Iron overload and HFE gene mutations in Polish patients with liver cirrhosis
2011-07-03KatarzynaSikorskaTomaszRomanowskiPiotrStalkeEwayckawieszewskaandKrzysztofPiotrBielawski
Katarzyna Sikorska, Tomasz Romanowski, Piotr Stalke, Ewa Iżycka-Świeszewska and Krzysztof Piotr Bielawski
Gdansk, Poland
Iron overload and HFE gene mutations in Polish patients with liver cirrhosis
Katarzyna Sikorska, Tomasz Romanowski, Piotr Stalke, Ewa Iżycka-Świeszewska and Krzysztof Piotr Bielawski
Gdansk, Poland
BACKGROUND:Increased liver iron stores may contribute to the progression of liver injury and fibrosis, and are associated with a higher risk of hepatocellular carcinoma development. Pre-transplant symptoms of iron overload in patients with liver cirrhosis are associated with higher risk of infectious and malignant complications in liver transplant recipients.HFEgene mutations may be involved in the pathogenesis of liver iron overload and influence the progression of chronic liver diseases of different origins. This study was designed to determine the prevalence of iron overload in relation toHFEgene mutations among Polish patients with liver cirrhosis.
METHODS:Sixty-one patients with liver cirrhosis included in the study were compared with a control group of 42 consecutive patients subjected to liver biopsy because of chronic liver diseases. Liver function tests and serum iron markers were assessed in both groups. All patients were screened forHFEmutations (C282Y, H63D, S65C). Thirty-six of 61 patients from the study group and all controls had liver biopsy performed with semiquantitative assessment of iron deposits in hepatocytes.
RESULTS:The biochemical markers of iron overload and iron deposits in the liver were detected with a higher frequency (70% and 47% respectively) in patients with liver cirrhosis. There were no differences in the prevalence of allHFEmutations in both groups. In patients with a diagnosis of hepatocellular carcinoma, no significant associations with iron disorders andHFEgene mutations were found.
CONCLUSIONS:Iron disorders were detected in patients with liver cirrhosis frequently but without significant association withHFEgene mutations. Only the homozygous C282Y mutation seems to occur more frequently in the selected population of patients with liver cirrhosis. As elevated biochemical iron indices accompanied liver iron deposits more frequently in liver cirrhosis compared to controls with chronic liver disease, there is a need for more extensive studies searching for the possible influence of non-HFEiron homeostasis regulators and their modulation on the course of chronic liver disease and liver cirrhosis.
(Hepatobiliary Pancreat Dis Int 2011; 10: 270-275)
liver cirrhosis; iron overload; gene mutations; iron deposits; hepatocytes
Introduction
The liver is the main store of iron and its overload is a factor triggering liver injury and progressive fibrosis through induction of oxidative stress. This leads to the development of chronic inflammation, cirrhosis and finally end-stage liver disease with organ failure or hepatocellular carcinoma (HCC).
Hereditary hemochromatosis (HH), one of the most frequently inherited metabolic diseases, occurs with a rate of 3 to 8 cases per 1000 among Caucasians.[1]HFE gene mutations, identified with type 1 HH, are responsible for changes of the HFE protein conformation and its cell surface expression, impairment of hepcidin expression (the key regulator of body iron turnover) and excessive iron accumulation.[2,3]In Europe, North America and Australia 60%-96% of HH cases are related to C282Y homozygosity and 5% to compound heterozygosity for C282Y and H63D polymorphism in theHFEgene.[4]In the caucasian population,HFEhomozygotes are detected with a high frequency (1∶200-1∶400) but phenotypic expression of HH with multiorgan iron overload islimited.[5]Based on meta-analyses of genetic association studies, a 4- to 11-fold risk of liver diseases including HCC, hepatitis C and non-alcoholic steatohepatitis has been shown in C282Y homozygotes.[6]
The clinical significance of iron metabolism disorders in different HFE genotypes (H63D, S65C) is unclear and the results of many studies are equivocal.[7-9]The role of mutations other than C282Y/C282Y or C282Y/H63D as an additional pathogenic factor that increases the risk of developing cirrhosis and liver cancer, in cases of concomitant hepatitis virus infections or alcohol abuse, is being explored.[10-14]
Secondary iron overload is observed in some chronic liver diseases: chronic hepatitis C, alcoholic liver disease, non-alcoholic fatty liver disease, and toxic liver injury.
