Micron-sized Magnetic Polymer Microspheres for Adsorption and Separation of Cr(VI) from Aqueous Solution*
2012-03-22WANGQiang王强GUANYueping官月平LIUXiang刘翔YANGMingzhu杨明珠andRENXiufeng任秀峰
WANG Qiang (王强), GUAN Yueping (官月平)**, LIU Xiang (刘翔), YANG Mingzhu (杨明珠)and REN Xiufeng (任秀峰)
School of Materials Science and Engineering, University of Science and Technology Beijing, Beijing 100083, China
1 INTRODUCTION
Heavy metals with the potential impact on environmental quality and human health cause a great risk.Amongst them Cr(VI) is dangerous for humans due to its toxicity and carcinogenic properties [1, 2]. In addition, as its special properties, chromium is extensively used in pigments and paints, leather tanning, fungicides, electroplating, cement, steel, ceramic and glass industries [3, 4]. Attempts to remove or recover Cr(VI)from the environment have utilized a variety of separation techniques, such as chemical precipitation, ion exchange, adsorption, and solvent extraction [5, 6]. However, these conventional technologies have a number of drawbacks: loss of extractant, environmental pollution, high cost, and complexity of separation process, etc.
With the rapid development of modern separation techniques, magnetic microsphere (MMS) plays an important role in many fields in recent years, especially for cell isolation [7], enzyme immobilization [8],protein separation and purification [9], etc. Over the past two decades, many methods have been proposed to prepare composite microspheres with inorganic magnetic core and polymer outer shell, which provide high magnetic susceptibility, appropriate size distribution, and abundant functional groups on surface for coupling affinity ligands. Several kinds of polymerization can be used, such as emulsion polymerization[10, 11], dispersion polymerization [12, 13], suspension polymerization [14, 15], and seed polymerization [16].Of these methods, suspension polymerization is simple and more suitable for large-scale production of magnetic polymer microspheres with higher saturation magnetization.
In the present work, we selected amino group to adsorb and separate Cr(VI) from aqueous solution.The magnetic poly-(MA-DVB) microspheres with micron size were synthesized by modified suspension polymerization based on previous works [17, 18],which have narrow size distribution and high magnetite contents. After being soaked and agitated in methylformamide (DMF) and ethylenediamine (EDA),the ester groups on the poly-(MA-DVB) microspheres were converted into amino groups, and the resulted microspheres were denoted as poly-(MA-DVB)-NH2.The above mentioned magnetic microspheres were applied for adsorption of Cr(VI) from aqueous solution. The advantages of this method over conventional techniques include the higher specific surface area,simple operation and rapid separation. The effects of pH value, adsorption time and adsorption temperature were investigated in a series of experiment.
2 EXPERIMENTAL
2.1 Reagents and instruments
Chemicals used were generally of reagent grade from commercial sources. Methacrylate (MA) and divinyl benzene (DVB) were distilled to remove the inhibitor prior to use. All other materials were of analytical grade and used without further purification including ferrous chloride tetrahydrate (FeCl2·4H2O),ferric chloride hexahydrate (FeCl3·6H2O), ammonium hydroxide [25% (by mass) NH3in water], oleic acid,benzoyl peroxide (BPO), poly(vinyl alcohol) (PVA),ethylenediamine (EDA), N,N-dimethylformamide(DMF), ethanol, sodium chloroacetate, sodium carbonate (Na2CO3), copper sulfate (CuSO4·5H2O), sodium hydroxide (NaOH), ethylene diamine tetraacetic acid (EDTA), potassium chromate (K2CrO4). Water is purified by deionization using ion exchange resins.AA-6800 atomic absorption spectroscopy (AAS) is from Shimadzu Co. (Japan).
