Efficacy of liver transplantation for acute hepatic failure: a single-center experience
2011-07-05XianJieShiHongBinXuWenBinJiYuRongLiangWeiDongDuanLeiHeMingJunWangandZhiMingZhao
Xian-Jie Shi, Hong-Bin Xu, Wen-Bin Ji, Yu-Rong Liang, Wei-Dong Duan, Lei He, Ming-Jun Wang and Zhi-Ming Zhao
Beijing, China
Efficacy of liver transplantation for acute hepatic failure: a single-center experience
Xian-Jie Shi, Hong-Bin Xu, Wen-Bin Ji, Yu-Rong Liang, Wei-Dong Duan, Lei He, Ming-Jun Wang and Zhi-Ming Zhao
Beijing, China
BACKGROUND:Acute hepatic failure (AHF) is a devastating clinical syndrome with a high mortality rate. The outcome of AHF varies with etiology, but liver transplantation (LT) can significantly improve the prognosis and survival rate of such patients. This study aimed to detect the role of LT and artificial liver support systems (ALSS) for AHF patients and to analyze the etiology and outcome of patients with this disease.
METHODS:A retrospective analysis was made of 48 consecutive patients with AHF who fulfilled the Kings College Criteria for LT at our center. We analyzed and compared the etiology, outcome, prognosis, and survival rates of patients between the transplantation (LT) group and the non-transplantation (N-LT) group.
RESULTS:AHF was due to viral hepatitis in 25 patients (52.1%; hepatitis B virus in 22), drug or toxic reactions in 14 (29.2%; acetaminophen in 6), Wilson disease in 4 (8.3%), unknown reasons in 3 (6.3%), and miscellaneous conditions in 2 (4.2%). In the LT group, 36 patients (7 underwent living donor LT, and 29 cadaveric LT) had an average model for endstage liver disease score (MELD) of 35.7. Twenty-eight patients survived with good graft function after a follow-up of 27.3± 4.5 months. During the waiting time, 6 patients were treated with ALSS and 2 of them died during hospitalization. The 30-day, 12-month, and 18-month survival rates were 77.8%, 72.2%, and 66.7%, respectively. In the N-LT group, 12 patients had an average MELD score of 34.5. Four patients were treated with ALSS and all died during hospitalization. The 90-day and 1-year survival rates were only 16.7% and 8.3%, respectively.
CONCLUSIONS:Hepatitis is the most prominent cause of AHF at our center. Most patients with AHF, who fulfill the Kings College Criteria for LT, did not survive longer without LT. ALSS did not improve the prognosis of AHF patients, but may extend the waiting time for a donor. Currently, LT is still the most effective way to improve the prognosis of AHF patients.
(Hepatobiliary Pancreat Dis Int 2011; 10: 369-373)
acute hepatic failure; liver transplantation; arti ficial liver support; prognosis; survival rate; etiology
Introduction
Acute hepatic failure (AHF) is a severe condition, which often affects young persons and is associated with a high mortality rate. Moreover, the outcome of AHF varies etiologically and geographically; however, the prognosis of this disorder improves dramatically after liver transplantation (LT). The most widely accepted definition of AHF includes evidence of coagulation abnormality, which is typically determined by the prothrombin time (prothrombin index <40% or international normalized ratio ≥1.5), and any degree of mental alteration (encephalopathy) in patients without pre-existing cirrhosis or with an illness of <26 weeks duration. Patients with Wilson disease, verticallyacquired HBV, or autoimmune hepatitis may be included in spite of the possibility of cirrhosis, if their disease is recognized for <26 weeks.[1]
This catastrophic illness can progress rapidly resulting in the placement of the patient in a coma and/or death from cerebral edema or multi-organ dysfunction. The reported survival rate of patients with AHF is less than 15% before LT, while the overall short-term survival rate after LT is more than 65%.[2]The present study was undertaken to assess the roleof LT and artificial liver support system (ALSS) in the treatment of AHF patients, and to analyze the etiology of the disease and the outcome of treatment at our center.
