Large regenerative nodules in a patient with Budd-Chiari syndrome after TIPS positioning while on the liver transplantation list diagnosed by Gd-EOB-DTPA MRI
2011-07-05MatteoRenzulliVincenzoLucidiCristinaMosconiChiaraQuarnetiEmanuelaGiampalmaandRitaGolfieri
Matteo Renzulli, Vincenzo Lucidi, Cristina Mosconi, Chiara Quarneti, Emanuela Giampalma and Rita Golfieri
Bologna, Italy
Case Report
Large regenerative nodules in a patient with Budd-Chiari syndrome after TIPS positioning while on the liver transplantation list diagnosed by Gd-EOB-DTPA MRI
Matteo Renzulli, Vincenzo Lucidi, Cristina Mosconi, Chiara Quarneti, Emanuela Giampalma and Rita Golfieri
Bologna, Italy
BACKGROUND:Large regenerative nodules (LRNs) are hyperplastic benign nodules most commonly associated with Budd-Chiari syndrome (BCS), caused by outflow obstruction of the hepatic veins or vena cava. To our knowledge, no cases of LRNs arising in BCS after transjugular intrahepatic portosystemic shunt (TIPS) positioning and detected by Gd-EOB-DTPA MRI have been reported in the literature.
METHODS:A 58-year-old woman with BCS, on the liver transplantation (LT) list, underwent a follow-up enhanced MRI. Two years earlier, a TIPS had been placed. In 2008, recurrent hepaticoencephalopathy resistant to medical treatment fulfilled the LT criteria for BCS treated with TIPS and the patient was therefore added to the LT list. CT performed before TIPS had not detected any hepatic lesions. CT performed six months after TIPS showed its complete patency but documented two indeterminate hypervascular liver lesions.
RESULTS:MRI performed with Gd-EOB-DTPA revealed additional hypervascular lesions with uptake and retention of the medium in the hepatobiliary phase, thus reflecting a benign behavior of hepatocellular composition. These MRI features were related to LRNs as confirmed by histopathologic analysis.
CONCLUSIONS:Gd-EOB-DTPA-enhanced MRI is potentially superior to standard imaging using gadolinium chelates or spiral CT, especially for the differential diagnosis of hypervascular lesions. Gd-EOB-DTPA MRI may become the imaging method of choice for evaluating LT list patients with BCS after TIPS placement.
(Hepatobiliary Pancreat Dis Int 2011; 10: 439-442)
Budd-Chiari syndrome; large regenerative nodules; Gd-EOB-DTPA-enhanced MRI
Introduction
Liver injury can result in several kinds of hepatocellular nodules. An expert group classified these lesions based on the type of cells within them (hyperplastic or dysplastic) and on the anatomical characteristics of the surrounding liver.[1]Accurate distinction is important because the clinical implications vary from totally benign to frankly malignant, with other nodules of intermediate concern.[2]The working group distinguished between nodular regenerative hyperplasia (NRH) and large regenerative nodules (LRNs) at the histological level. However, prior reports seem to have grouped NRH and LRNs together.[3]
NRH is defined as multiple benign regenerative lesions measuring approximately 1 mm in diameter, which involve most of the liver and occur in the absence of fibrous septa.[1]
In contrast to NRH, LRNs are hyperplastic benign nodules measuring between 5 mm and 5 cm in diameter that are larger than most cirrhotic nodules located in the liver that is otherwise abnormal (either with cirrhosis or severe disease of portal veins, hepatic veins, or sinusoids).[1]
LRNs are commonly associated with Budd-Chiari syndrome (BCS), caused by outflow obstruction of the hepatic veins or vena cava,[4,5]although they have been reported with cirrhosis, certain forms of congenital heart disease, and other conditions.[5-7]
We report a case of multiple LRNs in BCS arising after transjugular intrahepatic portosystemic shunt (TIPS) positioning and diagnosed by gadolinium-ethoxybenzyldiethylenetriamine pentaacetic acid (Gd-EOB-DTPA) MRI.
