the cDNA of rpL21 from the Ailuropoda melanoleuca
A cDNA fragment of about 600bp was amplified from the Ailuropoda melanoleuca with the primers rpL21-F and rpL21-R.The length of the cDNA cloned is 504bp.Blast research showed that the cDNA sequence cloned is highly homologous with the rpL21 from Homo sapiens and some other specieses reported.On the basis of the high identity,we concluded that the cDNA isolated is the cDNA encoding the Ailuropoda melanoleuca rpL21 protein.The rpL21 sequence has been submitted to Genbank(accession number:1393346).An ORF of 483bp encoding 160 amino acids was found in the cDNA sequence,encoding the Ailuropoda melanoleuca rpL21 protein.The initiation codon of rpL21 is ATG,and terminator codon is TGA.The average levels of the bases sequence is:A,34.7%;C,20.2%;G,23.6%;T,21.4%.
3.2 Analysis of the genomic sequence of rpL21 from the Ailuropoda melanoleuca
A DNA fragment of about 3000bp was amplified with primers rpL21-F and rpL21-R.The length of the DNA fragment cloned is 2254bp.Comparison between the cDNA sequence and the DNA fragment sequence of the rpL21 amplified from the Ailuropoda melanoleuca was performed by software Lasergene.The result indicated that the cDNA sequence is in full accord with 5 fragments in the DNA fragment,which mean that the DNA fragment amplified is the genomic sequence of the rpL21 from the Ailuropoda melanoleuca(Fig.1).The genomic sequence of the rpL21 has been submitted to Genbank(bankit1393347).A comparison of the nucleotide sequences of the genomic and cDNA sequences indicated that gene cloned contains 5 exons and 4 introns with Genscan software.The 5 exons distribute differently the details is as follows:16-82;342-403;971-1083;1834-1984;2165-2254.

Fig.1 Nucleotide sequence of cDNA encoding the Giant Panda rpL21 gene and the amino acid sequence deduced(* stands for the terminator codon.)
3.3 Prediction and analysis of protein functional sites in rpL21 protein of the Ailuropoda melanoleuca
Primary structure analysis revealed that the molecular weigh of the putative rpL21 protein of the Ailuropoda melanoleuca is 18.59kD with a theoretical pI 11.10,containing 45 positively charged amino acid residues(Arg,Lys and His),13-band negatively charged amino acid residues(Asp and Glu).The content of Lys is highest,and Trp is the lowest(only one).Topology prediction shows that there are 1 N-glycosylation site,1 cAMP-and cGMP-dependent protein kinase phosphorylation site,1 ribosomal protein L21e signature and 1 protein kinase C phosphorylation site,1 amidation site,1 N-myristoylation site in the rpL21 protein of the Ailuropoda melanoleuca(Fig.2).

Fig.2 Comparison of the rpL21 amino acid sequences among the different species
