In-situ Synthesis and Catalytic Properties of ZSM-5/Rectorite Composites as Propylene Boosting Additive in FluidCatalytic Cracking Process*
2012-03-22LIUHaiyan刘海燕CAOLiyuan曹丽媛WEIBaoying魏宝莹FANYu范煜SHIGang石冈andBAOXaojun鲍晓军
LIU Haiyan (刘海燕), CAO Liyuan (曹丽媛), WEI Baoying (魏宝莹), FAN Yu (范煜), SHI Gang (石冈) and BAO Xaojun (鲍晓军)**
State Key Laboratory of Heavy Oil Processing, China University of Petroleum, Beijing 102249, China
1 INTRODUCTION
Worldwide demand for propylene is growing faster than the anticipated supply from ethylene plants,and among the various routes to fill the propylene supply-demand gap, fluid catalytic cracking (FCC)units are considered as the largest source of propylene[1]. Currently, more than 30% of the worldwide propylene production is from FCC units and this figure will continue to increase [2]. For boosting propylene yield of FCC units, zeolite ZSM-5 has been widely employed as an additive in industrial practice since its first commercial use in 1983 [3-9]. Because of its unique pore structure, zeolite ZSM-5 can crack selectively gasoline-range linear or mono-branched hydrocarbons to C3-C4olefins, and its moderate acidity can largely suppress hydrogen-transfer reactions [4-6].Generally, propylene boosting FCC catalyst additives that contain ZSM-5 zeolite, as well as about 80% FCC main catalysts over the world, are mechanical mixtures made via the so-called semi-synthesis route that combines a pre-synthesized ZSM-5 or zeolite Y and a matrix (usually kaolin) using a binder (alumina or/and silica gel). On the other hand, about 20% FCC catalysts are prepared via the so-called in-situ synthesis(or in-situ crystallization) technique from kaolin clay,which are usually referred as in-situ synthesized catalysts [10-14]. Due to their improved accessibility of active sites to bulky heavy crude oil molecules, enhanced resistance to attrition, high tolerance to contaminant metals, and excellent stability to steam at high temperature, in-situ synthesized catalysts have been widely used in FCC units, especially in resid FCC units that take at least a portion of vacuum residue as feedstock [15]. However, at present all of ZSM-5 containing propylene boosting FCC catalyst additives are semi-synthesized ones and thus have much lower density than in-situ synthesized main catalysts used in resid FCC units. This leads to the much shorter residence time of ZSM-5 containing propylene boosting FCC additives compared to that of the main FCC catalysts, and therefore gives rise to the suppression of the secondary cracking reactions of5C+hydrocarbons in gasoline-range to C3-C4olefins and the enhancement of hydrogen transfer reactions, both of which are unfavorable for boosting propylene yields [7]. Intuitively, it is conceived that the best approach to overcome the problem is to fabricate ZSM-5 containing propylene boosting additives via the same synthesis route as used for preparing zeolite Y/kaolin based main FCC catalysts, i.e., via the in-situ synthesis technique from natural clay like kaolin.
Using kaolin clay as a starting material, the in-situ synthesis technique for fabricating FCC catalysts involves two steps [13]: (1) dehydroxylation of kaolin at 550-900 °C to form an X-ray amorphous material metakaolin that provides part or all of active SiO2and Al2O3species for zeolite synthesis (here the active SiO2and Al2O3are referred to those leachable from the metakaolin framework by alkali or acidic solutions);(2) using active SiO2and Al2O3species in metakaolin as well as a suitable amount of make-up silica when necessary, zeolite/kaolin composites are hydrothermally synthesized with the zeolite being overgrown on kaolin microspheres. Actually, ZSM-5/clay composites have also been synthesized via the in-situ crystallization method from kaolinite minerals [16-18]. However,with the fast expansion of FCC capacities over the world especially in Asian countries and thereby the increasing consumption of FCC catalysts, the production of zeolite/kaolin composites is suffering from the shortage in the supply of high-quality kaolin, the starting material forin-situsynthesis. Therefore, exploring new natural minerals that provide comparable or even better properties than kaolin for producingin-situcatalysts has been a focus of FCC catalyst research and development.
In the previous work [19], we have explored the possibility of using a natural aluminosilicate mineral,rectorite, as raw material for thein-situsynthesis of FCC catalysts/additives. The results showed that the rectorite mineral has suitable chemical composition,outstanding stability, and high chemical reactivity after thermal activation, and thus could be taken as an alternative of kaolin for thein-situsynthesis of FCC catalysts/additives. Most importantly, the synthesized ZSM-5/rectorite composite (As-Rec-800) has much better hydrothermal stability than the ZSM-5/kaolin composite (As-Kao-800) synthesizedviathe same method. In this article, we show that compared with a commercial ZSM-5 zeolite synthesized from chemical reagents and As-Rec-800 synthesized in Ref. [19], the ZSM-5/rectorite composite prepared from rectoriteviathein-situsynthesis method in this article has superior propylene boosting performance because of its unique pore structure and thus its great expectation as propylene boosting FCC catalyst additive is demonstrated.
2 EXPERIMENTAL
2.1 Materials
The natural rectorite mineral [containing >70%(by mass) rectorite] used in the present investigation was purchased from Hubei Celebrities Rectorite Company (Hubei Province, China) and its chemical composition in terms of oxides is given in Table 1. Sodium silicate [containing 26.3% (by mass) SiO2, used as a supplementary silica source for synthesizing ZSM-5/rectorite composites] was purchased from Beijing Aviation Material Institute (China). Silica gel [containing 25.0% (by mass) SiO2, used as the binder for fabricating the FCC catalysts] was purchased from Beijing Chemical Company (China). Reagent-grade NaOH,H2SO4and tetrapropylammonium bromide (TPABr)that was used as the organic template were purchased from the market and used without further purification.