HH is a rare indication for orthotopic liver transplantation (1%); however iron accumulation in the donor's liver increases the risk of fibrosis after transplantation which confirms the implication of iron metabolism disorders in post-transplantation failure.[15,16]
In many studies, the survival rate after liver transplantation for patients with HH compared with other cirrhotic patients was decreased due to cardiac complications, serious infectious and malignant complications with relapse of HCC and non-hepatic cancer development.[15-20]Susceptibility of liver transplant recipients with iron overload to serious fungal, bacterial and viral infections (aspergillosis, cryptococcosis, cytomegaly, infections with Staphylococcus aureus or Yersinia species) is explained by the special role that iron availability plays in the life cycle of pathogens and host defense.[21-24]
The purpose of this study was to determine the prevalence of iron overload in relation to HFE gene mutations among Polish patients with liver cirrhosis.
Methods
Sixty-one patients with liver cirrhosis admitted to the Department of Infectious Diseases, Medical University of Gdansk were included in the study. They were compared with a control group of 42 consecutive patients subjected to liver biopsy because of chronic liver diseases. The study protocol was approved by the Local Ethics Committee and informed consent for participation in it was given by all enrolled subjects.
The following biochemical factors were analyzed: blood morphology, alanine transaminase activity (ALT), serum concentrations of bilirubin, iron and ferritin, total iron binding capacity, and transferrin saturation. Elevated serum iron indices were defined as increased serum concentration of iron (above 28.3 μg/L), and transferrin saturation above 55%, and/or increased serum concentration of ferritin (men >300 ng/mL; women >200 ng/mL) according to clinical guidelines.[25]
The serological markers of HBV (HBsAg, HBcAb) and HCV (HCV-Ab) infections were measured in all subjects. In the positive cases of viral serology, the infection was confirmed by molecular assays according to the manufacturer's instructions.
Analysis ofHFEgene polymorphism
The genomic DNA from peripheral blood leukocytes was extracted by a High Pure PCR Template Preparation Kit (Roche Diagnostics) according to the manufacturer's instructions. The HFE mutations C282Y, H63D, and S65C were analyzed by PCR-RFLP assay.[26]The patients with homozygotic C282Y and combined heterozygotic C282Y/H63D HFE mutations were considered as a genetic manifestation of HH type 1.
Detection of iron deposits in liver specimens
Liver oligobiopsies (blind percutaneous needle biopsy, 1.4 mm in diameter) were done using the Menghini method in all controls and 36/61 cirrhotic patients. In 25/61 patients a biopsy was not done because of contraindications. The preparation of liver specimens was as previously described.[27]
Statistical analysis
Statistical analysis was carried out using STATISTICA version 8.0 (StatSoft Inc., USA). All statistical data are presented as a mean±standard error (SE) or median value. Analysis of differences between variations was done using nonparametric statistics: the Mann-Whitney'sUtest and Spearman's rank-order correlation test. The Chi-square test was used for comparing the etiology of chronic liver disease and relations of biochemical markers of iron overload to liver iron deposits and HFE mutations in the study and control groups. Logistic regression was used to determine factors independently associated with liver cirrhosis. A P value less than 0.05 was considered statistically significant.
Results
The most frequent etiology of liver cirrhosis was chronic hepatitis C and B (42/61 patients). HH was diagnosed in 3/61 patients (4.9%). In 2 patients, coexistence of HH and viral hepatitis was confirmed (Table 1).
The patients with cirrhosis were significantly older and had higher bilirubin and iron concentrations in serum. HCV infection was noted more frequentlyin the control group (Table 2). The distribution of HCV genotypes was similar in both groups with a predominance of genotype 1 (>80% in both groups). In HCV-infected cirrhotic patients, elevated serum iron metabolism indices and liver iron deposits were found in 21/33 and 10/20 patients respectively, with a frequency similar to non-HCV-infected cirrhotic patients.