2.2 Synthesis of magnetic poly-(MA-DVB) microspheres
The oleic acid-coated magnetic gel was prepared by a conventional co-precipitation method [19]. After the excess oleic acid was removed, the magnetic precipitate was re-dispersed in hexane to form magnetic fluid. The obtained Fe3O4magnetic fluid (3 g) and the inhibitor BPO (0.225 g) were dispersed in a mixture of MA (14.25 ml) and the cross-linker DVB (0.45 ml),and agitated until Fe3O4was dissolved completely.The mixture was then transferred to a 250 ml beaker containing the stabilizer PVA (3.75 g) and NaCl (4.5 g)dissolved in 150 ml deionized water. With rapid agitation, the mixture temperature was increased to reaction temperature under the protection of nitrogen. The resulted magnetic microspheres were thoroughly washed with deionized water and then with ethanol to remove the excess amount of stabilizer and other impurities.
2.3 Surface modification of magnetic poly-(MADVB) microspheres
Magnetic poly-(MA-DVB) microspheres (3 g)was washed with DMF two times and put in a mixture of DMF (150 ml) and EDA (150 ml). The mixture was agitated gently at 80 °C for 8 h. After cooled to room temperature, the microspheres were separated by magnetic decantation and washed with deionized water and then ethanol to remove the residual DMF. After modification, ester groups on the microspheres were converted into amino groups.
2.4 Adsorption procedure of Cr(VI) from aqueous solution
The Cr(VI) aqueous solutions were prepared by dissolving a weighed amount of K2CrO4in a known volume of deionized water. The pH value was adjusted to the desired value by adding 2 mol·L-1hydrochloric acid. A certain sample magnetic poly-(MADVB)-NH2microspheres were added into the prepared aqueous solutions with agitation for 1 h at specified temperature. The magnetic microspheres adsorbed Cr(VI) were separated by magnet. The concentration of the residual Cr(VI) aqueous solution was characterized by AAS. The adsorption efficiency of magnetic microspheres was calculated by the change of concentration of Cr(VI) before and after.
3 RESULTS AND DISCUSSION
3.1 Synthesis and surface modification of magnetic poly-(MA-DVB) microspheres
Micron-size magnetic poly-(MA-DVB) microspheres were synthesizedviaa modified suspension polymerization method as shown in Fig. 1.

Figure 1 Synthesis and modification of magnetic poly-(MA-DVB) microspheres
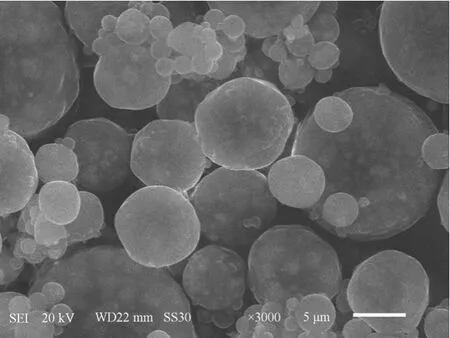
Figure 2 SEM picture of the magnetic poly-(MA-DVB)microspheres synthesized by the modified suspension polymerization method
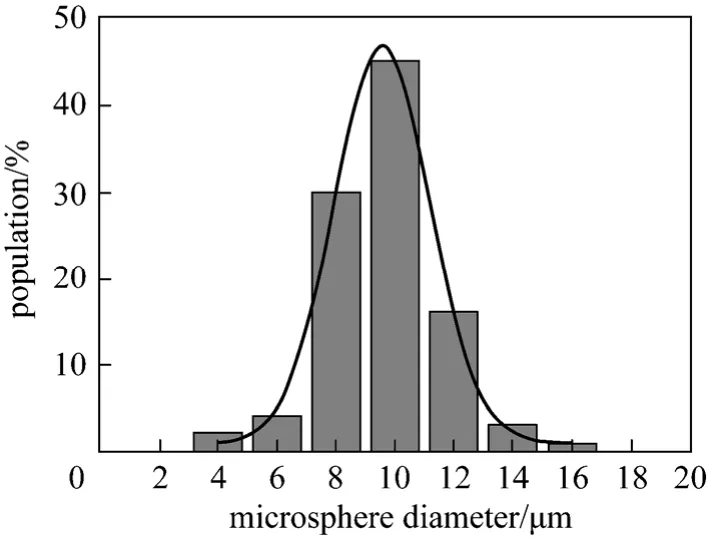
Figure 3 Size distribution of the magnetic poly-(MA-DVB)microspheres (Dm=9.8 μm, δ=0.18)
The morphology and size of magnetic microspheres are shown in Figs. 2 and 3. Magnetic poly-(MA-DVB) microspheres are spherical. The particle size distributions were calculated as statistics of 300 particles in different regions of several transmission electron microscope (TEM) photos. It indicates that these microspheres are of 9.8 μm in average diameter with narrow size distribution.