3.4 Comparison of sequence similarity of rpL21 gene sequence and encoding sequence
The gene sequence and coding sequence of rpL21 were compared with high similarity between the Ailuropoda melanoleuca and the other 5 eukaryotic.The molecular weight and isoelectric point(pI)of rpL21 also found a closer comparison.
There is some change been found through the comparison of functional site,especially the change of N-myristoylation site,others are the same(Table 1).
3.5 Comparison of rpL21 genome
The rpL21 gene was compared and found that there are different in length of genomes,introns among 5 species.However,the intron length was found to decide the length of the genome(Table 2).

Table 1 Differences of secondary structure among 5 mammal species
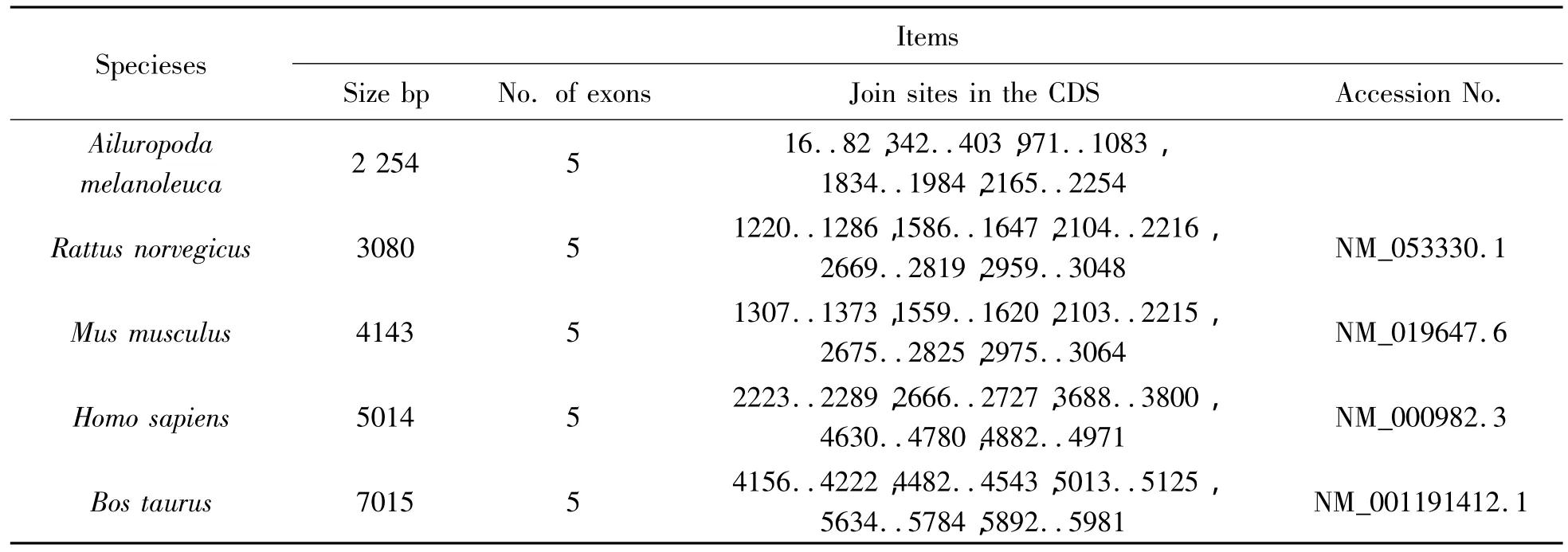
Table 2 The length comparision of the rpL21 genomic sequence between A.melanoleuca and other four species
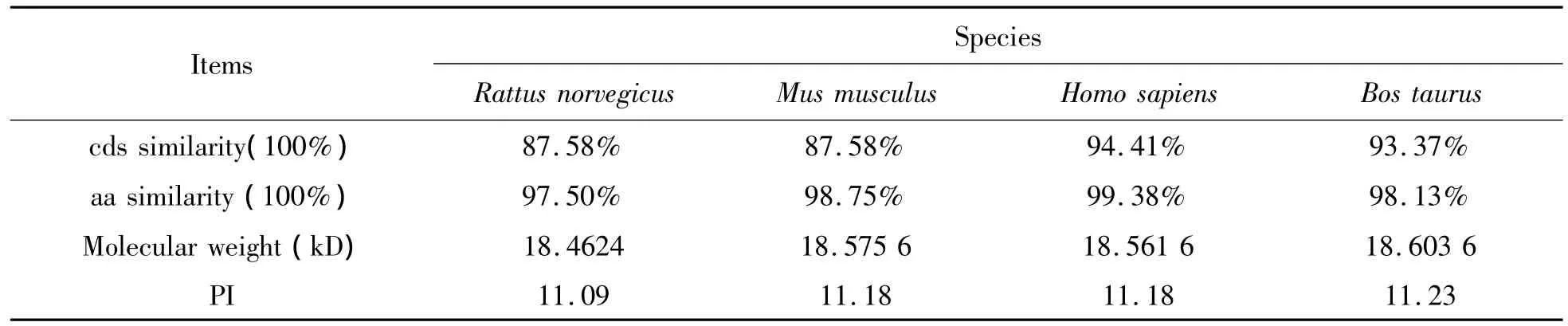
Table 3 Comparison of nucleotide,amino acid sequences and physicochemical property between A.melanoleuca and other 4 mammal species
5.DISCUSSION
Ribosomal protein L21 plays an important role in ribosome,being not only involved in several steps in protein synthesis,but also associated with nucleolin in the cell proliferation of tooth germ[3].Here,we cloned genomic sequence and cDNA sequence encoding rpL21 from the Ailuropoda melanoleuca.The genomic sequence of rpL21 is 2254bp in size and possesses 5 exons and 4 introns.Compared with some species incluing Rattus norvegicus,Mus musculus,Homo sapiens,Bos taurus,the number and length of introns are different.The largest intron of the rpL21 gene from the Ailuropoda melanoleuca is 750bp.The variations in lengths of the introns determine the lengths of the rpL21 gene(Table 2).
The length of cDNA fragment cloned is 504bp,containing an open reading frame of 483bp.Deduced protein was composed of 160 amino acids with an estimated molecular weight of 18.59 kD and pI of 11.10.The rpL21 shares high homology by comparision of cds sequence and amino acid sequences similarity.Alignment analysis indicated that many polymorphism sites exist in the cds sequence comparision of vertebrates’rpL21.Among these polymorphic sites,half is almost degeneration sites,half another is a single variable sites.It is transversion and transition that caused the variation,which do not affect amino acid sequences encoded.rpL21 is shares over 99.25%amino acid sequences with other vertebrates,as well as stay very similar amino acid identity with homologous sequences in Homo sapiens,in particular,the most middle amino acid sequences,is the variation arise largely in begin and end of L21 amino acid sequences.
