Long-term survival of a patient after resection of a gastrointestinal stromal tumor arising from the pancreas
2011-07-03FilipkaBohumilJonAlexanderFerkoZdenubrtDimitarNikolovandraTyov
Filip Čečka, Bohumil Jon, Alexander Ferko, Zdeněk Šubrt, Dimitar H Nikolov and Věra Tyčová
Hradec Králové, Czech Republic
Long-term survival of a patient after resection of a gastrointestinal stromal tumor arising from the pancreas
Filip Čečka, Bohumil Jon, Alexander Ferko, Zdeněk Šubrt, Dimitar H Nikolov and Věra Tyčová
Hradec Králové, Czech Republic
BACKGROUND:Gastrointestinal stromal tumors (GISTs) may arise in any part of the gastrointestinal tract; extragastrointestinal locations are extremely rare. Only a few cases of extragastrointestinal stromal tumor arising from the pancreas were reported. None of the reports described a long-term follow-up of the patients.
METHOD:This report describes an interesting and unusual case of GIST arising from the pancreas.
RESULTS:A 74-year-old female presented with a palpable abdominal mass. CT scan showed a large mass 11×8×4 cm originating from the tail of the pancreas. Percutaneous biopsy revealed a GIST predominantly with spindle cells, but some parts also contained epitheloid cells. The patient was treated by distal pancreatic resection with splenectomy. Immunohistochemistry of the tumor showed a staining pattern characteristic of GIST. The patient has achieved a long-term survival of five years and six months without any sign of recurrence of the disease.
CONCLUSION:This is the first reported case of an extragastrointestinal stromal tumor arising from the pancreas treated surgically, with a long-term survival.
(Hepatobiliary Pancreat Dis Int 2011; 10: 330-332)
gastrointestinal stromal tumor; pancreas; survival
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract.[1]They may arise in any part of the gastrointestinal tract, from the esophagus to anus. However, the stomach and small intestine are by far the most common sites. Mesenchymal tumors arising from extragastrointestinal locations (extragastrointestinal stromal tumors, EGISTs) with similar clinicopathological and molecular profiles have been reported. The authors report an extremely rare case of EGIST arising from the pancreas.
Case report
A 74-year-old woman was referred to our department for the treatment of a tumor in the pancreas. The patient was examined for an abdominal mass in the left mesogastrium. She had no other symptoms, bowel movements and appetite were normal, there was no weight loss, and the patient did not complain of abdominal pain. Physical examination showed a palpable mass in the left mesogastrium, with no tenderness. Routine laboratory tests were normal, and CA19-9 was also normal.
Ultrasound and CT scans revealed a large mass 11×8×4 cm originating from the tail of the pancreas. According to the CT scan (Fig. 1), the tumor infiltrated the splenic artery and vein and contacted the greater curvature of the stomach. The upper mesenteric vein and artery were intact. No other pathology was found on imaging. The findings were not typical for primary pancreatic tumor, and therefore percutaneous biopsy was performed without complications. The biopsy revealed a GIST predominantly with spindle cells, but some parts also contained epitheloid cells. Mitotic activity of the tumor could not be evaluated due to the small amount of examined material. Immunohistochemistry showeda staining pattern characteristic of GIST. C-Kit (CD117) and CD34 were positive, and S100 protein and smoothmuscle actin were negative.
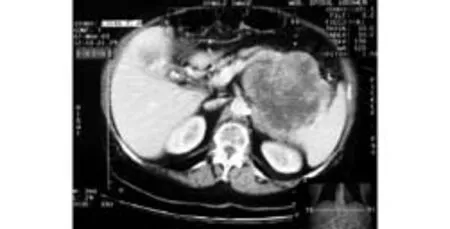
Fig. 1. Enhanced CT scan showing a large tumor arising from the tail of the pancreas.

Fig. 2. Histological examination of the surgical specimen: predominantly spindle cell type of GIST. The mitotic count was less than 5 mitoses per 50 HPFs (Hematoxylin-eosin staining, original magnification ×40).
Surgical resection of the tumor was perfomed with a bilateral subcostal incision. The lesser sac was opened through division of the gastrocolic ligament. The tumor was in the tail of the pancreas, infiltrating the greater curvature of the stomach. First, splenectomy was performed, with the tumor dissected from the retroperitoneum and the greater curvature of the stomach. Afterwards, the pancreas was transected in the tail and the specimen was removed. The postoperative course was uneventful and the patient was discharged on the 14th postoperative day. The final histological examination revealed an EGIST (Fig. 2). The mitotic count was less than 5 mitoses per 50 high power fields (HPFs). The tumor was evaluated as high risk because the size exceeded 10 cm. No adjuvant therapy was given to the patient, who was followed up in the surgical department regularly. Now she is alive and doing well 5 years and 6 months after the operation, with no evidence of recurrence of the disease.