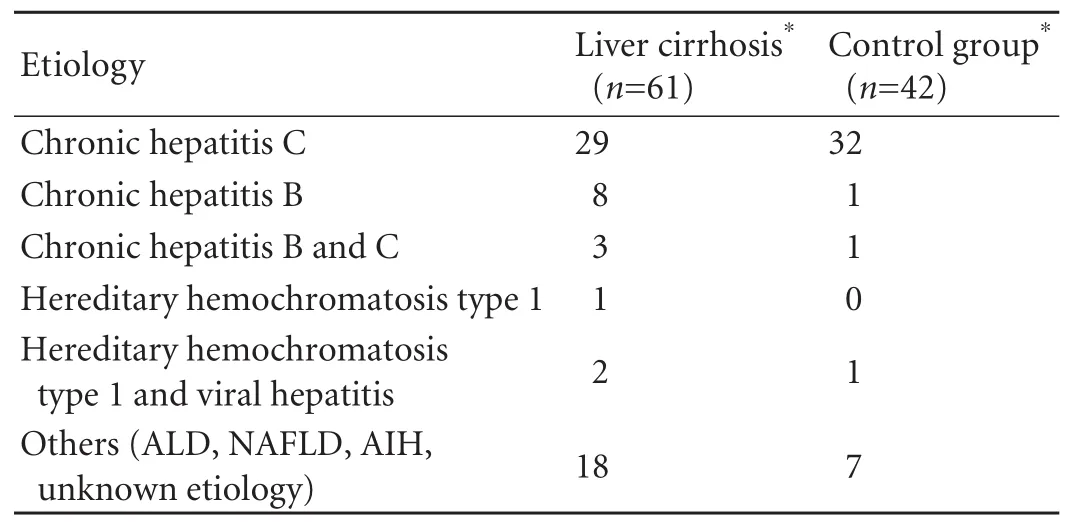
Table 1. Etiology of liver disease in the study and control groups
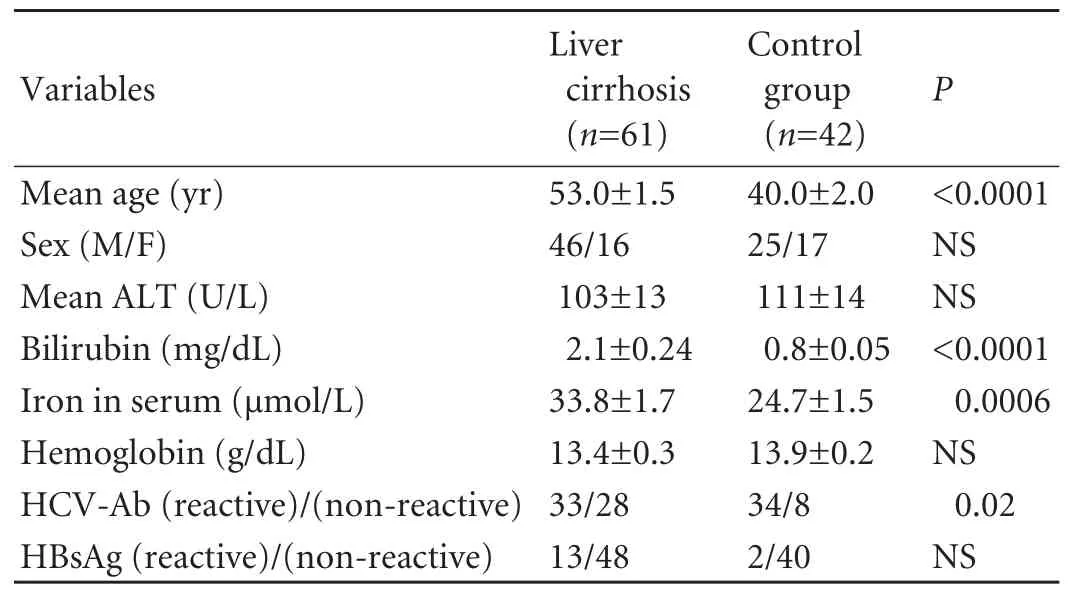
Table 2. Demographic and biochemical data in the study and control groups
The biochemical markers of iron overload and iron deposits in the liver were more frequent in patients with cirrhosis than in the control group (7% and 47%; P<0.0001 and P=0.04 respectively) (Table 3). Indices of biochemical iron overload were detected more frequently in the patients with cirrhosis than in the controls independently of the presence of liver iron deposits. Unlike the controls in cirrhotic patients, the detection of iron deposits was associated with elevated serum iron indices (χ2=8.2; P=0.004). Based on logistic regression analysis, age, serum concentration of bilirubin and occurrence of biochemical iron overload markers appeared to be independent predictors of liver cirrhosis (P<0.00001).