The genome sequence we cloned shared a high similarity with the published sequence(GL192357)of the Ailuropoda melanoleuca(97.97%).To begin with,GL192357 is 3629bp in size,but which is called rpL21 is 2254bp.Secondly,the GL192357 is the absence of termination code,correspondingly,the sequence we clone is TAA.More important,the 1th,4th,5th base of GL192357 is Thymine,Cytosine,Adenine,respectively.Correspondingly,HM047802 is Adenine,Guanine,and Cytosine.The difference leads to change in the 1th and 2th amino acid residue.The unknown protein sequence has been reported is L(Leu),Q(Gln),however,the sequence we clone is M(Met),A(Ala).We got the genome sequence is full accord with the clonings and sequencings three times.As for the reasons caused by these differences needs further study.
Topology prediction showed there are less changes been found through the comparison of functional sites(Figure 2).Only do Pongo abelii and Danio rerio have one more protein kinase C phosphorylation site than others’due to the change of 142 site(A→T)and of 150 site(C→R).The changes whether effect structure and the function of rpL21 need further studies.At the same time,the amino acid changes were also found in Sus scrofa and Rattus norvegicus,however,the variation of sites has no effect on the structure and function of rpL21 protein.
These facts suggest that rpL21 protein were high conserved in evolution.The results also accord with the previous study.Noteworthy is that the number of amino acid is not different(Figure 2).We found that the number of amino acid of rpL21 is sTable compared with the other species.
In summary,the cDNA and the genomic sequence of rpL21 were cloned successfully from the Ailuropoda melanoleuca,respectively,which were both sequenced and analyzed preliminarily.The data will enrich and supplement the information about rpL21.
6.ACKNOWLEDGEMENTS
This work is financially supported by Application Foundation Project in Sichuan Province(2009JY0061)and Youth Fund Project of Educational Committee of Sichuan province(09ZB088).
[1]H Wang,L N Zhao,K Z Li.Overexpression of ribosomal protein L15 is associated with cell proliferation in gastric cancer[J].BMC Cancer,2006(6):91.
[2]F Yang,W P Liu.Study on the Relation of Ribosomal Protein Gene and human diseases[J].Chinese journal of clinical and experimental pathology,2005(3):354.
[3]Ming Xie,Kobayashi,Kiyoshima,et al.In situ expression of ribosomal protein L21 in developing tooth germ of the mouse lower first molar[J].Journal of Molecular Histology,2010(4 -5):199.
[4]Albertsen,Smith,Mazoyer,et al.A physical map and candidate genes in the BRCA1 region on chromosome 17q12-21,Journal of Nat[J].Genet,1994(4):472.
[5]M J Liao,M Y Zhu,Z H Zhang,et al.Cloning and sequence analysis of FSH and LH in the Giant Panda(Ailuropoda melanoleuca)[J].Animal Reproduction Science,2003(1 -2):107.
[6]M J Liao,M Y Zhu,Z H Zhang,et al.cDNA cloning of growth hormone from giant panda(Ailuropoda melanoleuca)and its expression in Escherichia coli[J].Comp.Biochem.Phys.B.,2003(1):109.
[7]Jennie,Alison,Rong.Activins,inhibins and follistatins:further thoughts on a growing family of regulator[J].Proc Soc Exp Biol Med,1997,215:209.
[8]Z A Wu,W X Liu,Murphy,et al.Satellite DNA sequence from genomic DNA of the giant panda[J].Nucleic Acids Res,1990(4):1054.
[9]W R Hou,Y Chen,Z S Peng,et al.cDNA cloning and sequences analysis of ubiquinol_cytochrome c reductase complex ubiquinone_binding protein(QP_C)from Giant Panda[J].Acta.Theriologica.Sinica,2007(2):190.
[10]Y J Du,X Y Luo,Y Z Hao,et al.Cloning and overexpression of acidic ribosomal phosphoprotein P1 gene(RPLP1)from the Giant Panda[J].Inter J Biol Sci,2007(3):428.
[11]W R Hou,Y J Du,Y Chen,et al.Nucleotide sequence of cDNA encoding the mitochondrial precursor protein of the ATPase inhibitor from the Giant Panda(Ailuropoda melanoleuca)[J].DNA Cell Biol,2007(11):799.
[12]Y J Du,W R Hou,Z S Peng,et al.cDNA cloning and sequences analysis of acidic ribosomal phosphoprotein P1(RPLP1)from Giant Panda[J].Acta Theriologica Sinica,2008(1):75.
[13]W R Hou,X Y Luo,Y J Du,et al.cDNA Cloning and Sequences analysis of RPS15 from the Giant Panda[J].Recent Patent on DNA Sequence,2008(2):16.
[14]Y L Hou,W R Hou,Z L Ren,et al.cDNA,genomic sequence and overexpression of crystalline alpha_B Gene(CRYAB)of the Giant Panda[J].Inter J Bio Sci,2008(4):415.
[15]Y L Hou,Y J Du,W R Hou,et al.Cloning and sequence analysis of translocase of inner mitochondrial membrane 10 homolog(yeast)gene(TIMM10)from the giant panda[J].J Cell Anim Biol,2009(1):9.
[16]Y L Hou,W R Hou,Z L Ren,et al.cDNA Cloning and Overexpression of Ribosomal Protein S19 Gene(RPS19)from the Giant Panda[J].Cell Biol,2009(1):41.