Methods
We retrospectively reviewed 48 consecutive patients with AHF treated at our center from March 2005 to June 2009, who fulfilled the Kings College Criteria for LT. AHF was diagnosed by clinical, analytical, and histological results. Etiology of the disease, age and sex of the patients, MELD score, perioperative complications, artificial liver support, continuous renal replacement therapy (CRRT), waiting time, causes of death, and survival time were recorded and analyzed. Informed consent was obtained from the patients or their relatives before the study. The study was approved by the Ethics Committee of the General Hospital of PLA.
General information
Forty-eight patients (20 males and 28 females) were divided into two groups: a transplantation (LT) group of 36 patients, who had undergone LT, and a nontransplantation (N-LT) group of 12, who had not. The average age of the patients was 34.1±4.9 years, and the MELD score was 35.2±7.9. In the LT group, 6 patients were given ALSS, 6 were given CRRT before surgery, and 6 were given CRRT after surgery. In the N-LT group, 4 patients were given ALSS and 2 were given CRRT (Table 1).
Etiology
AHF was due to viral hepatitis in 25 patients (52.1%; hepatitis B virus in 22 patients), drug or toxic reactions in 14 (29.2%; acetaminophen in 6), Wilson disease in 4 (8.3%), unknown reasons in 3 (6.3%), and miscellaneous conditions in 2 (4.2%) (Fig.1).
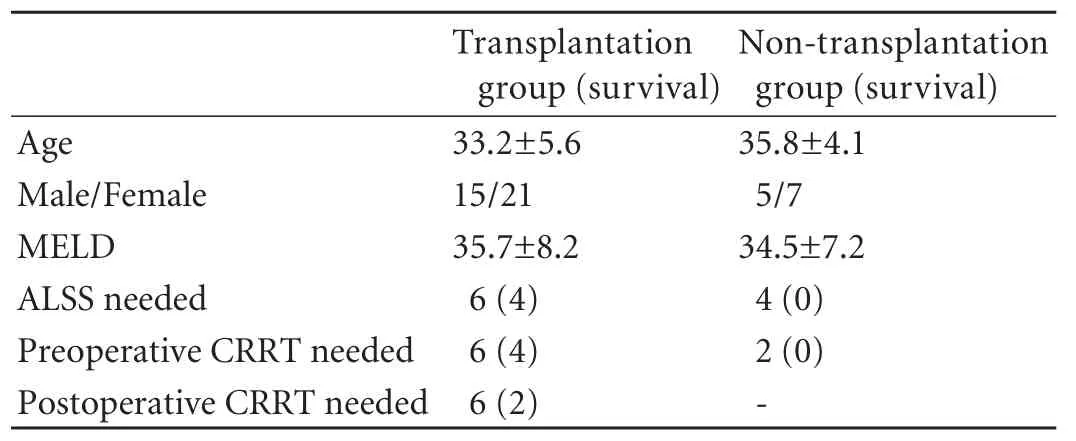
Table 1. General information of patients in two groups before operation
Treatment and follow-up
Seven patients underwent living donor LT, and 29 received a cadaveric whole liver. Thirty-four pairs of donors and recipients had the same ABO blood type, while the other two pairs were type-B recipients of type-O donors. Other operative data are shown in Table 2. Immunosuppression was based on a regimen of FK506, MMF, and prednisone in all patients. Other than LT, the medical treatments used in both groups were the same, including liver protection, anti-infection, nutritional support and anti-hepatic encephalopathy. The follow-up interval was from the date of diagnosis to death or December 2010.
Statistical analysis
Data were analyzed on a CHISS system (Windows version 3.0.0.21). Baseline data were presented as mean± SD. Actuarial survival rates were calculated by the Kaplan-Meier method.
Results
During the period of hospitalization, 19 patients died of brain edema and refractory intracranial hypertension, multi-organ failure (MOF), sepsis, renal failure, coagulation disorders (intracranial hemorrhage, upper digestive tract hemorrhage), acute respiratory distress syndrome, and primary graft non-function.