A reference ZSM-5 zeolite sample with a molar SiO2/Al2O3ratio of 34 and an ultra-stabilized Y (USY)zeolite sample with a molar SiO2/Al2O3ratio of 10.0 used as the primary active component in FCC catalysts for cracking gas oil were purchased from Nankai University, Tianjin, China. Kaolin clay [containing 95% (by mass) kaolinite, used as the matrix for preparing FCC catalysts] was kindly provided by Lanzhou PetroChemical Co., PetroChina Co. (China).
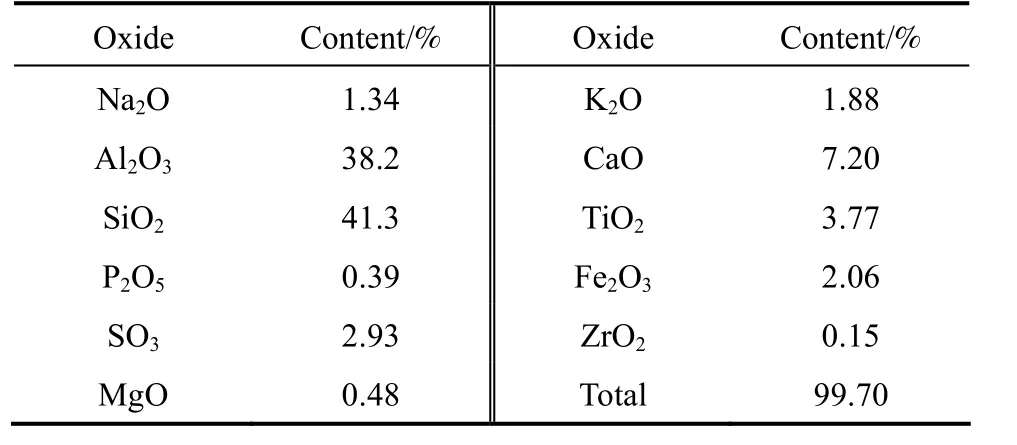
Table 1 Chemical composition of the rectorite mineral
2.2 Synthesis
Rectorite powders with their particle size ≤2 μm calcined at 900 °C for 1.5 h were mixed with suitable amounts of deionized water and the binder Al2O3, and extruded into sticks of 1.5 mm in diameter. After dried at 150 °C, the sticks were crushed and sieved to obtain rectorite particles of 80-110 μm in size, and put into a solution of 5 mol·L-1alkali hydroxide for thein-situsynthesis of ZSM-5/rectorite composites. After aged for 4 h, the pH value of the reaction mixture was adjusted to 8-11 by adding H2SO4. The synthesis was conducted in a Teflon-lined stainless steel autoclave at 170 °C for 48 h using TPABr as the template and sodium silicate as the supplementary silica source. In the synthesis system, the mass ratio of rectorite to SiO2(supplementary silica) was 1.43 and the molar ratios of (Na2O + K2O) to SiO2, H2O to SiO2, and TPABr to SiO2were 0.3, 100, and 0.09, respectively. After the autoclave was cooled down to room temperature, the resulting products were rinsed with deionized water and calcined at 550 °C to remove the template.
2.3 Characterization
The phase structure of the samples was characterized by X-ray diffraction (XRD) conducted on a Shimadu 6000 diffractometer (Japan). The instrument used CuKαradiation and was operated at 40 kV,30 mA, 2θranging from 5° to 50°. The morphology and size of the samples were identified by scanning electronic microscopy (SEM) conducted on a LEO-435VP instrument combined with energy dispersive spectrometer (EDS) (England). The specific surface area and pore structure of the samples were acquired on a Micromeritics ASAP 2405N instrument(USA) using N2adsorption-desorption at -196 °C.The chemical composition of the samples is determined by X-ray fluorescence (XRF) using a ZSX-100e4580 spectrometry (Japan).
Fourier transformed infrared spectroscopy of pyridine adsorption (Py-FTIR) spectra were obtained on a Magna-IR 560 E.S.P. FTIR spectrometer (USA).During the measurement, each sample (ca. 15 mg) was first pressed into a self-supported wafer and then evacuatedin-situin an IR cell at 350 °C for 2 h; after the temperature was decreased to room temperature,the IR spectra were recorded; pyridine was introduced into the cell to saturate the sample surface for 4 h, and finally the desorption of pyridine was performed by stepwise increasing the temperature from ambient to 200 °C and then to 350 °C at 10-3Pa and the spectra were recorded correspondingly. The peaks at 1540 and 1450 cm-1on the adsorption curves are related to the adsorption of pyridine molecules on Brönsted (B) acid sites and Lewis (L) acid sites, respectively.