The magnetic properties of poly-(MA-DVB) microspheres were recorded by vibrating sample magnetometer (VSM) at room temperature. Fig. 4 showed their magnetization curves. No hysteresis loop was observed at this temperature, suggesting that the magnetic microspheres were superparamagnetic, which indicated that there would be no magnetic interaction among magnetic microspheres in an environment of zero magnetic strength. This feature would result in easy dispersion of the magnetic microspheres. The saturation magnetization of poly-(MA-DVB) microspheres was 7.8 A·m2·kg-1. With such saturation magnetization, they could be easily and quickly separated from a suspension. This could be used to the magnetic separation of Cr(VI) on a large scale.

Figure 4 Magnetization curve of magnetic poly-(MA-DVB)microspheres obtained by VSM at room temperature (σs=7.8 A·m2·kg-1)
To quantitatively measure the capacity of the amino groups on the surface of magnetic poly-(MA-DVB)microspheres, the surface amino groups were transferred into iminodiethanoic acid (IDA) groups by reaction with sodium chloroacetate as shown in Fig. 5.
The IDA groups are ready for chelating metal ions such as Cu2+. Cu2+was selected as the metal chelating ligand because of its high chelation efficiency with IDA groups. When magnetic poly-(MA-DVB) microspheres with Cu2+immobilized were treated with 0.1 mol·L-1EDTA, the EDTA aqueous solution become blue, indicating that Cu2+already chelated on the surface of magnetic poly-(MA-DVB) microspheres. The capacity of Cu2+on the surface of magnetic poly(MA-DVB) microspheres was quantitatively measured by atomic absorption spectrophotometer (AAS).The capacity of the amino groups were calculated by the capacity of Cu2+immobilized on the surface of magnetic poly-(MA-DVB) microspheres. The results showed that the capacity of the amino groups was up to 1.67 mmol·g-1magnetic microspheres.