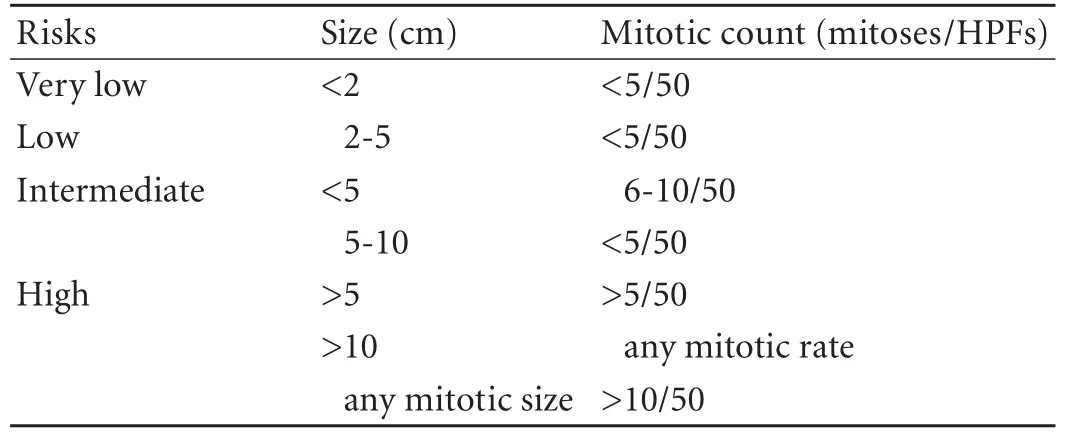
Table. Proposed approach for defining risk of aggressive behavior in GISTs
Discussion
The term gastrointestinal stromal tumor is now used for a group of specific mesenchymal tumors which arise from the pacemaker cells or interstitial cells of Cajal (ICCs). These cells are located in the muscle layer of the gastrointestinal tract. GISTs can occur anywhere in the gastrointestinal tract; 50%-60% of GISTs arise in the stomach, 20%-30% in the small bowel, 10% in the large bowel, 5% in the esophagus, and 5% elsewhere in the abdominal cavity.[1]The overall incidence of the tumor has been estimated for 10-20 cases per million per year.
Histologically, most GISTs have a remarkably uniform appearance and fall into one of three categories: spindle cell type (70%), epitheloid cell type (20%), or mixed type.[1]Regarding immunohistochemistry, almost all GISTs express the CD117 antigen, part of the Kit receptor tyrosine kinase, and this is therefore considered the defining feature. Aside from the positivity for Kit, 60%-70% of GISTs show an immunopositivity for CD34, 30%-40% for smooth-muscle actin, and around 5% for S100 protein.
Clinically, there are no precise symptoms for stromal tumors of the pancreas. If the tumors become large enough, they may present as a palpable abdominal mass,[2,3]as seen in our patient. Various symptoms involving the upper abdomen may appear, such as nausea and vomiting.[4]The tumor may even be incidentally found.[5]GISTs exhibit a broad spectrum of clinical behaviors, with some low-risk lesions remaining stable for years, while others progress rapidly to widely metastatic diseases. There has been extensive study on GIST to determine which factors predict the prognosis. A risk category assignment, which was proposed by Fletcher and has been widely accepted, has standardized prediction of the behavior of GIST. Suggested definitions for the risk categories are shown in Table.[1]The risk category is assigned using both tumor size and mitotic rate per 50 HPFs. If size alone is used, a wrong risk category can be assigned.
Before 2001, surgery was the only effective treatmentfor GISTs.[6]The five-year survival rates of patients with such a tumor ranged from 28% to 80%.[7]The patients were benefited a little from chemotherapy or radiation therapy. Dramatic improvement in GIST management occurred with the recognition that mutational activation of Kit stimulated the growth of GIST cells. This led to effective systemic therapies using small molecule inhibitors such as imatinib mesylate (Glivec, Novartis). Recent studies suggest that the prognosis for unresectable and/or metastatic GIST is poor, because imatinib alone does not provide a complete or longterm response. Therefore, surgical resection is advocated when feasible, even for metastasizing processes, because it is presently the only chance for cure.[6,8]Systematic lymph node dissection is not thought to be necessary, as lymphatic dissemination is rare. The overall survival appears to be related to radical surgical resection. Therefore, every effort should be made to achieve a complete resection with clear margins in some cases necessitating the resection of adjacent organs.
GISTs arising from outside the gut wall are termed extragastrointestinal stromal tumors (EGISTs). They are reported to be very rare. Isolated cases of EGISTs have also been reported at unusual sites such as the gallbladder,[9,10]omentum,[5]or liver.[11]EGISTs arising from the pancreas are extremely rare, and only a few cases have been reported.[2-5,12-14]However, no report has described the long-term survival of a patient with EGIST arising from the pancreas.