2.4 Catalytic performance assessment
Taking the catalytic cracking of Daqing atmospheric residue (AR) whose physicochemical properties are listed in Table 2 as a model reaction, the propylene boosting performance of the samples was evaluated in a fluidized microreactor with the size ofФ36×380 mm using steam as the fluidizing gas. Before the reaction, the H-form samples of the reference ZSM-5 and the ZSM-5/rectorite composite were obtained by ion exchange with a NH4NO3solution of 1.0 mol·L-1and calcination at 500 °C for 2 h twice. By adding each of the obtained H-form samples to a blank FCC catalyst consisting of a USY zeolite with a molar SiO2/Al2O3ratio of 10.0, the kaolin, and the binder silica gel, a series of catalysts were prepared and their compositions are listed in Table 3. All of the catalysts were made by mixing the ingredients sufficiently in slurry at the first, and then drying the mixture, and finally grinding the resulting solid products to 80-110 μm in size. Thus obtained catalysts (ca.50-60 g) were deactivated at 800 °C in 100% steam for 5 h prior to test. For comparison purpose, a blanktest was also conducted over the blank FCC catalyst.The reaction was carried out under the following conditions: 580 °C, feed injection time 20 s, catalyst to oil ratio (mass to mass) 11, water to oil ratio (mass to mass) 0.05.
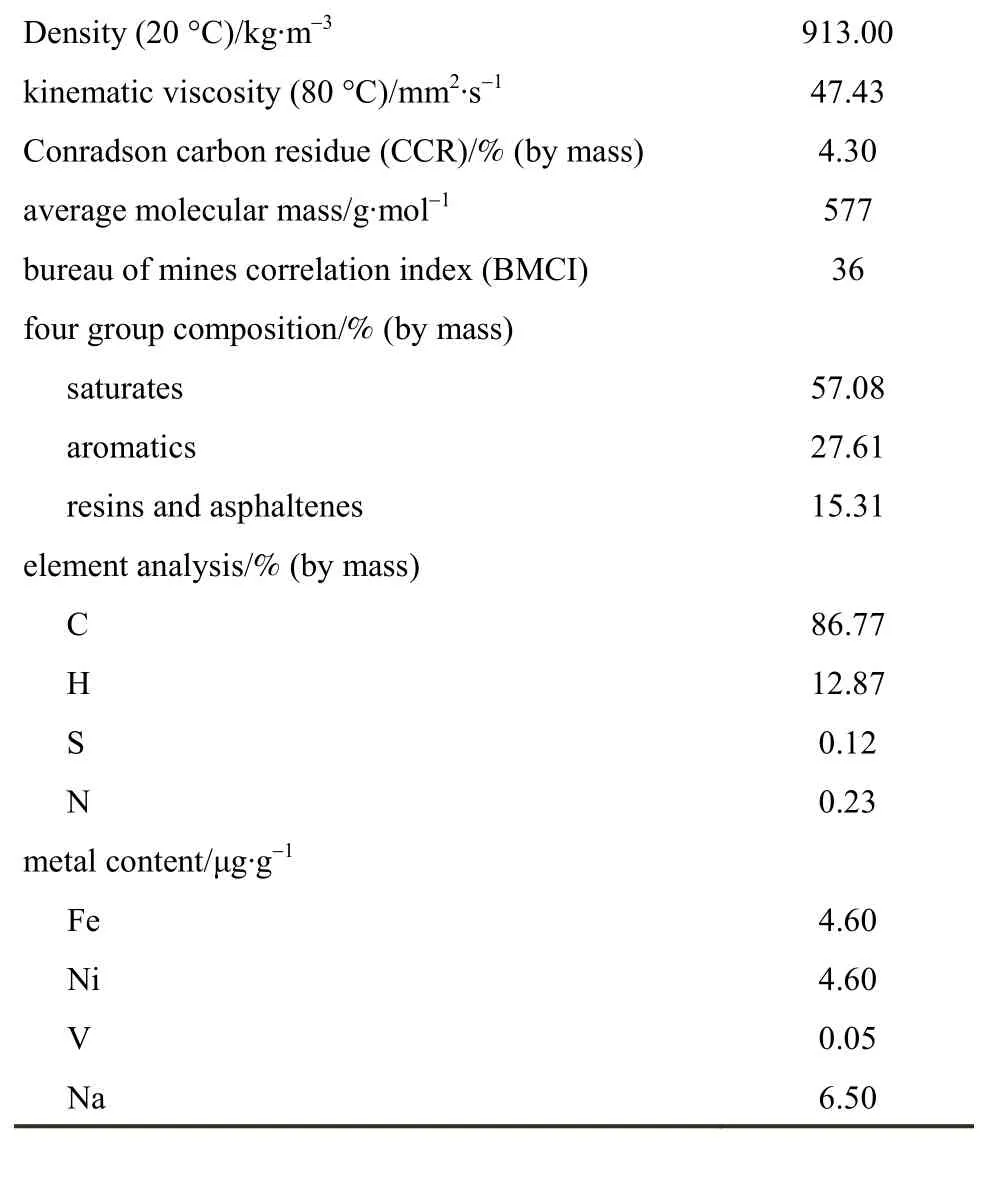
Table 2 Properties of Daqing AR

Table 3 Composition of the catalysts
The gaseous products obtained were analyzed on an Angilent 6890 gas chromatograph (GC) installed with a flame ionization detector (FID). The fractions of gasoline, diesel and heavy oil in liquid products were analyzed using the chromatographic simulated distillation method as described in the ASTM D2887 standard. Coke deposition on the catalysts was determined by combusting the catalysts after the cracking reaction and measuring the amount of CO2released using a GC installed with a thermal conductivity detector (TCD).
3 RESULTS AND DISCUSSION
3.1 Characterizations
3.1.1XRD and SEM/EDS
The XRD patterns in Fig. 1 indicate that the reference ZSM-5 sample [Fig. 1 (c)] has only the peaks ascribed to typical ZSM-5 zeolite, whereas the ZSM-5/rectorite sample synthesized from the rectorite particles shows not only the peaks ascribed to ZSM-5 zeolite, but also those of the calcined rectorite clay[Fig. 1 (a)]. From the SEM images of the samples shown in Fig. 2, it can be seen that in the composite the ZSM-5 phase overgrows on the rectorite particles in a layer of 2-3 μm in thickness, slightly larger than the size (ca. 1-2 μm) of the reference ZSM-5 crystals.