HFE gene mutations were detected in 24/61 (39%) and 16/42 (38%) in the study and control groupsrespectively. There were no differences in the prevalence of HFE mutations and HH genotype between the two groups. Neither the occurrence of elevated serum iron metabolism parameters nor liver iron deposits depended significantly on the presence ofHFEgene mutations (Table 3). A C282Y homozygous mutation was detected in only two patients with liver cirrhosis and hepatocellular carcinoma. HCC was diagnosed in 16/61 (26%) patients with cirrhosis. Fourteen of 16 patients with HCC were disqualified from orthotopic liver transplantation because the Milan criteria were not fulfilled. No HCC cases were found in the control group. No significant associations of HCC with iron disorders and HFE gene mutations were found in the study group.
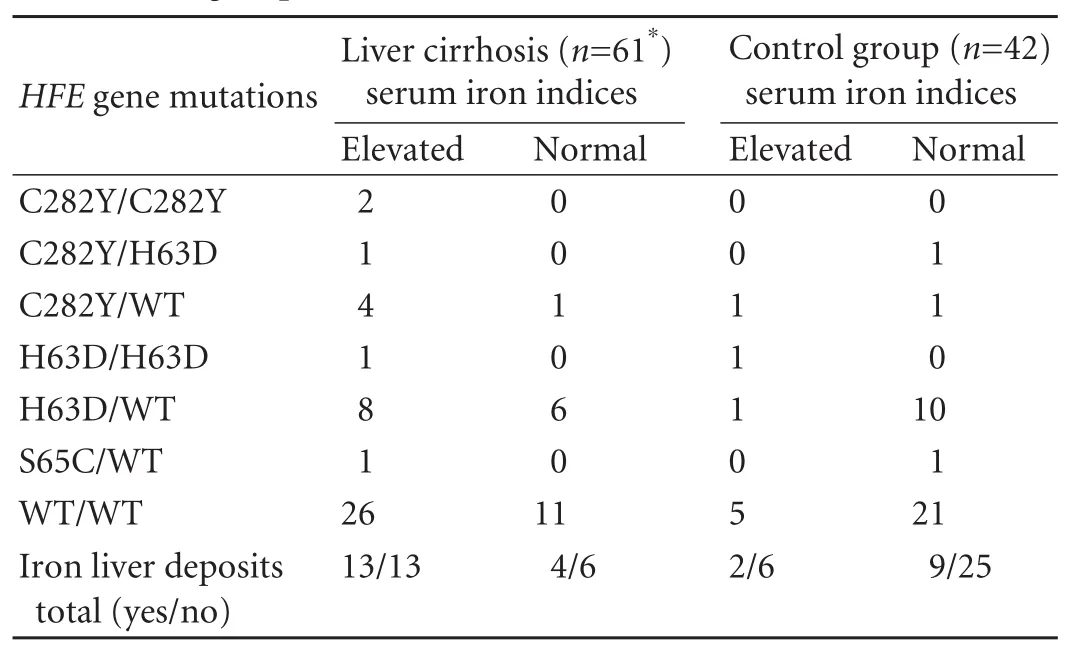
Table 3. HFE gene polymorphism and liver iron deposits in the study and control groups
Discussion
The disturbances in iron metabolism in our patients with liver cirrhosis were similar in frequency to Ludwig's report.[28]The biochemical markers of iron overload were detected in 70% and liver iron deposits in 47% of cases in the study group and appeared to be an exponent of the advanced phase of liver disease compared to the control group. Higher bilirubin concentration in serum and older age in the study group corresponded to the diagnosis of liver cirrhosis. Our case-control analysis of the patient populations was not a longitudinal study and in many cases patients with liver cirrhosis or not yet advanced chronic liver disease had been recruited at the moment of diagnosis. It was impossible to define exactly how long this liver disease had lasted and thus we omitted time of observation of chronic liver disease as a factor associated with morbidity.
