Solvent Extraction of Yttrium by Task-specific Ionic Liquids Bearing Carboxylic Group*
2012-03-22WANGWei王威LIUYu刘郁XUAimei徐爱梅YANGHualing杨华玲CUIHongmin崔红敏andCHENJi陈继
WANG Wei (王威), LIU Yu (刘郁), XU Aimei (徐爱梅), YANG Hualing (杨华玲), CUI Hongmin (崔红敏) and CHEN Ji (陈继),**
1 State Key Laboratory of Rare Earth Resources Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China
2 Graduate School of the Chinese Academy of Sciences, Beijing 100039, China
1 INTRODUCTION
Ionic liquids (IL) have been widely investigated in liquid-liquid extraction since the first report by Rogers and co-workers [1]. IL attract attention continually not only because of their unique properties but also a promising possibility of tuning their properties by modification of IL’s cation or anion [2-7]. The tuned properties makes it feasibility to find IL not only with desirable physicochemical properties but also adjusted for particular applications. It has been reported that IL could be used in metal ions extraction[2, 4, 8]. There exist two different types of IL in this field. The first one is using IL as diluents for extraction, in which the IL themselves have no extraction ability for metal ions and an organic coordinating compounds should be added to enhance the extraction efficiency. The second type lies in the concept of task-specific ionic liquds (TSIL), in which the ILs themselves contain functional groups on their cation or anion. Thus, the TSIL could extract metal ions from aqueous phase by the functional groups. Many TSIL incorporating functional groups has been reported for metal ions extraction and separation, especially in the filed of extraction and separation of actinides and lanthanides from nuclear waste because of the high stability of IL under α- and γ-irradiation [3, 9].
Rare earths are abundant in China. They are wildly applied in many fields and gained considerable recognition with the increasing demand for them [10].Liquid-liquids extraction is a most important way for the rare earths separation and purification. Although the rare earth elements are not radioactive, they always coexist with radioactive elements, such as thorium [8, 11]. So introducing the functional groups which could combine with rare earths ions into the IL to be TSIL and applying them in the rare earths extraction would be an improvement for the industrial safety. Our group has investigated several extraction system using TSIL as extractant for the rare earths extraction and separation, and good results were obtained [5, 12, 13].
In this paper, a new kind of TSIL containing carboxylic group are synthesized and characterized. And the extraction properties of Y(III) into this kind of task-specific ionic liquids were investigated.
2 EXPERIMENTAL
2.1 Reagents
1-Chlorobutyl, 1-bromohexane, 1-chlorooctane and hexafluorophosphoric acid were purchased from Sigma-Aldrich (Milwaukee, USA). Ethyl acetate,imidazole and dichloromethane were obtained from Beijing Beihua Fine Chemicals Co. (China). Arsenazo III (Beijing Shiying Chemical Works). [C8mim][PF6]was synthesized as previously reported [7]. Stock solution of Y(III) was prepared by dissolving its oxide(99.9%) in HNO3, and the concentration of Y(III) was standardized by EDTA (ethylenediamine tetraacetic acid) titration using xylenol orange as indicator. The other chemicals were all analytical grade reagents.
2.2 Apparatus and measurements
The pH values of the aqueous phase were measured by model PHS-3C pH meter (Leici, Shanghai,China). The concentration of Y(III) in aqueous phase was determined by UVmini-1240 UV-visible spectrophotometer (Shimadzu, Japan), and the concentration of Y(III) in organic phase was calculated by mass balance. The FTIR measurements were performed with a Bruker Vertex 70 FTIR spectrometer (Bruker,Switzerland). NMR spectra were measured on an AV-400 NMR spectrometer (Bruker, Switzerland) at 25 °C. Thermal stability was detected by thermogravimetry analysis (TGA) at 10 °C·min-1from 40 °C to 600°C on a Perkin-Elmer TGA 7 system (TA instrument, USA). Elemental analysis was carried out at the VarioEL(Elementar, Germany).
2.3 Procedure
The TSIL containing carboxylic group investigated in this paper were viscous, hence, the organic phase was produced by adding TSIL containing carboxylic group into [C8mim][PF6]. [C8mim][PF6] is always used as diluent in the liquid-liquid extraction[3, 14, 15]. Extraction experiments were performed by adding 1 ml of organic phase and 4 ml of aqueous phase in equilibrium tubes. The two-phase systems were shaken at room temperature [(298±2) K] with the help of mechanical shaker for 50 min, which was sufficiently long for complete equilibration as confirmed in our preliminary experiments. In the stripping experiments, 1 ml of Y(III) loaded organic phase was taken and contacted with 4 ml HNO3solution, followed by vigorous shaking about 60 min to reach equilibrium. In the salts concentration experiments,the salts was added in the aqueous phase to be definite concentration, and then mixed with organic phase. The extraction efficiency (E), distribution ratio (D) and stripping percentage (S) were defined as follows:

whereCtandCarepresent initial and final concentrations of Y(III) in the aqueous phase;Caqis the equilibrium concentration of Y(III) in stripping acid andCorgis the initial concentration of Y(III) in organic phase.All the determinations were performed in duplicate to get the average and the relative error is below 5%.