3.2 Adsorption of Cr(VI) from aqueous solution
3.2.1Effect of pH value
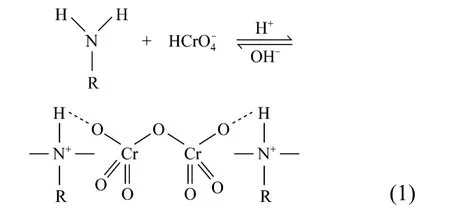
The initial pH of the Cr(VI) solution is an important parameter, which controls the adsorption process particularly the adsorption capacity. This parameter causes the change of surface charge of the sorbent, conversion of the chromium species and other ions present in the solution, and extent of dissociation of functional groups on the active sites of the adsorbent [20]. The distribution of Cr(VI) species is dependent on both the total concentration of Cr(VI) and pH of the equilibrium solution. Chromium exists in five main forms in aqueous solution. The reactions between these species and the reaction equilibrium constants (K) are shown in Reactions 2-5 [21]:
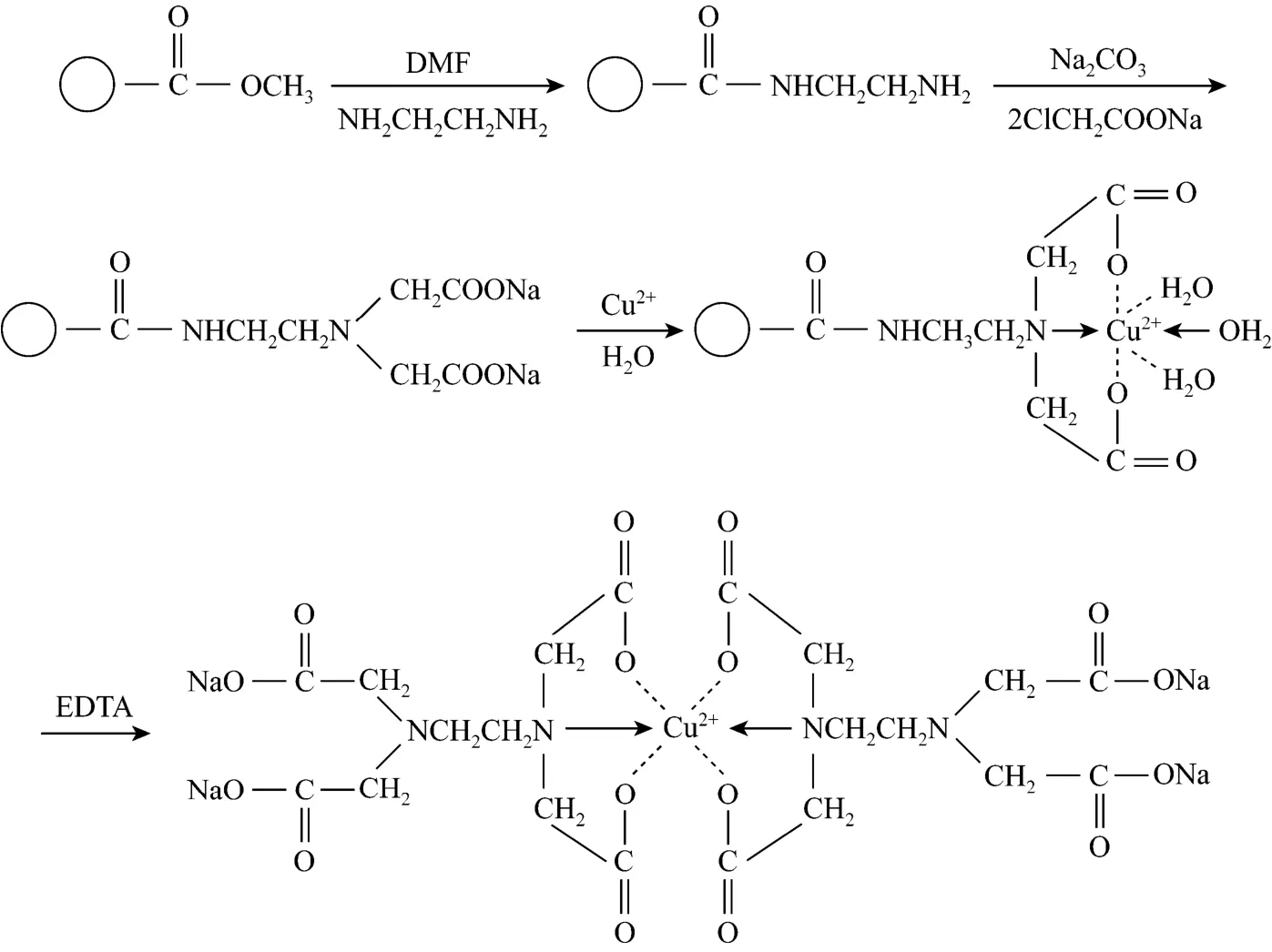
Figure 5 Chelation of IDA groups on magnetic poly-(MA-DVB) microspheres with Cu2+
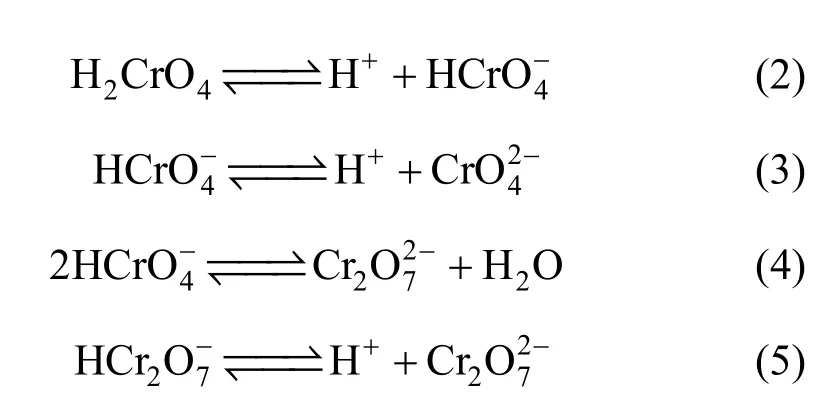
To determine a suitable pH value in Cr-containing aqueous solution, the experiments were carried out using different pH value ranging from 1 to 5. The other parameters were kept constant: mass of microspheres was 100 mg, temperature was 20 °C, initial Cr(VI)concentration was 26 mg·L-1and adsorption time was 60 min. The results are depicted in Fig. 6, showing that the maximum adsorption capacity was achieved at pH of 3. The low adsorption capacity at pH below and above 3 may be related to the structural change of amino groups and chromium species, respectively.Therefore, the optimum pH of 3 was selected for subsequent investigations.