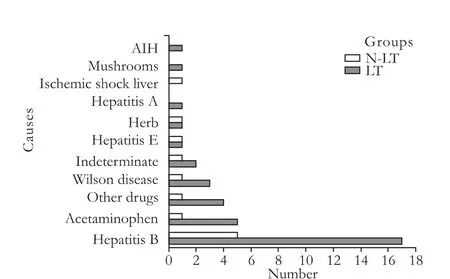
Fig. 1. Causes of 48 patients with AHF. LT: transplantation group; N-LT: non-transplantation group; AIH: autoimmune hepatitis; AHF: acute hepatic failure.
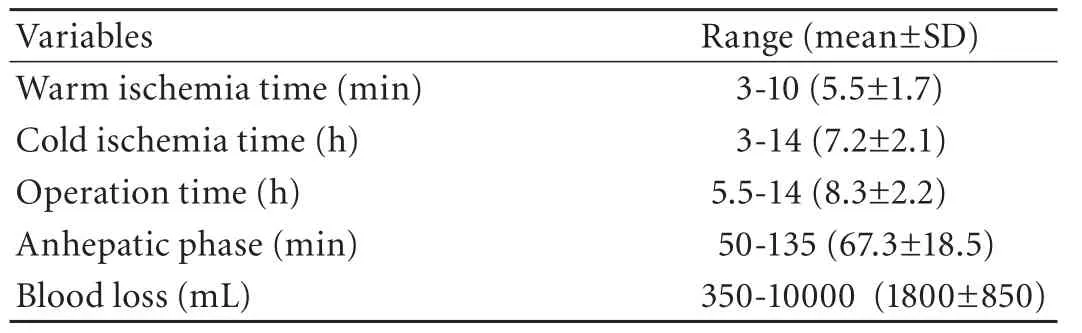
Table 2. Operative information
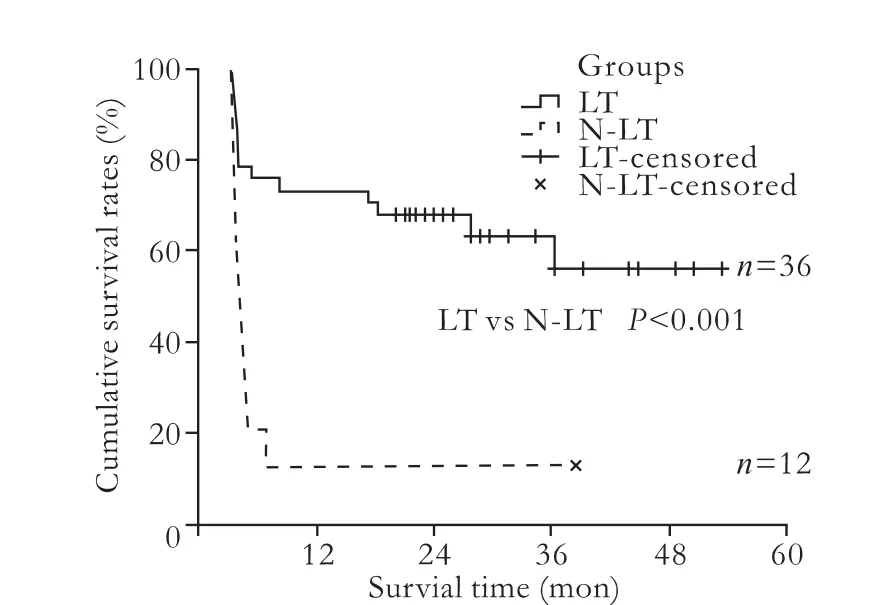
Fig. 2. The survival rate was significantly greater in the LT group than in the N-LT group (P<0.001). LT: transplantation group; N-LT: non-transplantation group.