2.4 Preparation of task-specific ionic liquids
1-Butylimidazole: The synthesis of 1-butylimidazole was according to a previous reference [16]. IR:υ(cm-1)=3108, 2960, 2934, 2874, 1465.1H NMR(400 MHz; DMSO; 25 °C ):δ(ppm)=7.451 (s, 1H),7.042 (s, 1H), 6.900 (s, 1H), 3.925 (t, 2H,J=7.2Hz),1.717-1.791 (m, 2H), 1.277-1.371 (m, 2H), 0.938 (t,3H, J = 7.2Hz).
[1-butyl-3(1-carboxymethylpropyl)im][Br]: Under an atmosphere of nitrogen, 2-bromobutanoic acid methyl ester 109 g (0.60 mol) was dropped in a mixture of 1-buthylimidazole 70 g (0.57 mol) and 65 ml ethanol. Then the system was stirred at 60 °C for 24 h,the viscous liquid obtained was washed with diethyl ether (3×100 ml) and dried in vacuum for 24 h to give 140 g product. Yield: 99%. IR:υ(cm-1)=3130, 3064,2962, 2876, 1747, 1558, 1462, 1171.1H NMR: ( 400 MHz; DMSO; 25 °C ) :δ(ppm)=9.409 (s, 1H), 7.910(s, 2H), 5.377-5.414 (m, 1H), 4.237 (t, 2H,J=7.2 Hz),3.743 (S, 3H), 2.137-2.221 (m, 2H), 1.781-1.836 (m,2H), 1.198-1.272 (m, 2H), 0.901 (t, 3H,J=7.6 Hz),0.828 (t, 3H,J=3.6 Hz). Elemental analysis calad (%)for C12H21BrN2O2: N 9.210, C 47.356, H 6.960.Found: N 8.987, C 47.385, H 7.174. Decomposition temperature 205 °C.

Figure 1 Synthetic routes to the task-specific ionic liquids
[1-butyl-3(1-carboxylpropyl)im][Br]: In 500 ml round-bottomed flask, 70 ml (0.48 mol) HBr was dropped in 120 g [1-butyl-3(1-carboxymethylpropyl)im][Br] (0.40 mol). The mixture was stirred for 4 h at 82 °C, then the water and remaining HBr was removed by the vacuum distillation. Yield: 113 g, 99%.IR:υ(cm-1)=3131, 3075, 2963, 2936, 2876, 1739,1558, 1463, 1168.1H NMR: (400 MHz; DMSO; 25°C):δ(ppm)=9.369 (s, 1H), 7.895 (S, 1H), 7.879 (S,1H), 5.223-5.260 (m, 1H), 4.227 (t, 2H,J=7.2 Hz),2.127-2.225 (m, 2H), 1.761-1.817 (m, 2H),1.216-1.272 (m, 2H), 0.901 (t, 3H,J=7.6 Hz), 0.828(t, 3H,J=7.6Hz). Elemental analysis calad (%) for C11H19BrN2O2: N 9.655, C 45.507, H 6.602. Found: N 8.479, C 42.330, H 6.538. Decomposition temperature 215 °C.
[1-butyl-3(1-carboxylpropyl)im][PF6]: 100 g [1-butyl-3(1-carboxylpropyl)im][Br] (0.34mol) was dissolved in distilled water, 50 ml 60% HPF6was drop in under ice bath, then the system was stirred 2 h under ice bath. A hydrophobic phase was obtained, which was washed with deioniced water more than ten times and dried in vacuum for 24 h to give 20 g production.Yield: 76%. IR:υ(cm-1)=3154, 3110, 2967, 2940,2879, 1737, 1558, 1465, 1169, 844.1H NMR (400 MHz; CDCl3; 25 °C):δ(ppm)=8.91 (s, 1H), 7.52 (d,1H,J=1.6 Hz), 7. 46 (d, 1H,J=1.6 Hz), 5.05-5.09(m, 1H), 4.18-4.22 (t, 2H,J=7.2 Hz), 2.08-2.32 (m,2H), 1.86-1.93 (m, 2H), 1.25-1.26 (m, 2H), 0.96-1.01(t, 6H,J=7.6 Hz).13C NMR (400 MHz; DMSO; 25°C):δ(ppm)=169.9, 136.6, 122.5, 122.1, 63.4, 48.8,31.3, 24.9, 18.8, 13.2, 10.2. Elemental analysis calad(%) for C11H19PF6N2O2: N 7.864, C 37.067, H 5.377.Found: N 7.704, C 37.386, H 5.456. Decomposition temperature 260 °C.