Case report
In November 2009, a 58-year-old woman with BCS, on the liver transplantation (LT) list, was admitted to our hospital to undergo a follow-up enhanced MRI. Two years earlier, a TIPS had been placed. Pertinent laboratory studies before TIPS showed: WBC 1.2×109/L (normal 3.8-10.6×109/L), hemoglobin 19 g/dL (normal 13.5-17.0 g/dL), platelets 93×109/L (normal 150-450×109/L), and normal ALT, AST, alkaline phosphatase, total bilirubin, and serum albumin. In 2008, recurrent hepaticoencephalopathy resistant to medical treatment fulfilled the LT criteria for BCS treated with TIPS and the patient was therefore added to the LT list.
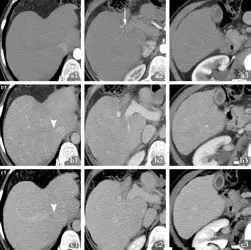
Fig. 1. Computed tomography (arterial phase a; portal phase b; venous phase c, in different hepatic planes 1-3) showing obstruction of the venous outflow tract of the liver in the Budd-Chiari syndrome (arrowhead in b1 and c1) but no focal liver lesion. a2 indicates a normal right hepatic artery (arrow).
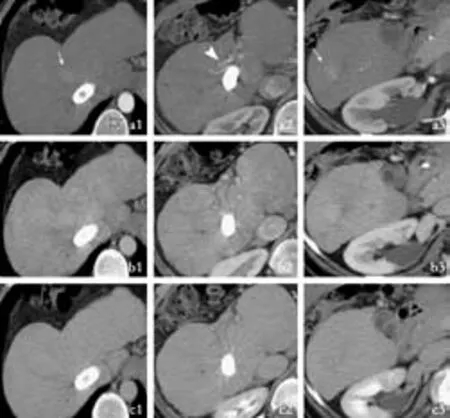
Fig. 2. Computed tomography (arterial phase a; portal phase b; venous phase c, in different hepatic planes 1-3) after TIPS showing the occurrence of two hypervascular lesions (arrows in a1 and a3). a2 indicates an hypertrophic right hepatic artery (arrowhead).
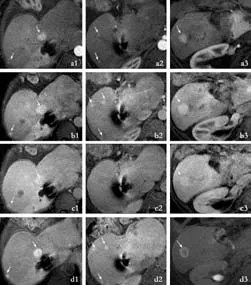
Fig. 3. Gd-EOB-DTPA MRI (arterial phase a; portal phase b; venous phase c; hepatobiliary phase d in different hepatic planes 1-3) showing many hypervascular lesions (arrows in a, b, c) with enhancement of nodules in hepatobiliary phase (arrows in d) that indicate their hepatocellular nature and delayed bile excretion from nodules.
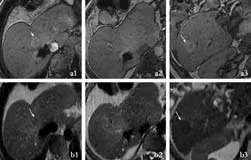
Fig. 4. Unenhanced MRI (T1-weighted image a; T2-weighted image b in different hepatic planes 1-3) showing two nodules hyperintense in the T1-weighted image (arrows in a) that appear hypointense in the T2-weighted image (arrows in b). The other small nodules, detected in dynamic phases (Fig. 3), are not visible.
CT performed before TIPS had not detected any hepatic lesions (Fig. 1). CT performed six months after TIPS showed its complete patency but documented two indeterminate hypervascular liver lesions (Fig. 2).
In addition to these CT-detected lesions, MRI performed with Gd-EOB-DTPA revealed additional hypervascular lesions whose dimensions ranged from a few millimeters to 20 mm. These lesions showed uptake and retention of the contrast medium in the hepatobiliary phase, thus reflecting a benign behaviour of hepatocellular composition with delayed clearance of bile from the nodules (Fig. 3). On unenhanced MRI, the larger nodules appeared hyperintense to the liver on T1-weighted images and isointense on unenhanced T2-weighted images; the smaller lesions were not clearly visualized (Fig. 4). These MRI features were related to LRNs as confirmed by histopathologic analysis of the specimens obtained by multiple needle biopsies of the larger nodules.