In the LT group, 8 patients died during their hospitalization, while 28 patients survived and showed good graft function during the median follow-up time of 27.3±4.5 months. The average waiting time for LT was 78±16 hours. During the waiting period, 6 patients were treated with ALSS and 2 of them died after LT. After surgery, 6 patients were given CRRT, but only 2 of these patients survived. Five patients who exhibited vascular or biliary complications recovered after interventional or surgical therapy. The 30-day, 12-month, and 18-month survival rates were 77.8%, 72.2% and 66.7%, respectively. In the N-LT group, only one patient survived longer. Four patients were given ALSS, but all died during the period of hospitalization. The 90-day and 1-year survival rates were only 16.7% and 8.3%, respectively. In all 29 long-term surviving patients, 19 experienced a good quality of life and resumed their normal activities. The survival rate was significantly greater in the LT group than in the N-LT group (χ2=14.846, P<0.001, Fig. 2). In patients who received ALSS, the level of serum bilirubin was significantly reduced, in addition to a marked regression of pruritus. Moreover, hepatic encephalopathy was also improved and no severe events occurred in patients receiving ALSS.
Discussion
AHF is a condition, which can progress rapidly with daily changes in consciousness and coagulation. Because AHF may result in such lethal complications as MOF, hepatic encephalopathy, and coagulation disorders, immediate etiological identification and intensive care are crucial. LT may remarkably improve the prognosis of patients with AHF;[3]however, not all patients with AHF should undergo transplantation. Because of the shortage of organ donors and the growing demand, patients should be evaluated carefully for LT.
The most frequently accepted criteria for LT in AHF patients are the Kings College Hospital criteria[4]and the Clichy criteria,[5]even though these criteria were formulated a decade ago. Compared to the Kings College Hospital criteria proposed by O'Grady, validation studies of the Clichy criteria are scarce, and its derivation was from a cohort of patients with AHF resulting from a single condition.[6]Hence, in our study, we used the Kings College Hospital criteria, which differentiate patients with acetaminophen-induced hepatotoxicity from those with other causes (nonacetaminophen-induced hepatotoxicity) of AHF,[6,7]to evaluate whether LT is needed or not.
Despite the improvement of therapeutic modalities in intensive care medicine, AHF is still an intractable condition with a high mortality rate.[8]In our patients, the most common causes of death were MOF, renal failure, sepsis, brain edema, and refractory intracranial hypertension. Although we took many measures such as CRRT, ALSS, and medical treatments to prevent or remedy these complications, many patients died.
In our experience, we found several important factors to consider when caring for patients with AHF. First, the maintenance of hemodynamic stability is a key for preventing renal failure. Although renal failure alone rarely leads to death, once it occurs the patient's prognosis is poor.[9]After surgery, 6 patients in our study required CRRT, but only 2 survived. Second, the pathogenesis of brain edema and refractory intracranial hypertension is poorly understood, but its incidence and severity are closely associated with hepatic encephalopathy. In patients with hepatic encephalopathy at stagesito II, brain edema is rare; yet patients at stage III exhibit a risk of 25%-35% for developing brain edema and the risk to patients at stage IV increases to 65%-75% or more.[10,11]These increased risks highlight the importance of monitoring intracranial pressure in patients with grade III/IV encephalopathy. Therefore, moderate hypothermia might be a promising treatment for patients with refractory intracranial hypertension,[12]other measures, such as head elevation, plasma colloid elevation, and/or application of mannitol may also be effective treatments.[13]Third, it is of utmost importance to immediately address any observed infections by shortening the time for the patient on mechanical ventilation, removing or reducing the factors which aggravate or induce infections, and adjusting doses of anti-infection agents.
AHF is a fatal condition that remains poorly understood, but defining the etiology is of great importance in predicting the patient's prognosis.[14]Theetiology of AHF is significantly different in geography and times. In east Asia and developing countries, AHF is mainly due to viral infections, such as hepatitis B, A, and E. In the United States and Western Europe, the incidence of virus-induced disease has declined substantially in recent years, with most cases now arising from drug-induced liver injury.[15]In Bangladesh, acute HEV infections are the leading cause of AHF.[16]In our study, more than half of the patients with AHF were attributed to hepatitis (19 patients in the LT group and 6 in the N-LT group) infections, especially hepatitis B (45.8%), and may be one of the reasons for the higher mortality in our N-LT group.