[1-hexyl-3(1-carboxylpropyl)im][PF6]: The same procedure was used as for [1-butyl-3(1-carboxylpropyl)im][PF6]. Yield: 68%. IR:υ(cm-1)=3164, 3116,2960, 2936, 2864, 1740, 1559, 1465, 1168, 842.1H NMR: (400 MHz; CDCl3; 25 °C) :δ( ppm )=8.788 (s,1H), 7.534 (S, 2H), 5.014-5.024 (m, 1H), 4.168-4.202(t, 2H,J=7.2 Hz), 2.049-2.301 (m, 2H), 1.878 (m,2H), 1.255-1.322 (m, 6H), 0.855-0.920 (m, 6H).13C NMR (400 MHz; DMSO; 25 °C):δ(ppm)=169.64,136.34, 122.53, 122.95, 63.61, 48.97, 30.38, 29.11,25.00, 24.84, 21.82, 13.72, 10.19. Elemental analysis calad (%) for C13H23PF6N2O2: N 7.289, C 40.630, H 6.032. Found: N 8.365, C 41.51, H 6.658. Decomposition temperature 270 °C.
[1-octyl-3(1-carboxylpropyl)im][PF6]: The same procedure was used as for [1-butyl-3(1-carboxylpropyl)im][PF6]. Yield: 85%. IR:υ(cm-1)=3162, 3114,2957, 2930, 2859, 1739, 1559, 1466, 841.1H NMR:(400 MHz; CDCl3; 25 °C) :δ(ppm)=8.77 (s, 1H),7.54 (S, 2H), 4.97-5.01 (m, 2H), 4.16-4.20 (t, 3H,J=7.2 Hz), 2.27-2.30 (m, 2H), 1.03-2.06 (m, 2H),1.26-1.32 (m, 10H), 0.85-0.92 (m, 6H).13C NMR(400 MHz; DMSO; 25 °C):δ(ppm)=169.50, 136.28,122.53, 121.86, 63.89, 48.93, 31.06, 29.17, 28.42,28.17, 25.36, 24.97, 21.99, 13.89, 10.23. Elemental analysis calad (%) for C15H27PF6N2O2: N 6.793, C 43.69, H 6.548. Found: N 7.304, C 43.56, H 7.046.Decomposition temperature 273 °C.
3 RESULTS AND DISCUSSION
3.1 Effects of 1-alkyl group of TSIL on Y(III) extraction efficiency
The comparison of Y(III) extraction with three[1-alkyl-3-(1-carboxylpropyl)im][PF6] was shown in Table 1. The data showed that the extraction efficiency for Y(III) increased with the length of the alkyl carbon chain under the same conditions. It also indicated that the extraction ability of [1-alkyl-3-(1-carboxylpropyl)im][PF6] could be improve by saponification obviously. However, the extraction system would be emulsification when [1-octyl-3-(1-carboxylpropyl)im][PF6] was used as extractant. So the [1-hexyl-3-(1-carboxylpropyl)im][PF6] was chosen as extractant for the extraction mechanism investigation.

Table 1 The effect of the length of alkyl on the extraction([TSIL]=0.2 mol·L-1, [Y(NO3)3]=7.2×10-4 mol·L-1)
3.2 The effect of the saponification degree on Y(III)extraction
Table 2 showed the results of the effect of the saponification degree on the Y(III) extraction while[1-hexyl-3-(1-carboxylpropyl)im][PF6] was used as extractant. The data showed that the extraction efficiency increased with saponification degree increasing,this trend was accord with the carboxylic extractant such as sec-octylphenoxy acetic acid, naphthenic acid.
