EmployingMixed Ligands to Construct 3d-4f Framework:Hydrothermal Synthesis,Structure and Magnetic Properties
2012-01-19LIUYangFENGYongLanCHENZhiMinLIJunHua
LIU Yang,FENG Yong-Lan,CHEN Zhi-Min,LI Jun-Hua
(Department of Chemistry and Materials Science,Hengyang Normal University,Hengyang Hunan 421008,China)
Synthesis of metal-organic frameworks(MOFs)has become an increasingly popular field over the past few decades owing to the many potential applications in the areas of sorption,separation,molecular magnets,and other multifunctional materials[1-4].In this respect,Sole-metal-based metal-organic framework was focused on and investigated extensively.Up to now,Compared to mono-metal MOFs,heterometal-organic frameworks(eg 3d-4fMOFs)is rare due to rigorous synthesis conditions resulting from the competitive reactions between lanthanide and transition metal ions with organic ligands.
It has been well established that lanthanide ions have a higher affinity for hard donor ligands and prefer to the O-donor ligands,while transition metals have a strong tendency to coordinate to N-donor ligands,as well as O-donor ligands.Therefore,choice of aromatic or nitrogen heteroaromatic polycarboxylates ligands is a rational candidates for the construction of heterometallic MOFs.As expected,a few heterometallic MOFs with fascinating structure and special property have been synthesized by use of these polydentate O-or N-containing ligands[5-8].
We have been interested in the study of benzene dicarboxylate-based 3d-4fheterometallic coordination polymers and related system,which has resulted in the formation of many interesting structures[9,10].Recently,we have synthesized a series of 3d-4fheterometallic coordination polymers by hydrothermal method successfully including Cu-Ln-Po networks based on the assembly of mixed 2,2'-bipyridine and isophthalic acid,apillared 3d-4fframework with ferromagnetic interactions and unique topological architecture.In continuation of the theme,Herein,we report a novel Dy-Co heterometallic 3Dmetal-organic framework constructed from pyridine dicarboxylic acids and oxalic acid organic ligands,namely,[DyCo(pydc)2(C2O4)0.5(H2O)3]·3H2O.
Preparation of the complex:Dy(NO3)3·6H2O(91.3mg,0.2mmol)was dispersed in water(6ml).Then pyridine-2,3-dicarboxylic acid(H2pydc,83.6mg,0.5mmol),oxalic acid(45mg,0.5mmol)and CoCl2·6H2O(119mg,0.5mmol)were added under continuous stirring.PH value of the reaction system was tuned to 6.5.The resulting mixture was homogenized for 30min at room temperature,sealed in a 7ml PTFE-lined autoclave and heated at 150℃for three days under autogenous pressure.Afterward,the reaction system was gradually cooled to room temperature.Salmon pink parallelepiped-like crystals suitable for single crystal X-ray diffraction analysis were collected.Single-crystal X-ray diffraction studies reveal that the compound 1crystallizes in the triclinic crystal system with P-1space group.Crystal data for the title compound:C15H18CoDyN2O16,Mr=703.74,Triclinic,P-1,a=9.972(2),b=10.599(2),c=10.917(2),α=80.65(3),β=72.30(3),γ=74.35(3),V=1054.4(3)Å3,Z=2,Dc=2.217g cm-3,μ(Mo Kα)=4.392,F(000)=686,T=293(2)K,10372measured reflections with 4778independent reflections.The final R1=0.0336,wR2=0.0947with(I>2σs(I)).The asymmetric unit of 1contains a Co2+ion,a Dy3+,two 2,3-pydc2-ligands,half an oxalic acid ligand and three free water molecules.Each Dy3+ion is a nine-coordination,being bound by nine oxygen atoms of which four come from two chelating 2,3-H2pydc ligands,one from a bridging 2,3-H2pydc ligand,two from oxalic acid ligand and two from two water molecules.The coordination sphere of Dy3+centers can be described as a slightly distorted tricapped trigonal prism.The bond lengths of Dy-O range from 2.287(3)to 2.554(4)Åwith an average value of 2.436Å,which is comparable to that found in the literature[11].The O-Dy-O bond angles fall in the range of 52.33(11)-150.46(13).Each Co2+ion is six-coordinated showing a nearly regular octahedral arrangement completed by three carboxylate oxygen atoms(O2,O4,O6),two nitrogen atoms(N1,N2)from three 2,3-pydc2-and one oxygen atom(O10)from a coordinated water molecular.
