Risk factors of severe ischemic biliary complications after liver transplantation
2011-07-05MingFengWangZhongKuiJinDaZhiChenXianLiangLiXinZhaoandHuaFan
Ming-Feng Wang, Zhong-Kui Jin, Da-Zhi Chen, Xian-Liang Li, Xin Zhao and Hua Fan
Beijing, China
Risk factors of severe ischemic biliary complications after liver transplantation
Ming-Feng Wang, Zhong-Kui Jin, Da-Zhi Chen, Xian-Liang Li, Xin Zhao and Hua Fan
Beijing, China
BACKGROUND:Ischemia-related biliary tract complications remain high after orthotopic liver transplantation. Severe ischemic biliary complications often involve the hepatic duct bifurcation and left hepatic duct, resulting finally in obstructive jaundice. Prevention and management of such complications remain a challenge for transplant surgeons.
METHODS:All 160 patients were followed up for at least 180 days after transplantation. One-way analysis of variance (ANOVA) and comparative univariate analysis were made using 3 groups (no complications; mild complications; severe complications), to analyze risk factors associated with biliary complications. Multiple logistic regression and linear regression analysis were used to analyze independent risk factors for severe ischemic biliary complications, after excluding other confounding factors.
RESULTS:By ANOVA and comparative univariate analysis, the risk factors associated with biliary complications were preoperative bilirubin level (P=0.007) and T-tube stenting of the anastomosis (P=0.016). Multiple logistic regression analysis showed that the use of T-tube and preoperative serum bilirubin were not independent risk factors for severe ischemic biliary complications after orthotopic liver transplantation. Chi-square analysis indicated that in the incidence of severe ischemic biliary lesions, bile duct second warm ischemic time longer than 60 minutes was a significant risk factor. Linear regression demonstrated a negative correlation between cold preservation time and warm ischemia time.
CONCLUSIONS:Preoperative serum bilirubin level and the use of T-tube stenting of the anastomosis were independent risk factors for biliary complications after liver transplantation, but not for severe ischemic biliary complications. The second warm ischemia time of bile duct longer than 60minutes and prolonged bile duct second warm ischemia time combined with cold preservation time were significant risk factors for severe ischemic biliary complications after liver transplantation with grafts from non-heart-beating donors.
(Hepatobiliary Pancreat Dis Int 2011; 10: 374-379)
liver transplantation; ischemic biliary complications; warm ischemia; cold preservation
Introduction
Orthotopic liver transplantation (OLT), first performed by Starzl in 1963, is now considered a standard operation for patients with acute liver failure and end-stage chronic liver disease.[1]Despite advances in organ preservation, surgical technique, immunosuppressive agents, and management of complications, ischemia-related biliary tract complications remain high after OLT and are the "Achilles's heel" of liver transplantation.[2]It was reported that an overall biliary complication rate is maintained at about 20.7%, including a bile leakage rate of 7.1% and an anastomotic stricture rate of 16.2%.[3,4]But reports on severe ischemic biliary complications are rare. Clavien's system, introduced in 1992, is the internationally validated classification system for describing the outcome of living donor liver grafts.[5]A modified version of the Clavien classification was presented in 2004 and is now used widely.[6]Tamura et al[7]believe that this classification should be considered whenever surgical complications of living donor liver grafts are discussed. Severe ischemic biliary complications occur when a port of the bile duct, or the bile duct in its entirety, is damaged by ishemic injury.[3]The damaged bile duct eventually atrophies, and necrosis and fibrotic stricture consequently involves the hepatic duct bifurcation as well as the left hepatic duct, finally resulting in obstructive jaundice.[8]Nevertheless, the prevention and management of ischemia-related bile duct complications remains a challenge for transplant surgeons.
In this study, we retrospectively analyzed 160 consecutive cases of OLT with grafts from non-heartbeating donors at our hospital from July 2000 to June 2009. We aimed to clarify the risk factors for severe ischemic biliary complications, and to investigate the roles of prolonged cold ischemia time (CIT) and warm ischemia time (WIT) in predicting the occurrence of these complications.