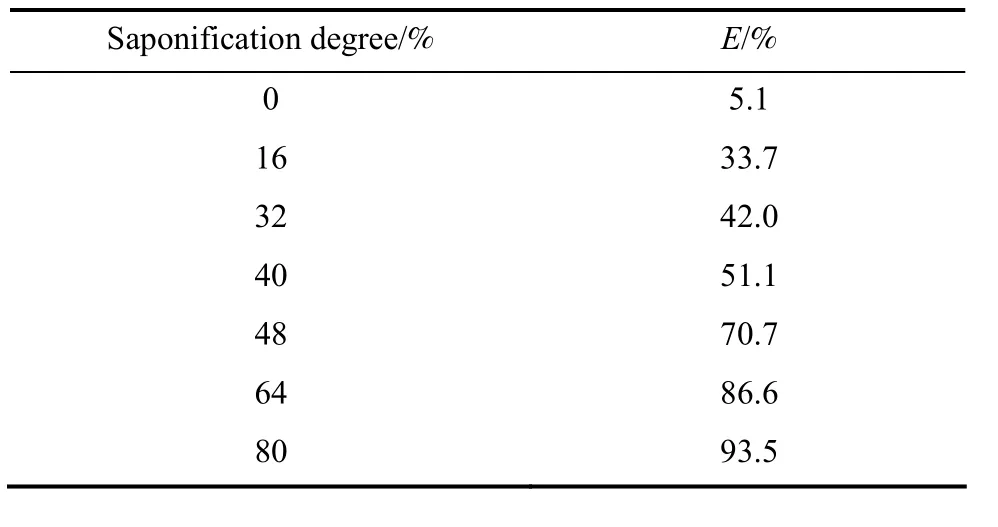
Table 2 The effect of saponification- degree of [1-hexyl-3(1-carboxyl propyl)im][PF6] in [C8mim][PF6] ([Y(NO3)3]=7.2×10-4 mol·L-1, [1-hexyl-3(1-carboxylpropyl)im][[PF6]=0.2 mol·L-1])
It has been reported that the carboxylic extractant would form dimer by strong hydrogen bonds O H- - -X interactions, and the saponification could destroy the dimer as reaction [17].
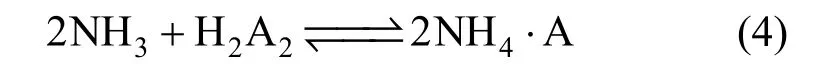
The TSIL investigated in this paper was a carboxylic extractant, too. So, the added NH3water would also destroy the dimer of TSIL in the organic phase.
3.3 The effect of aqueous phase acidity on Y(III)extraction
Figure 2 showed the effect of initial aqueous pH on the Y(III) extraction. The increase of lgDwith aqueous phase pH indicated that the coordinating and complexing abilities of [1-hexyl-3-(1-carboxylpropyl)im][PF6] with Y(III) are dependent on aqueous phase pH,just like the conventional carboxylic extractant [10, 18].At low pH values, the saponification of [1-hexyl-3-(1-carboxylpropyl)im][PF6] would be weakened,leading to lower extraction efficiency for Y(III). From Fig. 2, we could also see that lgDincreased slowly when the initial aqueous pH was higher than 2.5. And,in our works, the equilibrium pH of aqueous phase would be higher than 5.0 when the initial aqueous pH was higher than 2.5, which would result in the Y(III)hydrolysis. So, the effect of equilibrium pH of aqueous phase was discussed when the initial aqueous pH was lower than 2.5, and the results were shown in Fig. 3.The slope of LgDversusequilibrium pH was about 3 indicated that three moles of H+were released when one molar of Y(III) was extracted into the organic phase.

Figure 2 The effect of initial aqueous pH on the Y(III)extraction ([Y(NO3)3]=7.2×10-4 mol·L-1, [1-hexyl-3(1-carboxylpropyl)im][[PF6]=0.2 mol·L-1)
3.4 The effect of [1-hexyl-3-(1-carboxyl propyl)im][PF6] concentration on Y(III) extraction

Figure 3 The effect of equilibrium pH of aqueous phase on the Y(III) extraction ([Y(NO3)3]=7.2×10-4 mol·L-1,[1-hexyl-3(1-carboxylpropyl)im][[PF6]=0.2 mol·L-1)
The results of the effect of [1-hexyl-3-(1-carboxylpropyl)im][PF6] concentration were shown in the Fig. 4. We could see that the Y(III) extraction efficiency increased with the [1-hexyl-3-(1-carboxylpropyl)im][PF6] concentration, and there was a linear correlation between lgC0and lgD. The slope of lgC0versuslgD-3pH was about 3 indicated that the stoichiometry of [1-hexyl-3-(1-carboxyl propyl)im][PF6] to Y(III) in the organic phase was 3.