Figure 6 Effect of pH value in Cr(VI) aqueous solution on the adsorption capacity (T=20 °C, t=60 min, C0=26 mg·L-1)
3.2.2Adsorption equilibrium study
Figure 7 shows the change in the adsorption capacity of Cr(VI) by the given adsorption time at initial concentration of 26 mg·L-1(250 ml), 100 mg microspheres, pH at 3 and temperature of 20 °C. It can be seen that the adsorption capacity increases with adsorption time and levels off at 60 min. The adsorption capacity is considered saturated at 60 min. Saturated adsorption capacity is 37.0 mg·g-1. The linearity in Fig. 7 suggests that the adsorption is controlled by the chemical process related to adsorption rather than the mass transfer of Cr(VI) ions.
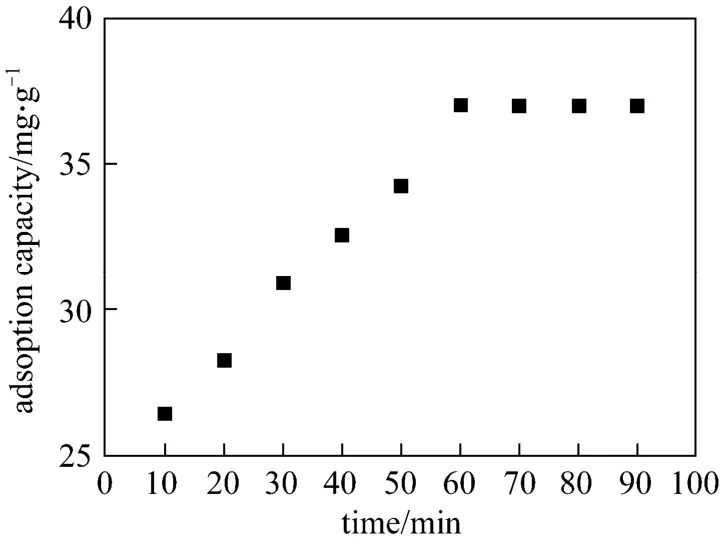
Figure 7 Effect of contact time on the adsorption capacity(T=20 °C, pH=3, C0=26 mg·L-1)

Figure 8 Kinetic fit for the adsorption of Cr(VI)aqueous solution on magnetic poly-(MA-DVB) microspheres (C0=26 mg·L-1, A=2∶5 g·L-1, pH=3, T=293 K)
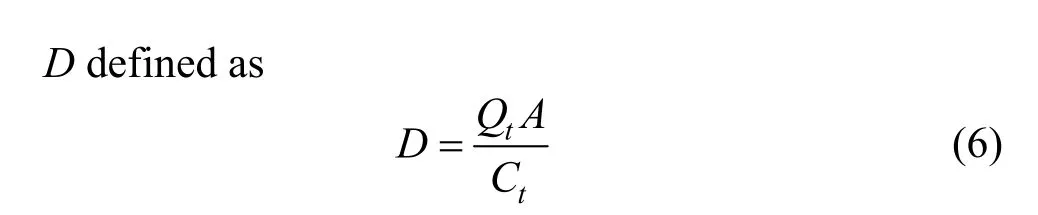
Figure 8 indicates the relationship of lnDversusadsorption time, with the solid-liquid distribution ratio whereQtis adsorption of Cr(VI) on solid (mg·g-1),Ctis the concentration of Cr(VI) in solution (mg·L-1),Ais solid-liquid ratio (g·L-1). The fitness of the straight line reveals that the adsorption process is a pseudo-first order reaction (R2=0.991).