It is well known that zeolite crystal size has strong effect on its catalytic performance. Tenget al.[20] showed that ZSM-5 zeolite with smaller crystal size has much better durability in cracking C4olefins to propylene due to its higher tolerance to coke; Zhang and Li [21] also indicated that different intercrystal diffusivities in ZSM-5 zeolites of different crystal sizes will affect their cracking performance of hydrocarbon. From the above analysis we can expect that the ZSM-5/rectorite composite that has smaller ZSM-5 crystal size (ca. 2-3 μm) will exhibit higher accessibility to hydrocarbon molecules and lower selectivity to coke in FCC process.
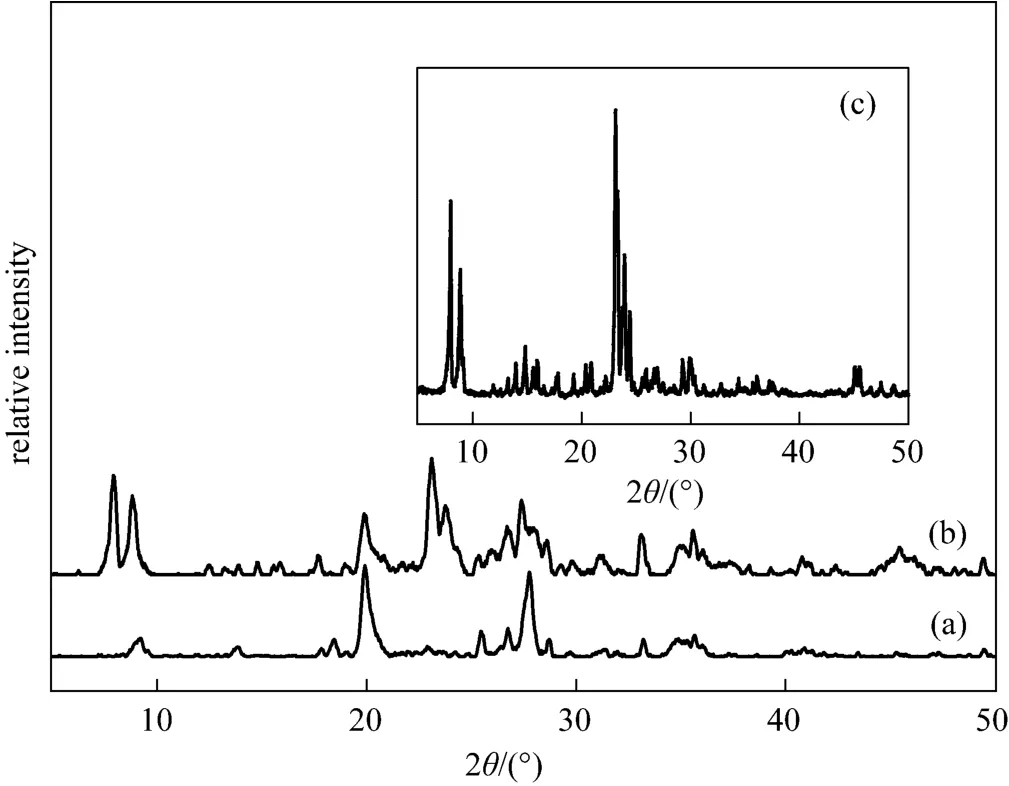
Figure 1 XRD patterns of (a) the calcined rectorite powder, (b) the ZSM-5/rectorite composite, and (c) the reference ZSM-5
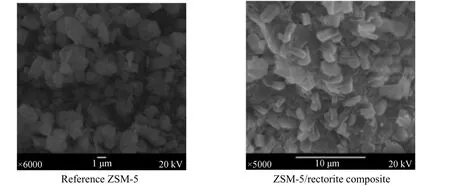
Figure 2 SEM images of the reference ZSM-5 and the ZSM-5/rectorite composite

Figure 3 Cross-sectional SEM image of the ZSM-5/rectorite composite
Figure 3 is the cross-sectional image of the ZSM-5/rectorite composite. The molar SiO2/Al2O3ratio at the positions marked by 1, 2, 3, and 4 (from the core to the surface of the composite, as shown in Fig. 3) determined by EDS are 1.99, 2.03, 28.0 30.0,respectively. This suggests that the composite has the following three-layer structure from the outer surface to the core: the densely packed ZSM-5 crystals, ZSM-5 plus rectorite below the surface, and the mother rectorite at the core, signifying that the sample synthesized from the rectorite particles is a composite consisting of both ZSM-5 phase and rectorite phase rather than a mechanical mixture of ZSM-5 and rectorite.The content of the ZSM-5 phase in the ZSM-5/rectorite composites measured by XRDviathe internal standard method [22] was 18% (by mass).
3.1.2N2 adsorption-desorption
The typical textural properties of the calcined rectorite powders, the reference ZSM-5, and the ZSM-5/rectorite composite are summarized in Table 4,and Fig. 4 gives their pore size distributions. FromTable 4, it can be seen that compared to the calcined rectorite powders, the ZSM-5/rectorite composite has a larger BET surface area and more micropores that can be ascribed to ZSM-5 crystals. From Fig. 4, it can be seen that different from the reference ZSM-5 (having a bimodal microporous-mesoporous structure) and the calcined rectorite powders (having a bimodal mesoporous-macroporous structure), the ZSM-5/rectorite composite presents a trimodal microporous-mesoporousmacroporous structure. These results are further confirmed by the N2adsorption-desorption results.