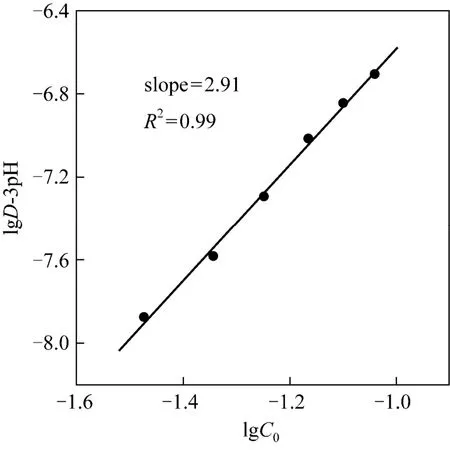
Figure 4 The effect of [1-hexyl-3(1-carboxylpropyl)im][[PF6] concentration on Y(III) extraction ([Y(NO3)3]=7.2×10-4 mol·L-1, the initial pH=2.01)
3.5 The effect of the salts concentration on Y(III)extraction
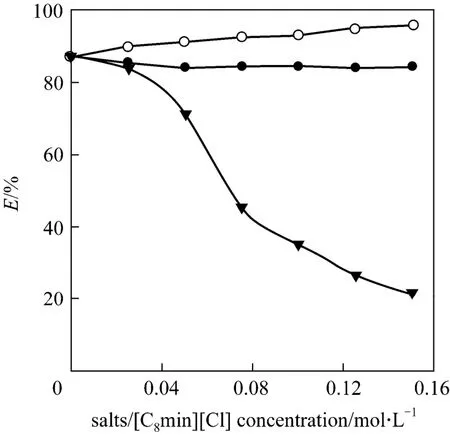
Figure 5 The effect of salts concentration ([1-hexyl-3(1-carboxylpropyl)im][[PF6]=0.2 mol·L-1, [Y(NO3)3]=7.2×10-4 mol·L-1, the initial pH=2.01)● NaNO3; ○ KPF6; ▼ [C8mim][Cl]
The effect of salts concentration were experimented to investigate the effect of different anion and cation on the extraction, the results were shown in Fig. 5. It showed that NaNO3had little effect on the Y(III) extraction while KPF6had positive effect on the extraction. It has been reported that [C8mim]+would participate in the extraction process, and the added[C8mim]+had the negative effect on the extraction [14,15]. So the effect of the concentration of [C8mim][Cl]on the extraction was also investigated in our work.The results showed that the Y(III) extraction efficiency decreased with the [C8mim][Cl] concentration obviously just in previous reports [17,18]. The reason that KPF6could improve the extraction efficiency for Y(III) might be the [PF6]-ions combining with[C8mim]+in the extraction system, which could weaken the effect of [C8mim]+.
3.6 The extraction mechanism
It has been reported that the TSIL bearing 2-hydroxybenzylamine groups could be used as extractant for the Am extraction, and the extraction reaction was given as Eq. (5) [3]:
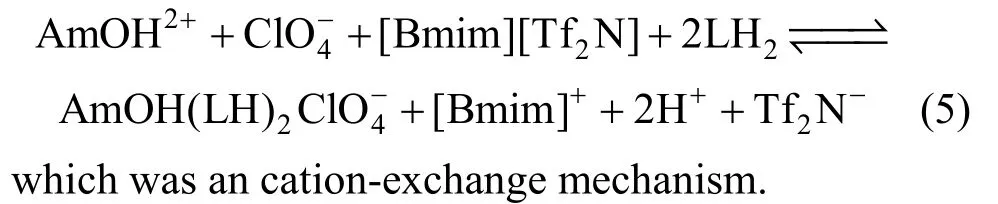
In this paper, the extraction mechanism of a acidic TSIL as extractant in [C8mim][PF6] was investigated. The results of equilibrium aqueous pH experiments, extractant concentration and salt effect experiments indicated that the extraction mechanism was also a cation-exchange mechanism, and the extraction reaction could be summarized as Eq. (6).
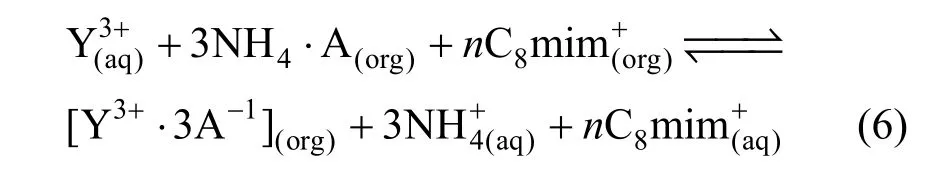
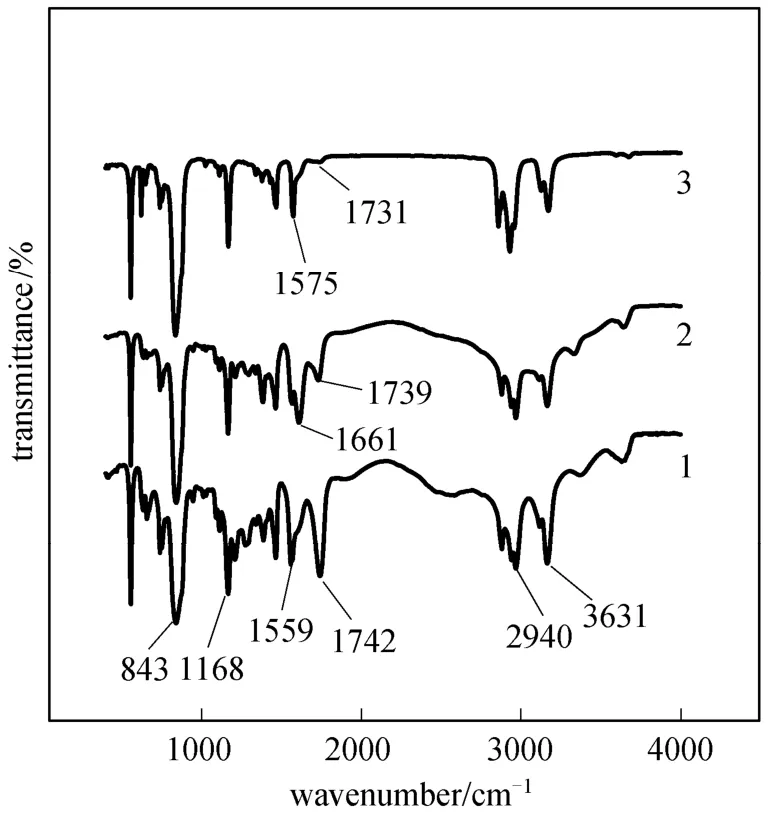
Figure 6 IR transmittance spectra of different systems1—[1-hexyl-3-(1-carboxylpropyl)im][PF6]; 2—[1-hexyl-3-(1-carboxylpropyl)im] [PF6] after saponification; 3—[1-hexyl-3-(1-carboxylpropyl)im][PF6] loaded with Y(III))