The lack of donor livers and other transplant-related problems leads to the death of many patients during the waiting period. In the United States, over 30% of patients listed for emergency LT will die while waiting for a graft.[17]Besides standard medical therapy, ALSS or hepatocyte transplantation is currently used for treating AHF.[18-20]In this study, we used the molecular adsorbent recirculation system, ALSS, which is an extracorporeal artificial liver support system that contains no biologic components. ALSS can remove endotoxins, bilirubin, and other toxic substances from the body, add a variety of plasma coagulation factors, treat hyperbilirubinemia, and improve blood coagulation and consciousness of patients with hepatic encephalopathy. In our LT group, 6 patients were treated with ALSS during the waiting time and 2 of them died during the period of hospitalization. In the N-LT group, 4 patients were treated with ALSS and all died during hospitalization. Apparently, ALSS may extend the waiting time for a donor and serves as a bridge for patients with AHF and needing a LT, but it does not improve the mortality and prognosis of AHF patients.[21]Of course, the use of ALSS as a therapeutic option is still under investigation and lacks proven longterm successful results, thus making LT the final and most effective therapy.
At times, AHF patients have to receive an ABO-incompatible donor liver to save their lives.[22]It is reported that ABO-incompatibility has no significant impact on patient survival. However, graft survival is significantly impaired by hyperacute rejection (20%), vascular thrombosis, and biliary injury (56%).[23]In our study, there were two type B recipients from type O donors, and both exhibited long-term survival. This result showed that even ABO-incompatible liver grafts can be used in the emergency, life-saving treatment of patients with AHF.
In summary, unlike in developed countries, viral infections, especially hepatitis B, were the most prominent causes of AHF in this study. In the N-LT group, all but one patient died. ALSS did not improve the prognosis of AHF patients, but did extend the waiting time for a donor. The use of ABO-incompatible liver grafts is a possible life-saving method for the emergency treatment of patients with AHF. Most patients with AHF, who fulfilled the Kings College Criteria for LT, would not survive longer if LT is not performed. In spite of great improvement in medication, LT is still currently the most effective way for improving the prognosis of AHF patients. Because of the limitations of LT, final decisions for LT should depend upon rapid diagnosis and accurate prognostic evaluation. Although new therapies for AHF are still being investigated, alternative treatment modalities also require further study.
Funding:None.
Ethical approval:The study was approved by the Ethics Committee of the General Hospital of PLA, China.
Contributors:SXJ proposed and designed the study. SXJ and LYR wrote the first draft. XHB and JWB analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. SXJ is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Polson J, Lee WM; American Association for the Study of Liver Disease. AASLD position paper: the management of acute liver failure. Hepatology 2005;41:1179-1197.
2 Ostapowicz G, Fontana RJ, Schiödt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002;137:947-954.
3 Lee WM, Squires RH Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology 2008;47:1401-1415.
4 O'Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989;97:439-445.
5 Bismuth H, Samuel D, Castaing D, Adam R, Saliba F, Johann M, et al. Orthotopic liver transplantation in fulminant and subfulminant hepatitis. The Paul Brousse experience. Ann Surg 1995;222:109-119.
6 Bernal W, Wendon J. Liver transplantation in adults with acute liver failure. J Hepatol 2004;40:192-197.
7 Riordan SM, Williams R. Use and validation of selection criteria for liver transplantation in acute liver failure. Liver Transpl 2000;6:170-173.
8 Mitzner SR, Stange J, Klammt S, Peszynski P, Schmidt R, Nöldge-Schomburg G. Extracorporeal detoxification using the molecular adsorbent recirculating system for critically ill patients with liver failure. J Am Soc Nephrol 2001;12:S75-82.