The number of patients with cirrhosis and chronic liver desease who were carriers of HFE mutations was too small for a mathematical analysis of differences inoccurrence of those polymorphisms between the groups (Table 3) (Limited funds did not allow us to include more patients in the study.). But the relation of patients with a homozygous C282Y genotype in the group with cirrhosis to the control group was 2∶0. Populations in Poland are relatively homogeneous ethnically. We decided to use the results of Polish population studies on the occurrence of HFE gene mutations to indicate a higher selective occurrence of only homozygous C282Y mutation in patients with liver cirrhosis (3.3% vs 0.12% or 0.13%; odds ratio=24.87; 95% confidence interval, 1.762-345.67; χ2=22.21; P<0.0001).[29,30]
We did not confirm an association of iron metabolism disorders with HFE gene mutations in the cirrhotic patients studied. Although liver iron deposits were detected more frequently in carriers of HFE gene mutations with cirrhosis, no significant association was found. There were some limitations, possibly influencing the results, in the accessibility of histological evaluation in all cirrhotic patients. Not all patients were submitted to the procedure because of contraindications (17/43 with elevated serum iron indices were not punctured, among whom 6 were carriers ofHFEgene mutations). In some cases, the histological material was not sufficiently representative to correctly assess the presence of iron due to a large amount of fibrous tissue and disintegration. That problem was previously described.[28,31]In consideration of the role of liver iron overload as a prognostic factor of survival after orthotopic liver transplantation, accurate liver iron assessment with noninvasive techniques (MRI) in pretransplant procedures seems to be indicated, especially when it is impossible to perform a histological examination.
Impaired expression of hepcidin may be one of the reasons for frequent iron loading in liver cirrhosis. Détivaud et al[32]have shown a negative correlation of liver fibrosis status with hepcidin mRNA and urinary hepcidin levels. Down-regulation of hepcidin leads to increased release of iron from macrophages and enterocytes, and then an increase of iron concentration and transferrin saturation in serum.[33,34]Iron loading is a strong inducer of hepcidin synthesis but probably this model of iron homeostasis regulation is insufficient in advanced cirrhosis when symptoms of progressive liver failure develop due to loss of functional hepatocytes that are both the main source of hepcidin and the main store of iron. It seems that the presence of iron deposits in cirrhotic liver not only accompanies local necroinflammatory processes as shown in chronic hepatits C.[35]It may signal long-term, intensified, systemic disorders of iron homeostasis preceding irreversible nodular liver transformation. This would explain the higher risk of infectious and malignant complications in liver transplant recipients with pretransplant symptoms of iron overload. Thus, excessive accumulation of iron in liver cirrhosis perceived as a systemic pathology warrants thorough diagnosis and treatment in the context of post-transplantation prognosis. It was reported in patients qualified for liver transplantation that iron reduction with preoperative venesections reduced the risk of cardiac and infection complications postoperatively.[16]
In the study group, cirrhotic patients chronically infected with HCV presented signs of increased body iron stores with a high frequency. Hepatitis C virus is the main reason for end-stage liver disease worldwide and the most common indication for liver transplantation in the United States and Europe. The outcome after liver transplantation depends on recurrence of hepatitis C infection that may lead to severe graft damage. Increased iron stores are associated with poor prognosis in chronic hepatitis C and resistance to antiviral treatment with interferon and ribavirin.[36,37]It is interesting to establish the role of iron overload syndrome as a prognostic factor in liver-transplanted chronic hepatitis C patients, particularly when the possible but not clearly explained influence of iron on HCV replication and HCV-induced iron metabolism disorders is taken into account.[38,39]Thus the precise assessment of iron metabolism in chronic hepatitis C patients qualified for liver transplantation is warranted.
In conclusion, iron disorders are frequently detected in patients with liver cirrhosis but without significant association with HFE gene mutations. Only the homozygous C282Y mutation seems to occur more frequently in the selected population of patients with cirrhosis. As elevated biochemical iron indices are accompanied liver iron deposits more frequently in cirrhosis patients compared with controls with chronic liver disease there is a need for more extensive studies searching for the possible influence of non-HFE iron homeostasis regulators and their modulation on the course of chonic liver disease and liver cirrhosis.
Funding: This study was supported by a grant from the Medical University of Gdansk (W-175).
Ethical approval:The study protocol was approved by the Local Ethics Committee (NKEB 270/2010).