To further elucidate the extraction mechanism,the infra-red spectra of [1-hexyl-3-(1-carboxylpropyl)im][PF6], [1-hexyl-3-(1-carboxylpropyl)im][PF6] after saponification and [1-hexyl-3-(1-carboxylpropyl)im][PF6] loaded with Y(III) are contrasted in Fig. 6. A comparison of (1) and (2) shows that the peak of C O stretching vibration at 1742 cm-1decreases obviously when the [1-hexyl-3-(1-carboxylpropyl)im][PF6] was saponified, which indicated that the saponification has effect on the C O of [1-hexyl-3-(1-carboxylpropyl)im][PF6]. And the C O stretching vibration at 1742 cm-1was almost disappeared when [1-hexyl-3-(1-carboxylpropyl)im][PF6] loaded with Y(III). These reveal a strong interaction of C O with Y(III).
3.7 The effect of the temperature on Y(III) extraction
The effect of temperature on the Y(III) extraction was studied in the rang 288 K-313 K, and the results were shown in Fig. 7. The Y(III) extraction efficiency increased as the temperature decreased, which indicated that the extraction system was more feasible at the room temperature. Then the enthalpy change of the reaction, ∆H, could be calculated by the van’t Hoff equation. The corresponding free energy ∆Gand entropy ∆Swere gotten using Eqs. (8) and (9) respectively, and their values were shown in the Table 3.

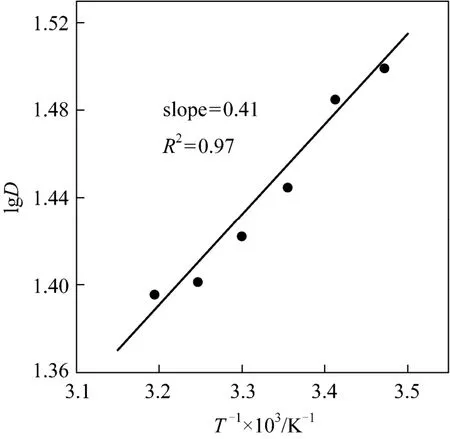
Figure 7 The effect of temperature on the Y(III) extraction([1-hexyl-3(1-carboxylpropyl)im][[PF6]=0.2 mol·L-1, [Y(NO3)3]=7.2×10-4 mol·L-1, the initial pH=2.01)
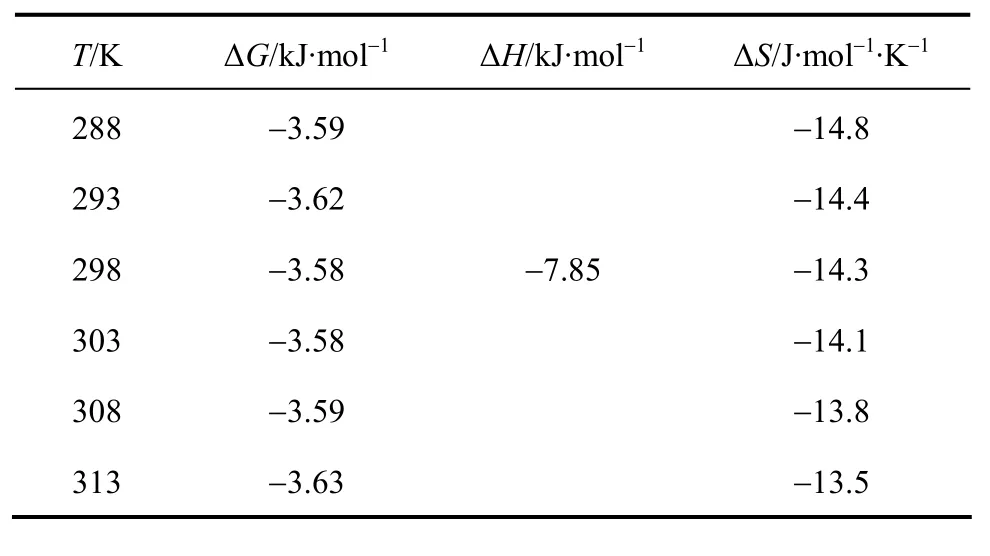
Table 3 Thermodynamic parameters of Y(III) extraction([1-hexyl-3(1-carboxylpropyl)im][[PF6]=0.2 mol·L-1, [Y(NO3)3]=7.2×10-4 mol·L-1, the initial pH=2.01)
WhereRis the universal gas constant,Cis an integral constant for the system. For estimating ∆G, the values ofDare used in place ofKas a common practice[15, 19]. In the liquid-liquid extraction, and in our work,the extractant concentration is much larger than Y(III)concentration. Hence,KandDfollow the same trend of variation, making this substitution reasonable.Table 3 lists the thermodynamic parameters, and it is clear that the extraction is an exothermic process.
3.8 Stripping experiments