Methods
Patients
Patients underwent OLT from non-heart-beating donors at our hospital from July 2000 to June 2009. Patients undergoing combined liver-kidney transplantation, transplantation with ABO blood type incompatible grafts, and second liver transplants were excluded. Pretransplantation diagnoses of all patients are listed in Table 1. The patients were divided into 3 groups (no complications; mild complications; severe complications) (Table 2).
Other non-ischemic factors that induce biliary complications after OLT were excluded by linear regression analysis, for example, anastomotic stricture caused by operation or removal of T-tube; biliary strictures due to bile duct circuitry, bile leakage, bacterial,and viral infections (especially cytomegalovirus); arterial vascular stenosis or thrombosis confirmed by ultrasound or CT scan; acute and chronic rejection on liver biopsy; hepatotoxic drugs; and recurrent viral or cholestatic disease.
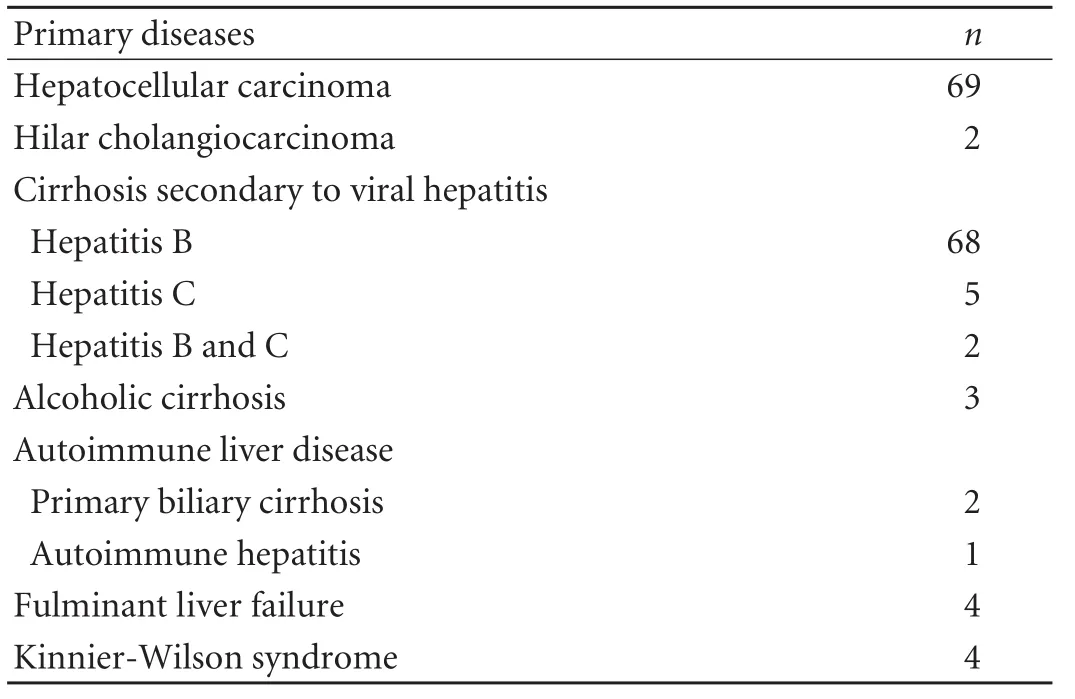