3.2.3Effect of temperature
The effect of temperature on the adsorption of Cr(VI) by magnetic poly-(MA-DVB) microspheres is shown in Fig. 9. Temperature ranged from 30 °C (303 K) to 0 °C (273 K). Initial Cr(VI) concentration was 26 mg·L-1(250 ml), mass of microspheres was 100 mg, pH was 3 and adsorption time was 60 min. Thermodynamically, parameters such as free energy change (∆G0), enthalpy change (∆H0) and entropy change (∆S0) can be calculated using the following Eqs. (7-10), whereKexis equilibrium constant,Qeis equilibrium adsorption amount (mg·g-1),Ceis the equilibrium Cr(VI) concentration in the solution(mg·L-1) andAis solid-liquid ratio (g·L-1). ∆G0, ∆H0and ∆S0can be calculated from a plot of lnKexversus1/T. As seen from Fig. 9, lnKex>0, so ∆G0<0 indicating the spontaneous nature of the adsorption. As calculated, ∆H0=1.47 kJ·mol-1, ∆S0=49.62 J·mol-1·K-1.The positive ∆H0shows that the adsorption on magnetic poly-(MA-DVB) microspheres is endothermic.The reason why adsorption capacity increased with increasing temperature can be explained by the value of ∆H0.

Figure 9 Effect of adsorption temperature on equilibrium constant (C0=26 mg·L-1, A=0.4 g·L-1, pH=3, t=60 min)
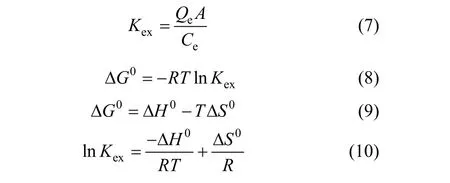
4 CONCLUSIONS
The magnetic poly-(MA-DVB) microspheres were synthesized by a modified suspension polymerization method. The amino groups on the surface of magnetic microspheres were modified, and detected by the method using IDA chelated Cu2+. The capacity of the amino groups was up to 1.67 mmol·g-1magnetic microspheres. The adsorption of Cr(VI) from aqueous solution by magnetic poly-(MA-DVB) microspheres with surface amination was investigated. The results show that the optimum pH for Cr(VI) adsorption was 3, the adsorption capacity increased with adsorption time and attained saturation at 60 min, and the adsorption capacity increased with increasing temperature.
metal affinity separation media and its use in the isolation of proteins”, J. Chromatogr. A, 795, 211-217 (1998).
1 Shu, Z.N., Du, R.J., Wang, X., Xiong, C.H., Li, T., “Adsorption of XSD-296 resin for Cr(VI)”,Trans.Nonferrous.Met.Soc.China, 17,869-873 (2007).
2 Elwakeel, K.Z., “Removal of Cr(VI) from alkaline aqueous solutions using chemically modified magnetic chitosan resins”,Desalination,250, 105-112 (2010).
3 Lin, S.H., Kiang, C.D., “Chromic acid recovery from waste acid solution by an ion exchange process: equilibrium and column ion exchange modeling”,Chem.Eng.J., 92, 193-199 (2003).
4 Kocaoba, S., Akcin, G.., “Removal of chromium (III) and cadmium(II) from aqueous solutions”,Desalination, 180, 151-156 (2005).
5 Venkateswaran, P., Palanivelu, K., “Studies on recovery of hexavalent chromium from plating wastewater by supported liquid membrane using tributyl phosphate as carrier”,Hydrometallurgy, 78,107-115 (2005).
6 Zhang, W., Liu, J., Ren, Z., Wang, S., Du, C., Ma, J., “Kinetic study of chromium(VI) facilitated transport through a bulk liquid membrane using tri-n-butyl phosphate as carrier”,Chem.Eng.J., 150,83-89 (2009).