Figure 8 showed the effect of HNO3concentration on the stripping ratio of Y(III) in [1-hexyl-3-(1-carboxylpropyl)im][PF6] system. As shown, the stripping ratio increased with the increasing of the aqueous phase acidity till Y(III) was almost wholly stripped from the loaded organic phase. It also showed that more than 95% of Y(III) could be stripped when the concentration of HNO3is higher than 0.07 mol·L-1,and the acid consumption for stripping was lower than both di-2-ethylhexylphosphoric acid (P204) and 2-ethylhexylphosphoric acid mono-2-ethylhexyl ester(P507) extraction system [20, 21]. This indicated that[1-hexyl-3-(1-carboxylpropyl)im][PF6] system investigated in our work had well stripping characters.

Figure 8 The stripping of Y(III) with HNO3 from the loaded organic phase ([Y(NO3)3](o)=2.5×10-3 mol·L-1, [1-hexyl-3(1-carboxylpropyl)im][[PF6]=0.2 mol·L-1])
4 CONCLUSIONS
A new kind of TSIL bearing carboxylic acidic groups were synthesized, and their extraction properties for Y(III) were investigated in this paper. The represent work shows that this kind of TSIL needs to be saponified before being used for the Y(III) extraction just like the conventional carboxylic acid extractants. The extraction is acid dependent in the [1-hexyl-3-(1-carboxylpropyl)im][PF6] system, and the extraction efficiency increases as the aqueous phase acidity decrease. The loaded organic phase is easy to be stripped, more than 95% Y(III) could be stripped from the loaded organic phase when the stripping acid is higher than 0.07 mol·L-1. A possible extraction mechanism is proposed, in which the extraction system is driven by a cation-exchange mechanism.
1 Huddleston, J.G., Willauer, H.D., Swatloski, R.P., Visser, A.E.,Rogers, R.D., “Room temperature ionic liquids as novel media for‘clean’ liquid-liquid extraction”,Chem.Commun., 16, 1765-1766 (1998).
2 Visser, A.E., Swatloski, R.P., Reichert, W.M., Davis Jr, J.H., Rogers,R.D., Mayton, R., Sheff, S., Wierzbicki, A., “Task-specific ionic liquids for the extraction of metal ions from aqueous solutions ”,Chem.Commun., 1, 135-136 (2001).
3 Ouadi, A., Gadenne, B., Hesemann, P., Moreau, J.J.E., Billard, I.,Gaillard, C., Mekki, S., Moutiers, G., “Task-specific ionic liquids bearing 2-hydroxybenzylamine units: Synthesis and americium-extraction studies”,Chem.Eur.J., 12 (11), 3074-3081 (2006).
4 Han, X., Armstrong, D.W., “Ionic liquids in separations”,Acc.Chem.Res., 40 (11), 1079-1086 (2007).
5 Sun, X., Ji, Y., Hu, F., He, B., Chen, J., Li, D., “The inner synergistic effect of bifunctional ionic liquid extractant for solvent extraction”,Talanta, 81 (4-5), 1877-1883 (2010).