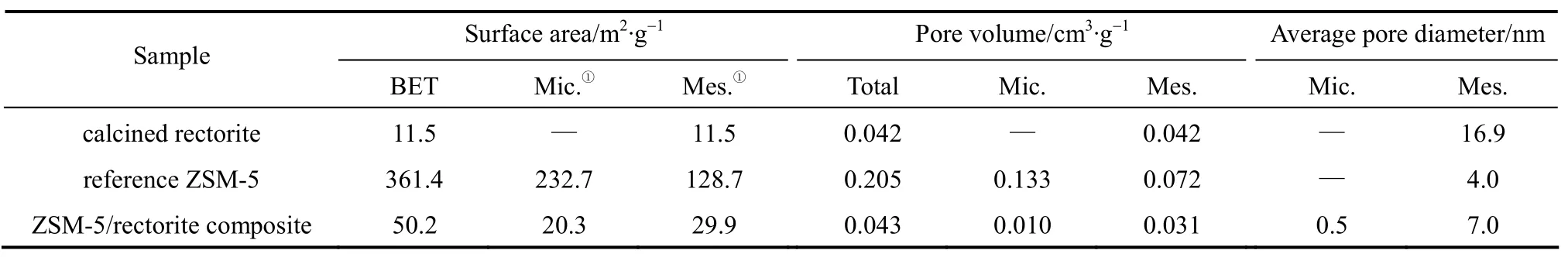
Table 4 Textural properties of the calcined rectorite powders, the reference ZSM-5, and the ZSM-5/rectorite composite
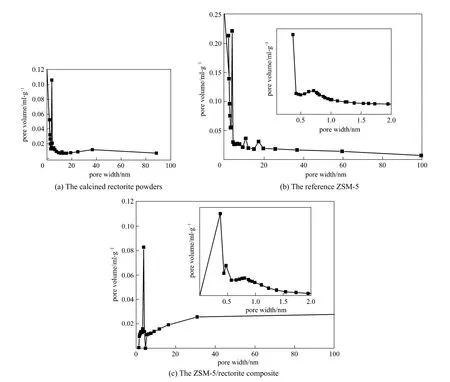
Figure 4 Pore size distributions of the calcined rectorite powders, the reference ZSM-5, and the ZSM-5/rectorite composite
Figure 5 presents the N2adsorption-desorption isotherms of the calcined rectorite powders, the reference ZSM-5, and the ZSM-5/rectorite composite. The N2adsorption-desorption isotherms of the calcined rectorite powders and the ZSM-5/rectorite composite[curves (b) and (a) in Fig. 5] have a typical hysteresis loop when the relative pressurep/p0is varied from 0.46 to 1.0, indicating that the presence of meso- and macroporous structures [17]. It is interesting to note that on the isotherm of the composite there is a sharp jump in the amount of N2adsorption when the relative pressurep/p0is at about 0 [curve (a) in Fig. 5], which is attributed to the filling of N2in micropores and thus indicates the presence of micropores ascribed to the ZSM-5 phase in the composite. These results suggest again that the ZSM-5/rectorite composite has a trimodal microporous-mesoporous-macroporous structure,wherein the micropores and mesopores are ascribed to the ZSM-5 crystals, whereas the macropores are ascribed to the mother rectorite. Most importantly, the size of micropores [Fig. 4 (c)] in the composite is centralized at about 0.4-0.5 nm, which will benefit the shape-selectivity for cracking linear olefins in the gasoline range to boost the propylene production in the FCC process [23-25].
Compared to those of the calcined rectorite powders and the ZSM-5/rectorite composite, the N2adsorption-desorption isotherm of the reference ZSM-5 shown by curve (c) in Fig. 5 is much similar to type I isotherm as classified by IUPAC and shows only a tiny hysteresis loop, indicating that the reference ZSM-5 has a relatively concentrated pore size distribution [22]. From Fig. 4 (b), we also note that in the commercial reference ZSM-5, supermicropores (0.7-2 nm) and smaller mesopores (2-3.5 nm) are dominant.
3.1.3Pyridine-FTIR
Table 5 shows the acidity properties of the samples measured by the Pyridine-FTIR technique. Compared to those of the reference ZSM-5 sample synthesized from the chemical reagents, the total amounts of B and L acid sites of the ZSM-5/rectorite composite are much lower. Furthermore, the ZSM-5/rectorite composite contains more L acid sites, with its L/B ratio for both total acid and medium and strong acid at about 1.11. It is widely accepted that the acid site density and B acid site amount of a catalyst can significantly influence its catalytic activity and selectivity in hydrocarbon cracking reactions [26, 27]. Low density of strong acid sites in ZSM-5 zeolite results in high cracking activity by boosting propylene and ethylene production while suppressing hydrogen transfer reactions which likely lead to the formation of paraffins,cyclo-olefins, aromatics, and coke-like polyaromatic molecules. Compared to L acid sites, strong B acid sites can provide protons to carbon-carbon double bonds of olefins, resulting in the easier formation of carbenium ions and the consequent hydrocarbon cracking.
From Table 5, it can be seen that the density of total medium and strong acid sites of the ZSM-5/rectorite composite is only 0.02 mmol·g-1, much smaller than that (0.16 mmol·g-1) of the reference ZSM-5. However, the unique trimodal microporousmesoporous-macroporous structure of the composite may endow it with the eminent catalytic performance and propylene selectivity, which will be further discussed in Section 3.2.