Table 1. Pre-transplantation diagnosis
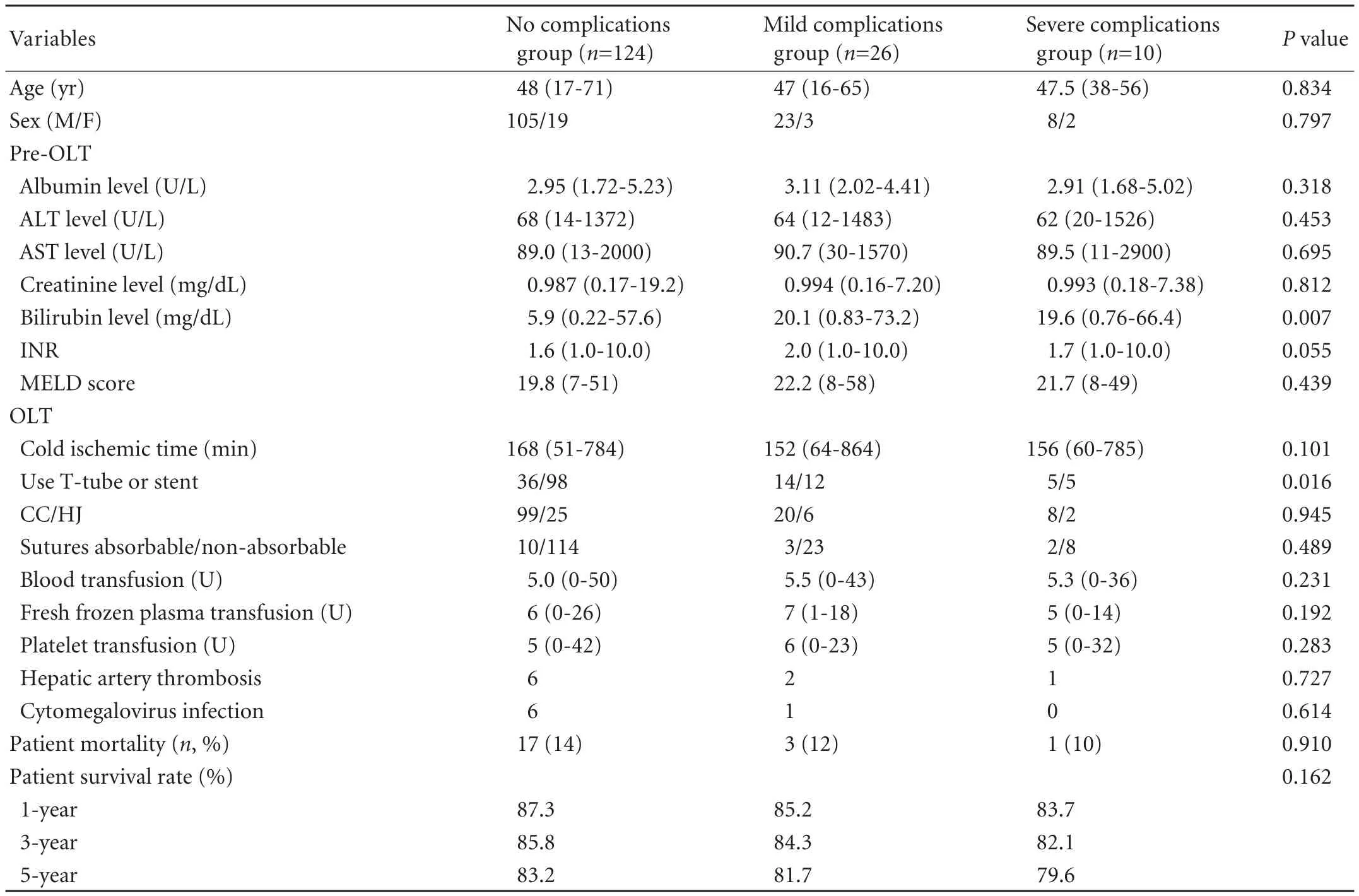
Table 2. Univariate analysis in the three groups
Operative techniques
All grafts were obtained from non-heart-beating donors. Donor and recipient blood types were compatible. Donor age was less than 50 years, without history of liver disease, evidence of malignancy, or liver steatosis. The combined liver and kidney allograft was harvested after perfusion of the abdominal aorta and superior mesenteric vein (SMV) with preservation solution. The abdominal aorta was flushed with 3000 mL of kidney preservation solution followed by 500 mL of University of Wisconsin (UW) solution. The SMV was flushed with 2000 mL of HTK (histidinetryptophan-ketoglutarate) followed by 1500 mL of UW solution. The bile duct was flushed with 250 mL of normal saline. Liver allografts were preserved in UW solution (0-4 ℃) after harvesting, and flushed again with HTK solution on the back table.