GISTs are believed to arise from ICCs because they share many characteristics with these cells, including strong expression of CD117 and CD34.[15]The pathogenesis of EGISTs remains controversial. Some researches believe that GISTs and EGISTs arise from a common precursor cell of ICCs and smooth muscle cells of the gut, which may account for their growth within and outside the gastrointestinal tract. On the other hand, others have proposed a simpler explanation suggesting that EGISTs are in fact mural GISTs with extensive extramural growth, resulting in eventual loss of their connection with the gut wall.
In conclusion, we report a rare case of an EGIST arising from the pancreas. The patient was treated surgically. Even though the tumor was evaluated as high risk because the size exceeded 10 cm, the patient achieved a long-term survival of five years and six months without any sign of tumor recurrence.
Funding: None.
Ethical approval:Not needed.
Contributors:ČF wrote the first draft of this commentary. All authors contributed to the intellectual context and approved the final version. ČF is the guarantor.
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-465.
2 Daum O, Klecka J, Ferda J, Treska V, Vanecek T, Sima R, et al. Gastrointestinal stromal tumor of the pancreas: case report with documentation of KIT gene mutation. Virchows Arch 2005;446:470-472.
3 Yamaura K, Kato K, Miyazawa M, Haba Y, Muramatsu A, Miyata K, et al. Stromal tumor of the pancreas with expression of c-kit protein: report of a case. J Gastroenterol Hepatol 2004; 19:467-470.
4 Yan BM, Pai RK, Van Dam J. Diagnosis of pancreatic gastrointestinal stromal tumor by EUS guided FNA. JOP 2008; 9:192-196.
5 Goh BK, Chow PK, Kesavan SM, Yap WM, Chung YF, Wong WK. A single-institution experience with eight CD117-positive primary extragastrointestinal stromal tumors: critical appraisal and a comparison with their gastrointestinal counterparts. J Gastrointest Surg 2009;13:1094-1098.
6 Pierie JP, Choudry U, Muzikansky A, Yeap BY, Souba WW, Ott MJ. The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Arch Surg 2001;136:383-389.
7 Pidhorecky I, Cheney RT, Kraybill WG, Gibbs JF. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol 2000;7:705-712.
8 Stratopoulos C, Soonawalla Z, Piris J, Friend PJ. Hepatopanc reatoduodenectomy for metastatic duodenal gastrointestinal stromal tumor. Hepatobiliary Pancreat Dis Int 2006;5:147-150.
9 Ortiz-Hidalgo C, de Leon Bojorge B, Albores-Saavedra J. Stromal tumor of the gallbladder with phenotype of interstitial cells of Cajal: a previously unrecognized neoplasm. Am J Surg Pathol 2000;24:1420-1423.
10 Park JK, Choi SH, Lee S, Min KO, Yun SS, Jeon HM. Malignant gastrointestinal stromal tumor of the gallbladder. J Korean Med Sci 2004;19:763-767.
11 Hu X, Forster J, Damjanov I. Primary malignant gastrointestinal stromal tumor of the liver. Arch Pathol Lab Med 2003;127:1606-1608.
12 Krska Z, Pesková M, Povysil C, Horejs J, Sedlácková E, Kudrnová Z. GIST of pancreas. Prague Med Rep 2005;106:201-208.
13 Showalter SL, Lloyd JM, Glassman DT, Berger AC. Extragastrointestinal stromal tumor of the pancreas: case report and a review of the literature. Arch Surg 2008;143:305-308.
14 Harindhanavudhi T, Tanawuttiwat T, Pyle J, Silva R. Extragastrointestinal stromal tumor presenting as hemorrhagic pancreatic cyst diagnosed by EUS-FNA. JOP 2009;10:189-191.
15 Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998;152:1259-1269.
Received December 16, 2009
Accepted after revision June 9, 2010
Author Affiliations: Department of Surgery (Čečka F, Jon B, Ferko A and Šubrt Z) and Fingerland Department of Pathology (Nikolov DH and Tyčová V), Faculty of Medicine and University Hospital Hradec Králové, Sokolská 581, 500 05 Hradec Králové, Czech Republic; Department of Field Surgery, Military Health Science Faculty, Hradec Králové, Defence University Brno, Třebešská 1575, 500 01 Hradec Králové, Czech Republic (Ferko A and Šubrt Z)
Filip Čečka, MD, Department of Surgery, Faculty of Medicine and University Hospital Hradec Králové, Sokolská 581, 500 05 Hradec Králové, Czech Republic (Tel: ++420-737-163931; Fax: ++420-495-832026, Email: filip.cecka@seznam.cz)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Emergency re-routing of anterior sector venous outflow for right lobe living donor liver transplantation including the middle hepatic vein
- Cytokine and apoptosis gene polymorphisms influence the outcome of hepatitis C virus infection
- Persistent port-site sinus in a patient after laparoscopic cholecystectomy: watch out for gallbladder tuberculosis
- Iron overload and HFE gene mutations in Polish patients with liver cirrhosis
- Predictive value and main determinants of abnormal features of intraoperative cholangiography during cholecystectomy