Contributors:SK designed the study. SK and SP collected, analysed, interpreted data and prepared the manuscript. IŚE performed histopathological examinations of liver biopsy specimens. RT performed genetic tests and revised the manuscript. BKP performed genetic tests and reviewed the manuscript. SK is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Phatak PD, Sham RL, Raubertas RF, Dunnigan K, O'Leary MT, Braggins C, et al. Prevalence of hereditary hemochromatosis in 16031 primary care patients. Ann Intern Med 1998;129:954-961.
2 Waheed A, Parkkila S, Zhou XY, Tomatsu S, Tsuchihashi Z, Feder JN, et al. Hereditary hemochromatosis: effects of C282Y and H63D mutations on association with beta2-microglobulin, intracellular processing, and cell surface expression of the HFE protein in COS-7 cells. Proc Natl Acad Sci U S A 1997;94:12384-12389.
3 Pietrangelo A. Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology 2010;139:393-408, 408.e1-2.
4 European Association For The Study Of The Liver. EASL clinical practice guidelines for HFE hemochromatosis. J Hepatol 2010;53:3-22.
5 Harrison SA, Bacon BR. Hereditary hemochromatosis: update for 2003. J Hepatol 2003;38:S14-23.
6 Ellervik C, Birgens H, Tybjaerg-Hansen A, Nordestgaard BG. Hemochromatosis genotypes and risk of 31 disease endpoints: meta-analyses including 66 000 cases and 226 000 controls. Hepatology 2007;46:1071-1080.
7 Gochee PA, Powell LW, Cullen DJ, Du Sart D, Rossi E, Olynyk JK. A population-based study of the biochemical and clinical expression of the H63D hemochromatosis mutation. Gastroenterology 2002;122:646-651.
8 Samarasena J, Winsor W, Lush R, Duggan P, Xie Y, Borgaonkar M. Individuals homozygous for the H63D mutation have significantly elevated iron indexes. Dig Dis Sci 2006;51:803-807.
9 Mura C, Raguenes O, Férec C. HFE mutations analysis in 711 hemochromatosis probands: evidence for S65C implication in mild form of hemochromatosis. Blood 1999;93:2502-2505.
10 Geier A, Reugels M, Weiskirchen R, Wasmuth HE, Dietrich CG, Siewert E, et al. Common heterozygous hemochromatosis gene mutations are risk factors for inflammation and fibrosis in chronic hepatitis C. Liver Int 2004;24:285-294.
11 Erhardt A, Maschner-Olberg A, Mellenthin C, Kappert G, Adams O, Donner A, et al. HFE mutations and chronic hepatitis C: H63D and C282Y heterozygosity are independent risk factors for liver fibrosis and cirrhosis. J Hepatol 2003;38: 335-342.
12 Fargion S, Stazi MA, Fracanzani AL, Mattioli M, Sampietro M, Tavazzi D, et al. Mutations in the HFE gene and their interaction with exogenous risk factors in hepatocellular carcinoma. Blood Cells Mol Dis 2001;27:505-511.
13 Machado MV, Ravasco P, Martins A, Almeida MR, Camilo ME, Cortez-Pinto H. Iron homeostasis and H63D mutations in alcoholics with and without liver disease. World J Gastroenterol 2009;15:106-111.
14 Burke W, Imperatore G, McDonnell SM, Baron RC, Khoury MJ. Contribution of different HFE genotypes to iron overload disease: a pooled analysis. Genet Med 2000;2:271-277.
15 Kilpe VE, Krakauer H, Wren RE. An analysis of liver transplant experience from 37 transplant centers as reported to Medicare. Transplantation 1993;56:554-561.
16 Dar FS, Faraj W, Zaman MB, Bartlett A, Bomford A, O'Sullivan A, et al. Outcome of liver transplantation in hereditary hemochromatosis. Transpl Int 2009;22:717-724.
17 Brandhagen DJ, Alvarez W, Therneau TM, Kruckeberg KE, Thibodeau SN, Ludwig J, et al. Iron overload in cirrhosis-HFE genotypes and outcome after liver transplantation. Hepatology 2000;31:456-460.
18 Farrell FJ, Nguyen M, Woodley S, Imperial JC, Garcia-Kennedy R, Man K,et al. Outcome of liver transplantation in patients with hemochromatosis. Hepatology 1994;20:404-410.
19 Fracanzani AL, Conte D, Fraquelli M, Taioli E, Mattioli M, Losco A, et al. Increased cancer risk in a cohort of 230 patients with hereditary hemochromatosis in comparison to matched control patients with non-iron-related chronic liver disease. Hepatology 2001;33:647-651.