Liver allografts were implanted by either a cavareplacing or piggyback technique. The portal vein and arterial reconstructions were usually performed via endto-end anastomosis. After vascular unclamping, allografts were reperfused first via the portal vein, followed by the hepatic artery. Either choledochojejunostomy (endto-side) or physiologic choledochocholedochostomy (end-to-end) were performed. The posterior bile duct wall was sutured continuously with 6-0 polypropylene or polydioxanone. The anterior bile duct wall was reconstructed with interrupted sutures. A T-tube placed as a stent was used at the beginning of this program and then rarely used after March 2005.
Immunosuppression regimens
Anti-interleukin 2 (IL-2) receptor antibodies (basiliximab) and methylprednisolone were administered for perioperative induction therapy. Maintenance therapy consisted of a triple drug regimen with either cyclosporine or tacrolimus (FK506), mycophenolate mofetil, and corticosteroids. A second dose of basiliximab was used to prevent rejection on postoperative day 4.
Diagnosis of biliary complications
Biliary complications were defined as an increase in liver function analysis with confirmed cholangiographic (endoscopic or percutaneous) or surgical evidence of an anastomotic stricture, leak, nonanastomotic biliary stricture (often associated with biliary casts), obstructing tumor, stones, or papillary stenosis.
According to the Clavien classification, severe biliary complications after OLT are defined as: grade III, complication with the need for surgical, endoscopic or radiological intervention (IIIa/b: without/with the need for general anesthesia); grade IV, life-threatening complication requiring intensive care; grade V, death.[6]
Patients treated empirically with biliary stents without clear cholangiographic evidence of a biliary problem were excluded. Papillary stenosis was identified in those with abnormal liver test results and a significantly dilated, congruent donor and recipient bile duct with no anastomotic narrowing and nor parenchymal or vascular explanation for the biochemical abnormalities.[9]Several radiographic imaging approaches were used to diagnose biliary complications,[10]such as magnetic resonance cholangiopancreaticography (MRCP), endoscopic retrograde cholangiopancreatography, multidetector computed tomography, percutaneous transhepatic cholangiography, or ultrasound. MRCP was the most useful method to assess biliary complications after OLT.[11]The serum levels of total bilirubin, direct bilirubin, gammaglutamyltranspeptidase, alkaline phosphatase, alanine aminotransferase, and aspartate transaminase were used to estimate allograft and bile duct injury. Percutaneous image-guided fine needle aspiration was used when necessary.
Statistical analysis
Comparative univariate analysis was made using 3 groups. One-way analysis of variance (ANOVA) was used to compare continuous variables among the three groups, and the Chi-square test was used to assess the differences among groups for categorical variables. Multiple logistic regression and linear regression analysis were used to analyze the independent risk factors for severe ischemic biliary complications, after excluding other confounding factors. Statistical analysis was carried out using SPSS software (version 13.0; SPSS Inc., Chicago, IL, USA). A P value less than 0.05 was considered statistically significant.
Results
Data from 160 patients (136 male, 24 female; median age 47.1±10.3, range 17-71 years), who had been followed up for at least 180 days after OLT, were retrospectively analyzed. Eighteen transplants were performed with a cava-replacing technique, and the modified piggybacktechnique was used in 142 patients. The portal vein and arterial reconstruction was done routinely by end-to-end anastomosis. The details of the arterial reconstruction were as follows: the donor proper hepatic artery to the recipient common hepatic artery in 145 patients; the donor proper hepatic artery to the recipient common hepatic artery at the level of the splenic artery in 3; and an interposition to the recipient abdominal aorta in 12. For bile duct reconstruction, end-to-side choledochojejunostomy was performed in 7 patients and physiologic end-to-end choledochocholedochostomy was performed in 153. A T-tube was placed as a stent in 69 recipients at the beginning of this program, and then rarely used after 2005.