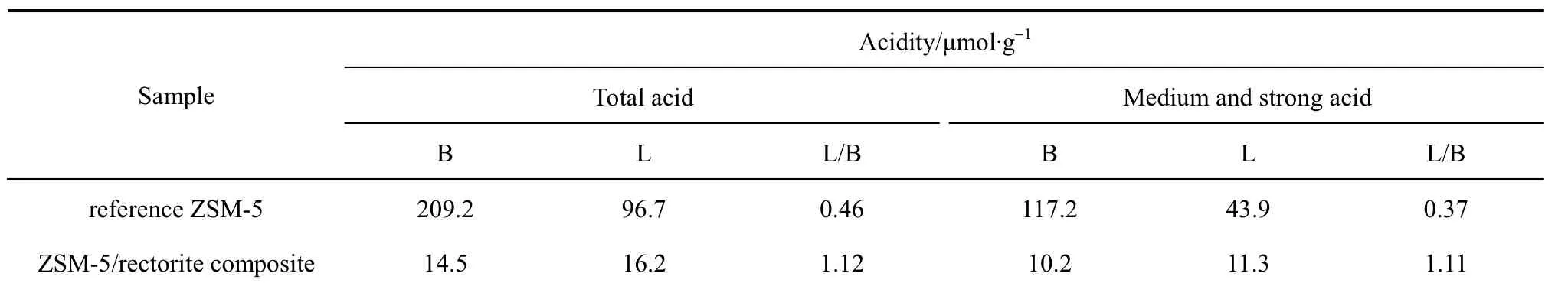
Table 5 Acidity properties of the reference ZSM-5 and the ZSM-5/rectorite composite
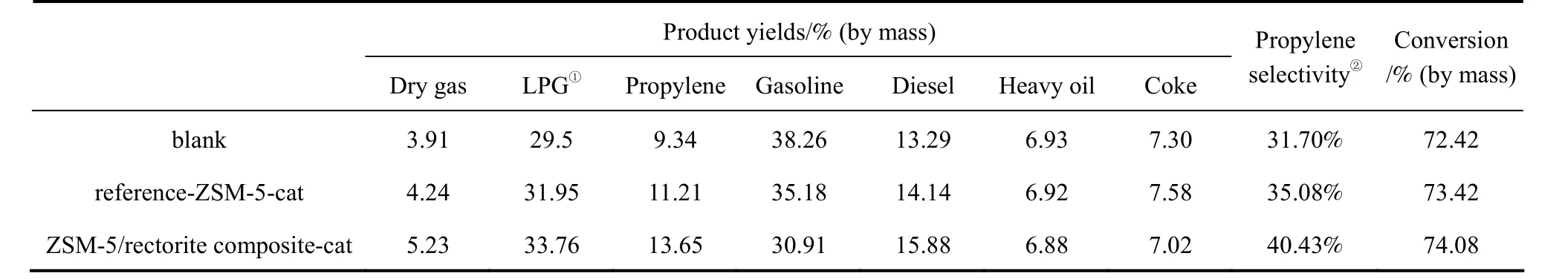
Table 6 Product distributions on the different catalysts in the FCC process
3.2 Catalytic performance
Using Daqing AR as the feedstock, the catalytic performance of the as-synthesized sample and the reference ZSM-5 sample for boosting propylene production in the FCC process was evaluated in a fluidized microreactor and the results are shown in Table 6. It can be seen that the addition of ZSM-5 as the additive to the base FCC catalyst increases propylene yield in the FCC process. Compared to that over the blank FCC catalyst, the propylene yields over the catalysts containing the reference ZSM-5 and the ZSM-5/rectorite composite were increased by 1.87% and 4.31%,respectively, correspondingly the gasoline yields decreased by 3.08% and 7.35%, respectively.
Furthermore, it can be seen that compared to the reference ZSM-5 sample, the ZSM-5/rectorite composite exhibits excellent catalytic activity: the total conversion over the composite incorporated catalyst[ZSM-5/rectorite composite-cat, which containing 10%(by mass) as-synthesized ZSM-5/rectorite composite,as shown in Table 3] is slightly higher than that over the reference ZSM-5 incorporated sample [Reference ZSM-5-cat, which containing 10% (by mass) reference ZSM-5, as shown in Table 3], and the yields of propylene and LPG over the former are 13.65% (by mass) and 33.76% (by mass), respectively, higher than those over the latter. Most importantly, compared to that over the reference ZSM-5-cat, the selectivity to propylene obtained over the ZSM-5/rectorite composite-cat was increased by 5.35%, demonstrating superior selectivity to propylene.
Given the very low density (0.02 mmol·g-1) of medium and strong acid sites of the ZSM-5/rectorite composite, the superior selectivity (40.43%) to propylene of the ZSM-5/rectorite composite incorporated catalyst should be attributed to its unique trimodal microporous-mesoporous-macroporous structure and much centralized micropore size of 0.4-0.5 nm. On the one hand, a good FCC catalyst needs such a relaying pore structure to fulfill the series reactions in a typical FCC process [28, 29]: (1) heavier molecules in residua are firstly pre-cracked to the medium-size molecules in macropores larger than 50 nm; (2) the medium-sized molecules diffuse from macropores into mesopores of 2-50 nm where they are further cracked to the middle distillate molecules; (3) the middle distillate molecules diffuse from mesopores to micropores (<2 nm)where they are finally cracked to light products. When adding ZSM-5 as the additive to the base FCC catalyst,gasoline-range olefins formed are cracked further to C3-C4olefins in ZSM-5’s micropores. To fulfill this reaction especially to boost the propylene production,an appropriate micropore dimension is needed. Altwasseret al. [25] proposed that the small pore zeolites show relatively high contributions of monomolecular Haag-Dessau cracking,i.e., high selectivity to C1-C3hydrocarbons. Cormaet al. [23] pointed out that the production of propylene requires pores of the size between 0.5 and 0.6 nm, and a pore size of above 0.6 nm should be adequate for producing butenes. According to the above analysis, we can conclude that just due to its unique trimodal microporous-mesoporousmacroporous pore structure and centralized micropore diameter of 0.4-0.5 nm, the FCC catalyst incorporated with the ZSM-5/rectorite composite as the additive exhibits excellent selectivity to propylene in FCC operation.