Discussion
Several types of benign vascular hepatocellular nodules have been described. This classification scheme is based on two primary sets of criteria: whether the hepatocytes are regenerative or dysplastic, and the anatomic characteristics of the adjacent hepatic stroma.[1]The distinction of these nodules is critical because the clinical significance varies. LRNs, a kind of benign liver nodule, are known to occur in response to abnormal perfusion of the liver, most commonly with BCS.[4,5]
To our knowledge, no cases of LRNs arising in BCS post-TIPS detected by Gd-EOB-DTPA MRI have been reported. In a previous study, Tanju et al[8]evaluated benign regenerative nodules following TIPS in BCS with contrast-enhanced MRI but not by using a liver-specific contrast agent.
We believe that, in our case, the placement of the TIPS produced an altered blood flow within a liver chronically injured by BCS, with reduction in the portal inflow and subsequent arterialization of the liver parenchyma (Fig. 2); these hemodynamic changes are thought to be related to LRN onset. So the postulated mechanism of LRN onset was related to TIPS insertion rather than BCS alone.
In unenhanced MRI, LRNs are hyperintense to the liver in T1-weighted images attributed to the presence of copper within the nodules, and isointense or hypointense on T2-weighted images and sometimes difficult to appreciate.[9,10]
In contrast-enhanced imaging, the LRN pattern typically appears as hypervascular nodules, which might potentially be misdiagnosed as hepatocellular carcinoma within a chronically injured liver.[5,11]There is little evidence to suggest that LRNs are pre-malignant or evolve into hepatocellular carcinoma.[5,11]
Gd-EOB-DTPA is a recently developed liver-specific MRI contrast agent with combined perfusion and hepatocyte-selective properties. A bolus injection of Gd-EOB-DTPA provides a dual mode of action which allows imaging both in the dynamic phases (as with standard gadolinium chelates) and in the delayed phase (hepatobiliary phase).[12,13]In the hepatobiliary phase, hepatic lesions lacking normally functioning hepatocytes, such as Hepatocellular carinoma, are imaged as a defect of hepatocyte-selective enhancement compared to the normal parenchyma.[14]In contrast, in the hepatobiliary phase, hyperplastic hepatocytes, such as those in LRNs which often contain ductular proliferation[4]as well as those in all focal nodular hyperplasia-like lesions, appear hepatocyte-selective iso- or more often hyper-enhanced compared to the normal parenchyma. The evaluation of vascularity and hepatocyte-specific uptake enables the accurate detection and characterization of focal liver lesions. Gd-EOB-DTPA-enhanced MRI is potentially superior to standard imaging using gadolinium chelates or spiral CT, especially for the differential diagnosis of hypervascular lesions.[15,16]
In conclusion, we believe that Gd-EOB-DTPA MRI may become the imaging method of choice for evaluating LT list patients with BCS after TIPS placement, especially in the era of long waiting lists.
Funding:None.
Ethical approval:Not needed.
Contributors:RM wrote the first draft of this report. All authors contributed to the intellectual context and approved the final version. RM is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Terminology of nodular hepatocellular lesions. International Working Party. Hepatology 1995;22:983-993.
2 Brancatelli G, Federle MP, Grazioli L, Golfieri R, Lencioni R. Large regenerative nodules in Budd-Chiari syndrome and other vascular disorders of the liver: CT and MR imaging findings with clinicopathologic correlation. AJR Am J Roentgenol 2002; 178:877-883.
3 Dachman AH, Ros PR, Goodman ZD, Olmsted WW, Ishak KG. Nodular regenerative hyperplasia of the liver: clinical and radiologic observations. AJR Am J Roentgenol 1987;148: 717-722.
4 Tanaka M, Wanless IR. Pathology of the liver in Budd-Chiari syndrome: portal vein thrombosis and the histogenesis of veno-centric cirrhosis, veno-portal cirrhosis, and large regenerative nodules. Hepatology 1998;27:488-496.