The overall biliary complication rate was 22.5% (36/160), including severe ischemic biliary complications in 6.25% (10/160). Non-ischemic factors inducing biliary complications were as follows: bile leakage 6.88% (11/160), biliary strictures 8.13% (13/160), and bacterial and viral infections 1.25% (2/160). By ANOVA and univariate analysis, the risk factors associated with biliary complications were preoperative serum bilirubin (P=0.007) and use of a T-tube (P=0.016) (Table 2). Multiple logistic regression analysis demonstrated that age, sex, Child-Pugh classification, operation duration, volume of blood loss, use of a T-tube, and preoperative serum bilirubin level were not independent risk factors for the development of severe ischemic biliary complications in OLT.
There were 16 patients with biliary complications due to ischemia/reperfusion injury: bile duct stones or sludge (5), nonanastomotic extrahepatic bile duct strictures (6), diffuse intrahepatic duct stenosis (5), and combined intrahepatic and extrahepatic bile duct strictures (2). Ten patients with primary graft dysfunction or nonfunction due to biliary ischemic injury were documented. Among them, there were 6 patients with postoperative graft nonfunction caused by nonanastomotic extrahepatic/intrahepatic bile duct strictures; 2 died and 4 underwent retransplantation. Four patients with primary graft dysfunction recovered after multi-interventional therapy. According to Clavien's system, severe ischemic biliary complication was defined in 6 patients (3.75%) as grade IIIa to IIIb, in 2 (1.25%) as grade IV, and in 2 (1.25%) as grade V.
The incidence of severe ischemic biliary lesions was 23.5% (8/34) if the second WIT of the bile duct was longer than 60 minutes, which was significantly decreased to 1.6% (2/126) if the time was less than 60 minutes. The Chi-square test also indicated that the incidence of severe ischemic biliary lesions was significantly increased when the second WIT of the bileduct was significantly longer than 60 minutes (Table 3).
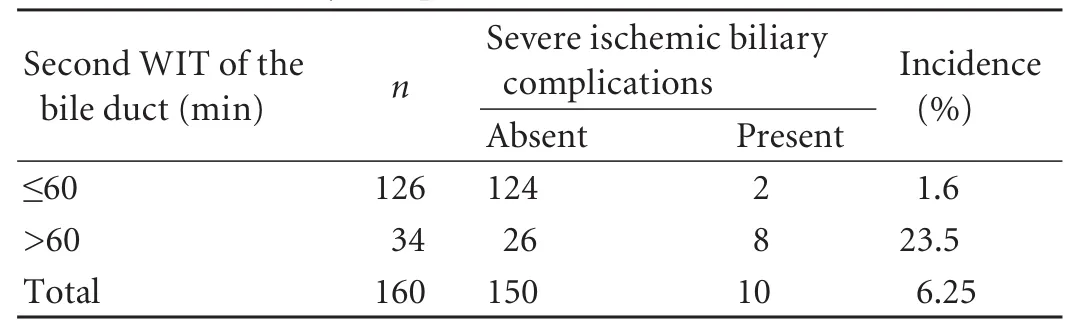
Table 3. Severe biliary complications and second WIT of the bile duct
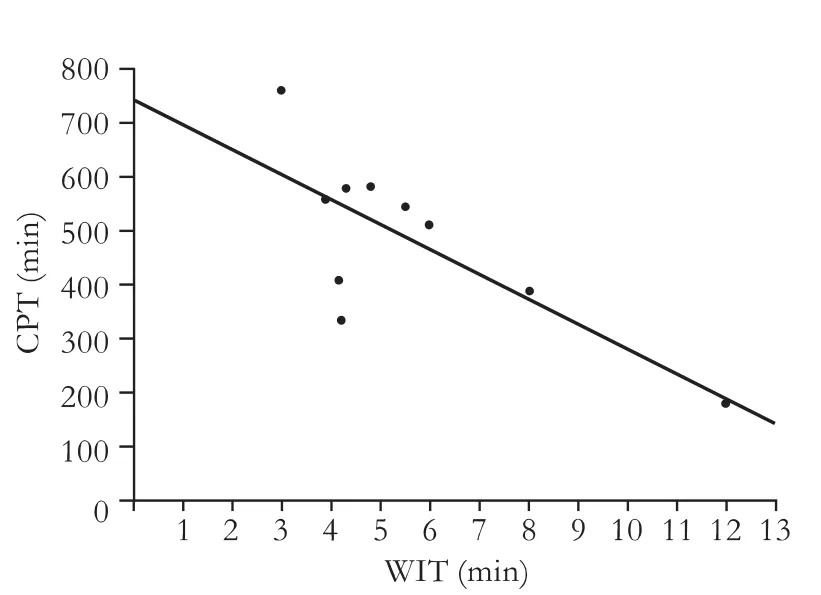
Fig. Linear regression showing a negative correlation between CPT and WIT. CPT=743.891-46.265×WIT; CPT: cold preservation time; WIT: warm ischemia time; F=10.608, P=0.012.
Donor WIT was 5.58±2.64 minutes (range 3-12), cold preservation time (CPT) was 485.50±162.03 minutes (range 180-762), and second WIT of the bile duct was 47.20±25.23 minutes (range 15-150). Logistic regression analysis identified a prolonged second WIT of the bile duct as a risk factor for severe ischemic biliary complications; if the second WIT of the bile duct was longer than 60 minutes, the effect was significant.