Moreover, the yield of coke over the ZSM-5/rectorite composite incorporated catalyst is 7.02% (by mass), slightly lower than that of the reference ZSM-5 incorporated catalyst. This result may be attributed to the relatively smaller ZSM-5 crystal size in the composite that endows the corresponding catalyst with improved accessibility to hydrocarbon molecules and therefore lower coke selectivity, in line with the analysis in Section 3.1.1.
Based on the above discussion, we can conclude that the unique trimodal microporous-mesoporousmacroporous structure, appropriate micropore diameter and relatively smaller ZSM-5 crystal size of the ZSM-5/rectorite composite contribute to the improved catalytic activity, superior propylene selectivity, and lower coke selectivity of the ZSM-5/rectorite composite incorporated catalyst.
In the previous work [19], we explored the potential of using rectorite mineral as raw material for the in-situ synthesis of FCC catalysts and prepared a core-shell type ZSM-5/rectorite composite (As-Rec-800)using activated rectorite as the starting material. In this work, we prepared a ZSM-5/rectorite composite via the same method. The results show that compared to As-Rec-800, the ZSM-5/rectorite composite prepared in this work has moderate acidity, unique trimodal microporous-mesoporous-macroporous structure and appropriate micropore diameter and thus exhibits superior propylene boosting performance in FCC process. At the almost equivalent propylene yield[13.69% (by mass)], the propylene selectivity over the catalyst containing 30% (by mass) As-Rec-800 as additive is 36.33%, much lower than that of (40.43%)the catalyst prepared in this work which containing 10% (by mass) as-synthesized ZSM-5/rectorite composite (as shown in Table 3).
4 CONCLUSIONS
Using XRD, SEM/EDS, N2adsorption-desorption and Py-FTIR techniques, we characterized elaborately the physicochemical properties of the ZSM-5/rectorite composite prepared from rectorite particles via the in-situ crystallization method and compared them with those of a commercial ZSM-5 sample, so as to understand the effect of the acidic properties and pore structure of the ZSM-5/rectorite composite on the propylene boosting performance of the ZSM-5/rectorite composite incorporated catalyst. The characterization results showed that ZSM-5/rectorite composite consists of both ZSM-5 and rectorite phases, with the ZSM-5 phase in-situ growing on rectorite particles as a layer of 2-3 μm in thickness. Because of its unique trimodal microporous-mesoporous-macroporous structure, appropriate micropore diameter of about 0.4-0.5 nm, and the relatively smaller crystal size, the ZSM-5/rectorite composite exhibits high catalytic activity,superior propylene selectivity, and lower coke selectivity when used as propylene boosting additive. Compared to those obtained over the commercial ZSM-5 incorporated catalyst, the yield and selectivity of propylene over the ZSM-5/rectorite composite incorporated catalyst were increased by 2.44% and 5.35%,respectively.
1 Radcliffe, C., “The FCC unit as a propylene source”, Petroleum Technology Quarterly, Q3, 45-50 (2007).
2 Liu, C.H., Zhao, H.J., Gao, X.H., Ma, J.T., “A novel FCC catalyst synthesized via in situ microspheres for maximizing propylene yield”, Catal. Today, 125, 163-168 (2007).
3 Wallenstein, D., Harding, R.H., “The dependence of ZSM-5 additive performance on the hydrogen-transfer activity of the REUSY base catalyst in fluid catalytic cracking”, Appl. Catal. A Gen., 214, 11-29(2001).
4 Buchanan, J.S., “The chemistry of olefins production by ZSM-5 addition to catalytic cracking units”, Catal. Today, 55, 207-212 (2000).
5 den Hollande, M.A., Wissink, M., Makkee, M., Moulijn, J.A., “Gasoline conversion: reactivity towards cracking with equilibrated FCC and ZSM-5 catalysts”, Appl. Catal. A Gen., 223, 85-102 (2002).
6 den Hollander, M.A., Wissink, M., Makkee, M., Moulijn, J.A.,“Synergy effects of ZSM-5 addition in fluid catalytic cracking of hydrotreated flashed distillate”, Appl. Catal. A Gen., 223, 103-119(2002).
7 Degnan, T.F., Chitnis, G.K., Schipper, P.H., “History of ZSM-5 fluid catalytic cracking additive development at Mobil”, Micropor.Mesopor. Mater., 35-36, 245-252 (2000).
8 Buchanan, J.S., “Gasoline selective ZSM-5 FCC additives: model reactions of C6-C10 olefins over steamed 55∶1 and 450∶1 ZSM-5”, Appl. Catal. A Gen., 171, 57-64 (1998).
9 Blasco, T., Corma, A., Martínez-Triguero, J., “Hydrothermal stabilization of ZSM-5 catalytic-cracking additives by phosphorus addition”, J. Catal., 237, 267-277 (2006).