9 Murphy EJ, Davern TJ, Shakil AO, Shick L, Masharani U, Chow H, et al. Troglitazone-induced fulminant hepatic failure. Acute Liver Failure Study Group. Dig Dis Sci 2000;45:549-553.
10 Vaquero J, Chung C, Cahill ME, Blei AT. Pathogenesis of hepatic encephalopathy in acute liver failure. Semin Liver Dis 2003;23:259-269.
11 Cordoba J, Blei AT. Cerebral edema and intracranial pressure monitoring. Liver Transpl Surg 1995;1:187-194.
12 Detry O, De Roover A, Honore P, Meurisse M. Brain edema and intracranial hypertension in fulminant hepatic failure: pathophysiology and management. World J Gastroenterol 2006;12:7405-7412.
13 Blei AT. Monitoring cerebral blood flow: a useful clinical tool in acute liver failure? Liver Transpl 2005;11:1320-1322.
14 MacQuillan G. Predicting outcome in acute liver failure: are we there yet? Liver Transpl 2007;13:1209-1211.
15 Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet 2010;376:190-201.
16 Mahtab MA, Rahman S, Khan M, Mamun AA, Afroz S. Etiology of fulminant hepatic failure: experience from a tertiary hospital in Bangladesh. Hepatobiliary Pancreat Dis Int 2008;7:161-164.
17 Lee WM. Acute liver failure in the United States. Semin Liver Dis 2003;23:217-226.
18 Wu CX, Zou Q, Zhu ZY, Gao YT, Wang YJ. Intrahepatic transplantation of hepatic oval cells for fulminant hepatic failure in rats. World J Gastroenterol 2009;15:1506-1511.
19 Yagi H, Parekkadan B, Suganuma K, Soto-Gutierrez A, Tompkins RG, Tilles AW, et al. Long-term superior performance of a stem cell/hepatocyte device for the treatment of acute liver failure. Tissue Eng Part A 2009;15: 3377-3388.
20 Pareja E, Cortes M, Bonora A, Fuset P, Orbis F, Lopez R, et al. New alternatives to the treatment of acute liver failure. Transplant Proc 2010;42:2959-2961.
21 Kjaergard LL, Liu J, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure: a systematic review. JAMA 2003;289:217-222.
22 Saliba F, Ichai P, Azoulay D, Habbouchi H, Antonini T, Sebagh M, et al. Successful long-term outcome of ABO-incompatible liver transplantation using antigen-specific immunoadsorption columns. Ther Apher Dial 2010;14:116-123.
23 Farges O, Kalil AN, Samuel D, Saliba F, Arulnaden JL, Debat P, et al. The use of ABO-incompatible grafts in liver transplantation: a life-saving procedure in highly selected patients. Transplantation 1995;59:1124-1133.
Received September 30, 2010
Accepted after revision April 18, 2011
Author Affiliations: Department of Hepatobiliary Surgery (Shi XJ, Xu HB, Ji WB, Liang YR, Duan WD, Heland Zhao ZM), and Department of Anesthesiology (Wang MJ), General Hospital of PLA, Beijing 100853, China
Xian-Jie Shi, MD, Department of Hepatobiliary Surgery, General Hospital of PLA, Beijing 100853, China (Tel: 86-10-68241383; Email: shixianjie301@yahoo.com.cn)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Risk factors of severe ischemic biliary complications after liver transplantation
- Health-related quality of life in living liver donors after transplantation
- Surgical treatment of Budd-Chiari syndrome: analysis of 221 cases
- Combined invagination and duct-to-mucosa techniques with modifications: a new method of pancreaticojejunal anastomosis
- Large regenerative nodules in a patient with Budd-Chiari syndrome after TIPS positioning while on the liver transplantation list diagnosed by Gd-EOB-DTPA MRI
- One hundred and seventy-eight consecutive pancreatoduodenectomies without mortality: role of the multidisciplinary approach