Other than the prolonged second WIT of the bile duct, the combination of prolonged WIT and graft CPT significantly increased the incidence of severe biliary complications. The equation used for linear regression analysis was: CPT=743.891-46.265×WIT, indicating a prolonged bile duct WIT combined with CPT significantly increased with regard to the incidence of severe biliary complications (Fig.).
Discussion
The biological characteristics and clinical pathology of biliary tract epithelial cells, especially their correlation with postoperative biliary complications, have been well studied.[12]Biliary strictures are the most frequently reported complications in the late postoperative period, with retransplantation and mortality rates of 37.5% and 31.2%, respectively.[13]Although multiple factors wereassociated with nonanastomotic bile duct strictures in our study, donor CPT and WIT were shown to be major causes. In our series, there were 34 cases of biliary tract complications caused by second warm ischemia of the bile duct mostly in the early phase of this program, and then significantly decreased after 2005. The results indicated that the experience and technical skills of the transplant surgeon were important to prevent the second warm ischemia injury of the bile duct. In several other series, ischemic biliary lesions occurred in up to 2%-19% of patients. Bile duct injury and stenosis resulted in damage to the duct and graft loss.[14]
Biliary tract epithelial cells are more sensitive to ischemia/reperfusion damage than hepatocytes; furthermore, the composition of bile might also damage the biliary tree in these patients.[15]A retrospective analysis of the incidence of severe ischemic biliary complications showed that WIT was the main cause, when comparing non-heart-beating and brain-dead donor groups.[16]Foley et al[17]also found that CIT longer than 8 hours was the strongest predictor for the development of ischemic cholangiopathy. The mechanism of biliary strictures and necrosis may be correlated with direct injury of bile duct epithelial cells by ischemic preservation or indirect injury caused by microvascular destruction. In our study, the combination of prolonged WIT and CPT was harmful to allografts and significantly increased the incidence of severe biliary complications when analyzed by linear regression.
Based on the linear equation, we estimated relatively safe limits of the preservation time. The equation used for linear regression analysis was reliable when WIT remained between 3 and 12 minutes, and if the WIT was longer than 12 minutes, verification was necessary.