7 Sun, L., Zborowski, M., Chalmers, J.J., “Continuous, flow-through immunomagnetic cell sorting in a quadrupole field”,Cytometry, 33,469-475 (1998).
8 Li, X.H., Sun, Z.H., “Synthesis of magnetic polymer microspheres and application for immobilization of proteinase of balillus sublitis”,J.Appl.Polym.Sci., 58, 1991-1997 (1995).
9 Abudiab, T., Beitle, R.R., “Preparation of magnetic immobilized
10 Noriko, Y., Hiromichi, N., Hideki, A., Tatsuo, S., “Preparation of magnetic latex particles by emulsion polymerization of styrene in the presence of a ferrofluid”, J. Polym. Sci., 50,765-776 (1993).
11 Kondo, A., Kamura, H., Higashitahi, K., “Development and application of thermosensitive magnetic immunomicrospheres for antibody purification”, Appl. Microbiol. Biotechnol., 41, 99-105 (1994).
12 Horak, D., Shapoval, P., “Reactive poly(glycidyl methacrylate) microspheres prepared by dispersion polymerization”, J. Polym. Sci.Part A Polym. Chem., 38, 3855-3863 (2000).
13 Horak, D., “Magnetic polyglycidylmethacrylate microspheres by dispersion polymerization”, J. Polym. Sci. A Polym. Chem., 39,3707-3715 (2001).
14 Cocker, T.M., Fee, C.J., Evans, R.A., “Preparation of magnetically susceptible polyacrylamide/magnetite beads for use in magnetically stabilized fluidized bed chromatography”, Biotechnol. Bioeng., 53,79-87 (1997).
15 Lee, Y., Rho, J., Jung, B., “Preparation of magnetic ion-exchange resins by the suspension polymerization of styrene with magnetite”,J. Appl. Polym. Sci., 89, 2058-2067 (2003).
16 Lee, J., Senna, M., “Preparation of monodispersed polystyrene microspheres uniformly coated by magnetite via heterogeneous polymerization”, Colloid. Polym. Sci., 273, 76-82 (1995).
17 Ma, Z.Y., Guan, Y.P., Liu, X.Q., Liu, H.Z., “Preparation and characterization of micron-sized non-porous magnetic polymer microspheres with immobilized metal affinity ligands by modified suspension polymerization”, J. Appl. Polym. Sci., 96, 2174-2180 (2005).
18 Liu, X., Guan, Y.P., Shen, R., Liu, H.Z., “Immobilization of lipase onto micron-size magnetic beads”, J. Chromatogr. B, 822, 91-97(2005).
19 Liu, X.Q., Guan, Y.P., Xing, J.M., Ma, Z.Y., Liu, H.Z., “Synthesis and properties of micron-size magnetic polymer spheres with epoxy groups”, Chin. J. Chem. Eng., 11, 731-735 (2003).
20 Hosseini, M.S., Hosseini-Bandegharaei, A., Raissi, H., Belador, F.,“Sorption of Cr(VI) by Amberlite XAD-7 resin impregnated with brilliant green and its determination by quercetin as a selective spectrophotometric reagent”, J. Hazard. Mater., 169, 52-57 (2009).
21 Cabatingan, L.K., Agapay, R.C., Rakels, J.L.L., Ottens, M., Vander Wielen, L.A.M., “Potential of biosorption for the recovery of chromate in industrial wastewaters”, Ind. Eng. Chem. Res., 40, 2302-2309(2001).
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Retrospect and Perspective of Micro-mixing Studies in Stirred Tanks*
- In-situ Synthesis and Catalytic Properties of ZSM-5/Rectorite Composites as Propylene Boosting Additive in FluidCatalytic Cracking Process*
- Low-temperature Electrodeposition of Aluminium from Lewis Acidic 1-Allyl-3-methylimidazolium Chloroaluminate Ionic Liquids*
- Solvent Extraction of Yttrium by Task-specific Ionic Liquids Bearing Carboxylic Group*
- Liquid-Liquid-Liquid Three Phase Extraction Apparatus: Operation Strategy and Influences on Mass Transfer Efficiency*
- Study on Kinetics of Iron Oxide Reduction by Hydrogen*