10 Haden, W.L., Metuchen, Jr., Dzierzanowski, F.J., Somerset, N.J.,“Microspheric zeolitic molecular sieve composite catalyst and preparation thereof”, U.S. Pat., 3506594 (1970).
11 Haden, W.L., Metuchen, Jr., Dzierzanowski, F.J., Somerset, N.J.,“Microspheric zeolitic molecular sieve composite catalyst and preparation thereof”, U.S. Pat., 3647718 (1972).
12 Gibson, C.W., Willis, M.J., Gantt, G.E., Baenes, R.E., Stockwell,D.M., “Fliud catalytic cracking catalyst manufacturing process”, U.S.Pat., 6696378 B2 (2004).
13 Chandrasekhar, S., Pramada, P.N., “Kaolin-based zeolite Y, a precursor for cordierite ceramics”, Appl. Clay Sci., 27, 187-198 (2004).
14 Basaldella, E.I., Bonetto, R., Tara, J.C., “Synthesis of NaY zeolite on preformed kaolinite spheres. Evolution of zeolite content and textural properties with the reaction time”, Ind. Eng. Chem. Res., 32,751-752 (1993).
15 Gonzalez, R.E., “Catalyst industry trends”, Petroleum Technology Quarterly (Catal.), 12, 7-8 (2007).
16 Komarneni, S., katsuki, H., Furuta, S.J., “Novel honeycomb structure: a microporous ZSM-5 and macroporous mullite composite”, J.Mater. Chem., 8, 2327-2329 (1998).
17 Wang, P., Shen, B.J., Shen, D.D., Peng, T., Gao, J.S., “Synthesis of ZSM-5 zeolite from expanded perlite/kaolin and its catalytic performance for FCC naphtha aromatization”, Catal. Commun., 8,1452-1456 (2007).
18 Feng, H., Li, C.Y., Shan, H.H., “In-situ synthesis and catalytic activity of ZSM-5 zeolite”, Appl. Clay Sci., 42, 439-445 (2009).
19 Wei, B.Y., Liu, H.Y., Li, T.S., Cao, L.Y., Fan, Y., Bao, X.J., “Natural rectorite mineral: A promising substitute of kaolin for in-situ synthesis of fluid catalytic cracking catalysts”, AIChE J., 56, 2913-2922 (2010).
20 Teng, J.W., Zhao, G.L., Xie, Z.K., Chen, Q.L., “Effect of ZSM-5 crystal size on propylene production from catalytic cracking of C4olefins”, Chinese J. Catal., 25, 602-606 (2004). (in Chinese)
21 Zhang, L.H., Li, Z.T., “Effect of HZSM-5 molecular sieve intracrystal diffusion on the cracking of hydrocarbons”, Acta Petrolei Sinica(Petroleum Processing Section), 11, 29-35 (1995). (in Chinese)
22 Xin, Q., Luo, M.F., Modern Characterization Techniques in Catalysis, Science Press, Beijing (2009). (in Chinese)
23 Corma, A., Gonzάlez-Alfaro, V., Orchilles, A.V., “The role of pore topology on the behavior of FCC zeolite additives”, Appl. Catal. A Gen., 187, 245-254 (1999).
24 Castañeda, R., Corma, A., Fornés, V., Martínez-Triguero, J., Valencia,S., “Direct synthesis of a 9×10 member ring zeolite (Al-ITQ-13): A highly shape-selective catalysts for catalytic cracking”, J. Catal.,238, 79-87 (2006).
25 Altwasser, S., Welker, C., Traa, Y., Weitkamp, J., “Catalytic cracking of n-octane on small pore zeolites”, Micropor. Mesopor. Mater., 83,345-356 (2005).
26 Dzikh, I.P., Lopes, J.M., Lemos, F., Ramôa Ribeiro, F., “Mixing effect of USHY + HZSM-5 for different catalyst ratios on the n-heptane transformation”, Appl. Catal. A Gen., 176, 239-250 (1999).
27 Bortnovsky, O., Sazama, P., Wichterlova, B., “Cracking of pentenes to C2-C4light olefins over zeolites and zeotypes role of topology and acid site strength and concentration”, Appl. Catal. A Gen., 287,203-213 (2005).
28 O’Connor, P., Gerritsen, L.A., Pearce, J.R., Desal, P.H., Yanik, S.J.,Humphries, A., “Improve resid processing”, Hydro. Proc., 70, 76-81(1991).
29 Kubicek, N., Vaudry, F., Chiche, B.H., Hudec, P., Renzo, F.D.,Schulz, P., Fajula, F., “Stabilization of zeolite beta for FCC application by Embedding in amorphous matrix”, Appl. Catal., 175, 159-171(1998).
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Low-temperature Electrodeposition of Aluminium from Lewis Acidic 1-Allyl-3-methylimidazolium Chloroaluminate Ionic Liquids*
- Performance Appraisal of Controlled Low-strength Material Using Sewage Sludge and Refuse Incineration Bottom Ash*
- Solvent Extraction of Yttrium by Task-specific Ionic Liquids Bearing Carboxylic Group*
- An Experimental Study of Liquid-Liquid Microflow Pattern Maps Accompanied with Mass Transfer*
- Liquid-Liquid-Liquid Three Phase Extraction Apparatus: Operation Strategy and Influences on Mass Transfer Efficiency*
- Micron-sized Magnetic Polymer Microspheres for Adsorption and Separation of Cr(VI) from Aqueous Solution*