The bile duct second WIT was defined as an interval between the time of portal vein unclamping and hepatic artery reperfusion, which significantly increased the incidence of severe ischemic biliary complications. Fisher and Miller[2]reported that after portal vein reperfusion, extensive microthrombi formed in hepatic arteries because of blood regression during hepatic artery reconstruction, resulting in bile duct ischemic strictures. How to prevent the biliary tract ischemia complication deserves further study. In rabbit experiments, we found that transfusion with autologous bone marrow mononuclear cells promotes neovascularization and improves the blood supply to the ischemic bile duct. This method provides a new way to diminish or prevent ischemic type biliary lesions after liver transplantation but is far from use in clinical situations.[18]Swine transplantation experiments have demonstrated that reperfusion injury can be prevented by earlier arterial reperfusion (hepatic artery 20 minutes before portal vein).[19]Others have proposed that unclamping and reperfusion of the portal vein and hepatic artery should be done simultaneously. However, according to a prospective study by Polak et al, simultaneous unclamping and reperfusion of the portal vein and hepatic artery, compared to a sequential procedure, does not prevent ischemic-type biliary lesions.[20]Furthermore, the incidence of anastomotic strictures is higher in the former.
Busuttil et al[21]reported that a bile duct WIT longer than 55 minutes is an independent predictor of graft function. They also claimed that the second WIT of longer than 60 minutes is an independent risk factor for severe biliary complications, but with no obvious synergistic effect between WIT and CIT. In our series, when the second WIT was less than 60 minutes, and there was no difference in the incidence of severe ischemia biliary complications. Possible reasons may be: 1) after portal vein unclamping, ischemic biliary injury was relieved by perfusion and reoxygenation; 2) recipient hypocoaguability in early reperfusion reduced the chance of microthrombi formation in biliary arterioles, with bile duct blood supply and oxygen recovery not influenced by hepatic artery reperfusion; and 3) in 99.38% of patients, CPT was less than 12 hours (159/160). Although it is less of a risk, the synergistic effect of CPT and the second WIT might deserve more attention in relation to severe biliary complications and needs to be confirmed by further investigation.
In conclusion, we demonstrated a direct correlation between severe ischemic biliary complications and the combination of prolonged WIT and CPT, which could be significantly influenced by the skill and experience of transplant surgeons. This conclusion is based on a single-center experience, so more clinical observations would be helpful to verify this conclusion.
Funding:None.
Ethical approval:Not needed.
Contributors:CDZ proposed the study. WMF and CDZ wrote the first draft. ZX analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. CDZ is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Starzl TE, Marchiore TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet 1963;117:659-676.
2 Fisher A, Miller CH. Ischemic-type biliary strictures in liver allografts: the Achilles heel revisited? Hepatology 1995;21: 589-591.
3 Qian YB, Liu CL, Lo CM, Fan ST. Risk factors for biliary complications after liver transplantation. Arch Surg 2004; 139:1101-1105.
4 Němec P, Ondrásek J, Studeník P, Hökl J, Cerny J. Biliary complications in liver transplantation. Ann Transplant 2001; 6:24-28.
5 Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 1992;111:518-526.
6 Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-213.
7 Tamura S, Sugawara Y, Kaneko J, Yamashiki N, Kishi Y, Matsui Y, et al. Systematic grading of surgical complications in live liver donors according to Clavien's system. Transpl Int 2006;19:982-987.
8 Yuan D, Wei YG, Lin HM, Li FQ, Yang M, Liu XL, et al. Risk factors of biliary complications following liver transplantation: retrospective analysis of a single centre. Postgrad Med J 2009;85:119-123.
9 Buxbaum JL, Biggins SW, Bagatelos KC, Ostroff JW. Predictors of endoscopic treatment outcomes in the management of biliary problems after liver transplantation at a high-volume academic center. Gastrointest Endosc 2011;73: 37-44.
10 Caiado AH, Blasbalg R, Marcelino AS, da Cunha Pinho M, Chammas MC, da Costa Leite C, et al. Complications of liver transplantation: multimodality imaging approach. Radiographics 2007;27:1401-1417.