5 Vilgrain V, Lewin M, Vons C, Denys A, Valla D, Flejou JF, et al. Hepatic nodules in Budd-Chiari syndrome: imaging features. Radiology 1999;210:443-450.
6 Grazioli L, Alberti D, Olivetti L, Rigamonti W, Codazzi F, Matricardi L, et al. Congenital absence of portal vein with nodular regenerative hyperplasia of the liver. Eur Radiol 2000; 10:820-825.
7 Menon KV, Shah V, Kamath PS. The Budd-Chiari syndrome. N Engl J Med 2004;350:578-585.
8 Tanju S, Erden A, Ceyhan K, Soygür IT, Bozkaya H, Erden I. Contrast-enhanced MR angiography of benign regenerative nodules following TIPS shunt procedure in Budd-Chiari syndrome. Turk J Gastroenterol 2004;15:173-177.
9 Ames JT, Federle MP, Chopra K. Distinguishing clinical and imaging features of nodular regenerative hyperplasia and large regenerative nodules of the liver. Clin Radiol 2009;64: 1190-1195.
10 Kim T, Baron RL, Nalesnik MA. Infarcted regenerative nodules in cirrhosis: CT and MR imaging findings with pathologic correlation. AJR Am J Roentgenol 2000;175:1121-1125.
11 Takayasu K, Muramatsu Y, Moriyama N, Wakao F, Makuuchi M, Takayama T, et al. Radiological study of idiopathic Budd-Chiari syndrome complicated by hepatocellular carcinoma. A report of four cases. Am J Gastroenterol 1994;89:249-253.
12 Hamm B, Staks T, Mühler A, Bollow M, Taupitz M, Frenzel T, et al. Phaseiclinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology 1995;195:785-792.
13 Vogl TJ, Kümmel S, Hammerstingl R, Schellenbeck M, Schumacher G, Balzer T, et al. Liver tumors: comparison of MR imaging with Gd-EOB-DTPA and Gd-DTPA. Radiology 1996;200:59-67.
14 Reimer P, Rummeny EJ, Shamsi K, Balzer T, Daldrup HE, Tombach B, et al. Phase II clinical evaluation of Gd-EOBDTPA: dose, safety aspects, and pulse sequence. Radiology 1996;199:177-183.
15 Zech CJ, Herrmann KA, Reiser MF, Schoenberg SO. MR imaging in patients with suspected liver metastases: value of liver-specific contrast agent Gd-EOB-DTPA. Magn Reson Med Sci 2007;6:43-52.
16 Narita M, Hatano E, Arizono S, Miyagawa-Hayashino A, Isoda H, Kitamura K, et al. Expression of OATP1B3 determines uptake of Gd-EOB-DTPA in hepatocellular carcinoma. J Gastroenterol 2009;44:793-798.
Received May 7, 2010
Accepted after revision December 11, 2010
Author Affiliations: Radiology Unit (Renzulli M, Lucidi V, Mosconi C, Giampalma E and Golfieri R) and Internal Medicine (Quarneti C), Department of Internal Medicine and Gastroenterology, University-Hospital Sant'Orsola-Malpighi Bologna, Bologna, Italy
Matteo Renzulli, MD, U.O. Radiologia Golfieri, via Albertoni 15, 40138 Bologna, Italy (Tel: 00390516362307; Fax: 00390516362699; Email: dr.matteo.renzulli@gmail.com)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Risk factors of severe ischemic biliary complications after liver transplantation
- Health-related quality of life in living liver donors after transplantation
- Surgical treatment of Budd-Chiari syndrome: analysis of 221 cases
- Efficacy of liver transplantation for acute hepatic failure: a single-center experience
- Combined invagination and duct-to-mucosa techniques with modifications: a new method of pancreaticojejunal anastomosis
- One hundred and seventy-eight consecutive pancreatoduodenectomies without mortality: role of the multidisciplinary approach