The two 2,3-pydc2-can be classified into two different types on the basis of their coordination modes with the metal atom.In coordination mode a,the nitrogen and one of the 2-carboxylate oxygen atom chelate one Co1ion.One of the 3-carboxylate oxygen atoms coordinates to one Co1and the other of the 3-carboxylate oxygen atoms is bonded to one Dy1ion.In b coordination mode,O2atom from 2-carboxylate has U3connectivity linking Dy and Co metal centers,while the 3-carboxylate group chelate a Dy ion through its two oxygen atoms.Co and Dy metal centers are connected alternately via-U3-O atoms and carboxylate groups to form an Co-O-Dy-O-C-O-Co type 12-memenber ring,which is further linked to peripheral four 12-memenber rings by eight 2,3-pydc ligands to 2D layers.These 2Dlayers are bridged by oxalic acid to form 3Dframework architecture(Figure 1).All carboxyl groups of 2,3-H2pydc ligand are deproto-nated,in agreement with the IR data(Figure 2)in which no strong absorption peaks around 1700cm-1for-COOH are observed.The IR spectrum of compound 1also shows characteristic bands of groups at near 1580cm-1for the antisymmetric stretching and at near 1480and 1420cm-1for the symmetric stretching.The very strong broad bond at around 3370cm-1is assigned to the vibrations of coordinating and noncoordinating water molecules in 1.
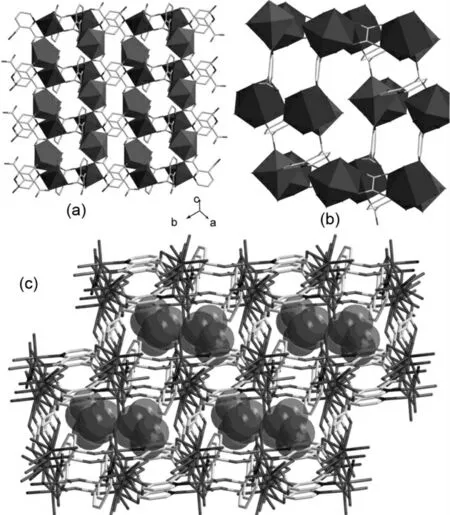
Figure 1 (a)view of the 2Dlayer structure constructed by Co,Dy and 2,3-pydc2-(b)overall 3D-framework of 1 with free water molecules shown in space filling mode occupying the channels.(c)schematic drawing of 3Dcoordination polyhedral framework pillared by oxalic acid.
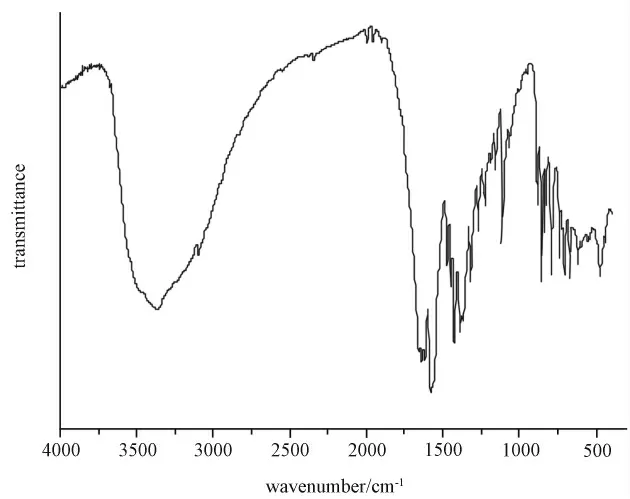
Figure 2 IR patterns of compound 1
In order to characterize the compound more fully in terms of thermal stability,their thermal behaviors were studied by TGA.The experiments were performed on samples consisting of numerous single crystals under a N2atmosphere with a heating rate of 10℃/min,as shown in Figure 3in the supporting information.The TG curve of compound 1shows two distinct weight loss steps.The first weight loss(≈16%)step between 80-160℃may be due to the loss of lattice water and coordinated water molecules,and the second weight loss,which occurs in two steps in the temperature range of 370-560℃,corresponds to the loss of the carboxylate moieties:38%(cacld 37.14%).
Solid-state dc magnetic susceptibility measurement for compound 1was performed in the range of 2-300Kunder a field of 1000Oe,and the magnetic behavior is shown in Firgure 4as plots of χMT andχM-1verse T.

Figure 3 the TG plot of compound 1
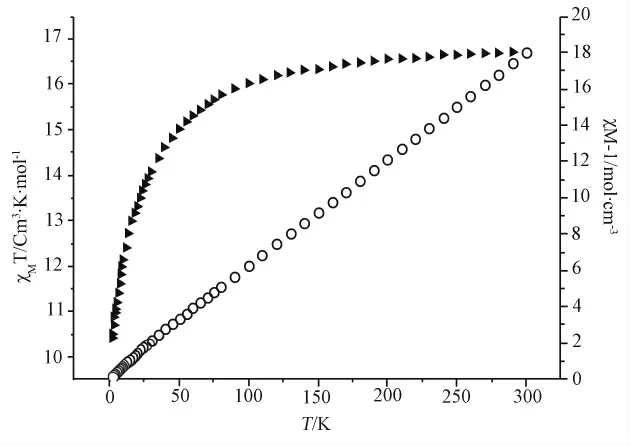
Figure 4 Plots ofχMT verse T and 1/χM Tverse T in a 1000Oe field
At 300K,theχMT value of 1is 16.72cm3.K.mol-1,which is slightly larger than the spin-only value of 16.04cm3.k.mol-1expected for isolated one Dy(Ⅲ)(SDy=5/2)and one Co(Ⅱ)(SCo=3/2)ion(assuming gDy=4/3and gCo=2.0),indicative of the presence of the large unquenched orbital angular momentum of Dy(Ⅲ)and Co(Ⅱ)ions in the crystal field[12].There is a continuous decrease in theχMT value as the temperature is lowered from 300Kto 2.0K,at which point theχMT value is 10.4cm3.K.mol-1.The linear fit viaχM=C/(Tθ)in the whole temperature range gives the Weiss constantθ=-4.5436Kand C=16.903cm3·K·mol-1.Despite the decrease ofχMT value on cooling and the presence of negativeθvalue,the nature of the magnetic interaction between Dy(Ⅲ)and Co(Ⅱ)ions cannot be unambiguously interpreted as antiferromagnetic because of the strong spin-orbit coupling of the Dy(Ⅲ)ion[13-15].In summary,we have synthesized a 3d-4f3Dcoordination polymer under solvothermal condition via mixed ligands.These results provide a new method to construct 3D3d-4fframework.