11 Valls C, Alba E, Cruz M, Figueras J, Andía E, Sanchez A, et al. Biliary complications after liver transplantation: diagnosis with MR cholangiopancreatography. AJR Am J Roentgenol 2005;184:812-820.
12 Huang ZQ. Bile duct anatomical physiology and biliary complications after liver transplantation. Zhonghua Wai Ke Za Zhi 2006;44:289-291.
13 Nakamura N, Nishida S, Neff GR, Vaidya A, Levi DM, Kato T, et al. Intrahepatic biliary strictures without hepatic artery thrombosis after liver transplantation: an analysis of 1,113 liver transplantations at a single center. Transplantation 2005;79:427-432.
14 Abou-Rebyeh H, Veltzke-Schlieker W, Radke C, Steinmüller T, Wiedenmann B, Hintze RE. Complete bile duct sequestration after liver transplantation, caused by ischemic-type biliary lesions. Endoscopy 2003;35:616-620.
15 Pirenne J, Monbaliu D, Aerts R, Desschans B, Liu Q, Cassiman D, et al. Biliary strictures after liver transplantation: risk factors and prevention by donor treatment with epoprostenol. Transplant Proc 2009;41:3399-3402.
16 Abt P, Crawford M, Desai N, Markmann J, Olthoff K, Shaked A. Liver transplantation from controlled non-heart-beating donors: an increased incidence of biliary complications. Transplantation 2003;75:1659-1663.
17 Foley DP, Fernandez LA, Leverson G, Anderson M, Mezrich J, Sollinger HW, et al. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann Surg 2011;253:817-825.
18 Qu ZW, Chen DZ, Sheng QS, Lang R, He Q, Wang MF. Preventive effects of autologous bone marrow mononuclear cell implantation on intrahepatic ischemic-type biliary lesion in rabbits. Hepatobiliary Pancreat Dis Int 2010;9:593-599.
19 van As AB, Lotz Z, Tyler M, Kahn D. Effect of early arterialization of the porcine liver allograft on reperfusion injury, hepatocellular injury, and endothelial cell dysfunction. Liver Transpl 2001;7:32-37.
20 Polak WG, Miyamoto S, Nemes BA, Peeters PM, de Jong KP, Porte RJ, et al. Sequential and simultaneous revascularization in adult orthotopic piggyback liver transplantation. Liver Transpl 2005;11:934-940.
21 Busuttil RW, Farmer DG, Yersiz H, Hiatt JR, McDiarmid SV, Goldstein LI, et al. Analysis of long-term outcomes of 3200 liver transplantations over two decades: a single-center experience. Ann Surg 2005;241:905-918.
Received March 11, 2011
Accepted after revision May 25, 2011
Author Affiliations: Department of Hepatobiliary and Pancreaticosplenic Surgery, Beijing Chaoyang Hospital, Capital Medical University, Beijing 100020, China (Wang MF, Jin ZK, Chen DZ, Li XL, Zhao X and Fan H)
Da-Zhi Chen, Professor, Department of Hepatobiliary and Pancreaticosplenic Surgery, Beijing Chaoyang Hospital, Capital Medical University, Beijing 100020, China (Tel: 86-10-85231504; Fax: 86-10-87502615; Email: chendazhi@medmail.com.cn)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Prognostic role of diabetes mellitus in hepatocellular carcinoma patients after curative treatments: a meta-analysis
- Health-related quality of life in living liver donors after transplantation
- Surgical treatment of Budd-Chiari syndrome: analysis of 221 cases
- Efficacy of liver transplantation for acute hepatic failure: a single-center experience
- Combined invagination and duct-to-mucosa techniques with modifications: a new method of pancreaticojejunal anastomosis
- Large regenerative nodules in a patient with Budd-Chiari syndrome after TIPS positioning while on the liver transplantation list diagnosed by Gd-EOB-DTPA MRI