6 Davis, J. J., “Task-specific ionic liquids”,Chem.Lett., 33 (9),1072-1077 (2004).
7 Huddleston, J.G., Visser, A.E., Reichert, W.M., Willauer, H.D., Broker, G.A., Rogers, R.D., “Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation”,GreenChem., 3 (4), 156-164 (2001).
8 Luo, H.M., Dai, S., Bonnesen, P.V., “Solvent extraction of Sr2+and Cs+based on room-temperature ionic liquids containing monoazasubstituted crown ethers”, Anal. Chem., 76 (10), 2773-2779 (2004).
9 Ouadi, A., Klimchuk, O., Gaillard, C., Billard, I., “Solvent extraction of U(vi) by task specific ionic liquids bearing phosphoryl groups”,Green Chem., 9 (11), 1160-1162 (2007).
10 Li, W., Wang, X., Meng, S., Li, D., Xiong, Y., “Extraction and separation of yttrium from the rare earths with sec-octylphenoxy acetic acid in chloride media”, Sep. Purif. Technol., 54 (2), 164-169 (2007).
11 Zuo, Y., Chen, J., Li, D., “Reversed micellar solubilization extraction and separation of thorium(IV) from rare earth(III) by primary amine N1923 in ionic liquid”, Sep. Purif. Technol., 63 (3), 684-690 (2008).
12 Liu, Y.H., Zhu, L.L., Sun, X.Q., Chen, J., “Toward greener separations of rare earths: Bifunctional ionic liquid extractants in biodiesel”, AlChE J., 56 (9), 2338-2346 (2010).
13 Sun, X., Ji, Y., Zhang, L., Chen, J., Li, D., “Separation of cobalt and nickel using inner synergistic extraction from bifunctional ionic liquid extractant (Bif-ILE)”, J. Hazard. Mater., 182 (1-3), 447-452(2010).
14 Dietz, M.L., Dzielawa, J.A., “Ion-exchange as a mode of cation transfer into room-temperature ionic liquids containing crown ethers:implications for the ‘greenness’ of ionic liquids as diluents in liquid-liquid extraction”, Chem. Commun., 20, 2124-2125 (2001).
15 Sun, X., Wu, D., Chen, J., Li, D., “Separation of scandium(III) from lanthanides(III) with room temperature ionic liquid based extraction containing Cyanex 925”, J. Chem. Technol. Biotechnol, 82 (3),267-272 (2007).
16 Bonhote, P., Dias, A.P., Papageorgiou, N., Kalyanasundaram, K.,Gratzel, M., “Hydrophobic, highly conductive ambient-temperature molten salts”, Inorg. Chem., 35 (5), 1168-1178 (1996).
17 Xu, G.X., Yuan, C.Y., Solvent Extraction of Rare Earth, Science Press, Beijing, 153-153 (1987). (in Chinese)
18 Zhang, X., Yin, G., Hu, Z., “Extraction and separation of gallium,indium and thallium with several carboxylic acids from chloride media”, Talanta, 59 (5), 905-912 (2003).
19 Yang, H.L., Wang, W., Zhang, D.L., Deng, Y.F., Cui, H.M., Chen, J.,Li, D.Q., “Recovery of trace rare earths from high-level Fe3+and Al3+waste of oil shale ash (Fe-Al-OSA) ”, Ind. Eng. Chem. Res., 49,11645-11651 (2010).
20 Wu, D.B., Zhang, Q., Bao, B.R., “Solvent extraction of Pr and Nd(III) from chloride-acetate mediumby 8-hydroquinoline with and without 2-ethylhexyl phosphoric acidmono-2- ethylhexyl ester as an added synergist in heptane diluent”, Hydrometallurgy, 88, 210-215(2007).
21 Moraisa, C.A., Ciminelli, V.S.T., “Process development for the recovery of high-grade lanthanum by solvent extraction”, Hydrometallurgy, 73, 237-244 (2004).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Retrospect and Perspective of Micro-mixing Studies in Stirred Tanks*
- In-situ Synthesis and Catalytic Properties of ZSM-5/Rectorite Composites as Propylene Boosting Additive in FluidCatalytic Cracking Process*
- Low-temperature Electrodeposition of Aluminium from Lewis Acidic 1-Allyl-3-methylimidazolium Chloroaluminate Ionic Liquids*
- An Experimental Study of Liquid-Liquid Microflow Pattern Maps Accompanied with Mass Transfer*
- Liquid-Liquid-Liquid Three Phase Extraction Apparatus: Operation Strategy and Influences on Mass Transfer Efficiency*
- Micron-sized Magnetic Polymer Microspheres for Adsorption and Separation of Cr(VI) from Aqueous Solution*