[1]Kagan H.Introduction:frontiers in lanthanide chemistry[J].Chem.Rev,2002,102:1805-1806.
[2]Wickleder M.Inorganic lanthanide compounds with complex anions[J].Chem.Rev.,2002,102(6):2011-2088.
[3]Lombardi J,Davis B.Periodic properties of force constants of small transition-metal and lanthanide clusters[J].Chem.Rev.,2002,102(6):2431-2460.
[4]Barbour L.Crystal porosity and the burden of proof[J].Chem.Commun.,2006,1163-1168.
[5]Imdert D,Cantuel M,Bünzli J C G,et al.Extending lifetimes of lanthanide-based near-infrared emitters(Nd,Yb)in the millisecond range through Cr(III)sensitization in discrete bimetallic edifices[J].J.Am.Chem.Soc.,2003,125(51):15698-15699.
[6]Guillou O,Daiguebonne C,Camara M,et al.New 3-D La(III)-Cu(II)-containing coordination polymer with a high potential porosity[J].Inorg.Chem,2006,45(21):8468-8470.
[7]Mori F,Nyui T,Ishida T,et al.Oximate-bridged trinuclear Dy-Cu-Dy complex behaving as a single-molecule magnet and its mechanistic investigation[J].J.Am.Chem.Soc,2006,128(5):1440-1441.
[8]Gao H L,Zhao B,Zhao X Q,et al.Structures and magnetic properties of ferromagnetic coupling 2DLn-M heterometallic coordination polymers(Ln=Ho,Er;M=Mn,Zn)[J].Inorg.Chem,2008,47(23):11057-11061.
[9]Luo F,Yang Y T,Che Y X,et al.Construction of Cu(II)–Gd(III)metal–organic framework by the intro-duction of a small amino acid molecule:hydrothermal synthesis,structure,thermostability,and magnetic studies[J].CrystEngComm,2008,10:1613-1616.
[10]Yang Y T,Luo F,Che Y X,et al.Employing an unprecedented ferromagnetic molecular building block(MBB)of[Co2Ln(μ3-OH)(CO2)5(N3)]2(Ln=Tb,Gd,Dy,Eu,Sm)to construct a 6-connectedα-Po Net[J].Crystal Growth &Design,2008,8(10):3508-3510.
[11]Chandrasekhar V,Pandian B M,Vittal J J,et al.Synthesis,structure,and magnetism of heterobimetallic trinuclear complexes{[L2Co2Ln][X]}[Ln=Eu,X=Cl;Ln=Tb,Dy,Ho,X=NO3;LH3=(S)P[N(Me)N=CH-C6H”3-2-OH-3-OMe]3]:A 3d-4ffamily of single-molecule magnets[J].Inorg.Chem,2009,48(3):1148-1157.
[12]Benelli C,Gatteschi D.Magnetism of lanthanides in molecular materials with transition-metal ions and organic radicals[J].Chem.Rev,2002,102(6):2369-2388.
[13]Tang J K,Li Y Z,Wang Q L,et al.Synthesis,crystal structure,and magnetic properties of the first nonanuclear lanthanide(III)-copper(II)complexes of macrocyclic oxamide[NaLn2Cu6](macrocyclic oxamide=1,4,8,11-tetraazacyclotradecanne-2,3-dione,Ln=Pr,Nd)[J].Inorg.Chem,2002,41(8):2188-2192.
[14]Kahn M L,Sutter J P,Golhen S,et al.Systematic investigation of the nature of the coupling between a Ln(III)Ion(Ln=Ce(III)to Dy(III))and its aminoxyl radical ligands.structural and magnetic characteristics of a series of{Ln(organic radical)2}compounds and the related{Ln(nitrone)2}derivatives[J].J.Am.Chem.Soc,2000,122(14):3413-3421.
[15]Yue Q,Yang J,Li G H,et al.Three-dimensional 3d-4fheterometallic coordination polymers:synthesis,structures,and magnetic properties[J].Inorg.Chem,2005,44(15):5241-5246.