20 Fenton H, Torbenson M, Vivekanandan P, Yeh MM, Hart J, Ferrell L. Marked iron in liver explants in the absence of major hereditary hemochromatosis gene defects: a risk factor for cardiac failure. Transplantation 2009;87:1256-1260.
21 Singh N, Sun HY. Iron overload and unique susceptibility of liver transplant recipients to disseminated disease due to opportunistic pathogens. Liver Transpl 2008;14:1249-1255.
22 Singh N, Wannstedt C, Keyes L, Mayher D, Tickerhoof L, Akoad M, et al. Hepatic iron content and the risk of Staphylococcus aureus bacteremia in liver transplant recipients. Prog Transplant 2007;17:332-336.
23 Alexander J, Limaye AP, Ko CW, Bronner MP, Kowdley KV. Association of hepatic iron overload with invasive fungal infection in liver transplant recipients. Liver Transpl 2006;12: 1799-1804.
24 de Sousa M, Porto G. The immunological system in hemochromatosis. J Hepatol 1998;28:1-7.
25 Qaseem A, Aronson M, Fitterman N, Snow V, Weiss KB, Owens DK, et al. Screening for hereditary hemochromatosis: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2005;143:517-521.
26 Sikorska K, Stalke P, Jaskiewicz K, Romanowski T, Bielawski KP. Could iron deposits in hepatocytes serve as a prognostic marker of HFE gene mutations? Hepatogastroenterology 2008;55:1024-1028.
27 Sikorska K, Bielawski KP, Stalke P, Lakomy EA, Michalska Z, Witczak-Malinowska K, et al. HFE gene mutations in Polish patients with disturbances of iron metabolism: an initial assessment. Int J Mol Med 2005;16:1151-1156.
28 Ludwig J, Hashimoto E, Porayko MK, Moyer TP, Baldus WP. Hemosiderosis in cirrhosis: a study of 447 native livers. Gastroenterology 1997;112:882-888.
29 Moczulski DK, Grzeszczak W, Gawlik B. Frequency of the hemochromatosis C282Y and H63D mutations in a Polish population of Slavic origin. Med Sci Monit 2001;7:441-443.
30 Raszeja-Wyszomirska J, Kurzawski G, Suchy J, Zawada I, Lubinski J, Milkiewicz P. Frequency of mutations related to hereditary haemochromatosis in northwestern Poland. J Appl Genet 2008;49:105-107.
31 Villeneuve JP, Bilodeau M, Lepage R, Coté J, Lefebvre M. Variability in hepatic iron concentration measurement from needle-biopsy specimens. J Hepatol 1996;25:172-177.
32 Détivaud L, Nemeth E, Boudjema K, Turlin B, Troadec MB, Leroyer P, et al. Hepcidin levels in humans are correlated with hepatic iron stores, hemoglobin levels, and hepatic function. Blood 2005;106:746-748.
33 Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004;306:2090-2903.
34 Delaby C, Pilard N, Goncalves AS, Beaumont C, Canonne-Hergaux F. Presence of the iron exporter ferroportin at the plasma membrane of macrophages is enhanced by iron loading and down-regulated by hepcidin. Blood 2005;106: 3979-3984.
35 Guyader D, Thirouard AS, Erdtmann L, Rakba N, Jacquelinet S, Danielou H, et al. Liver iron is a surrogate marker of severe fibrosis in chronic hepatitis C. J Hepatol 2007;46:587-595.
36 Fujita N, Sugimoto R, Urawa N, Araki J, Mifuji R, Yamamoto M, et al. Hepatic iron accumulation is associated with disease progression and resistance to interferon/ribavirin combination therapy in chronic hepatitis C. J Gastroenterol Hepatol 2007;22:1886-1893.
37 Sikorska K, Stalke P, Izycka-Swieszewska E, Romanowski T, Bielawski KP. The role of iron overload and HFE gene mutations in the era of pegylated interferon and ribavirin treatment of chronic hepatitis C. Med Sci Monit 2010;16: CR137-143.
38 Theurl I, Zoller H, Obrist P, Datz C, Bachmann F, Elliott RM, et al. Iron regulates hepatitis C virus translation via stimulation of expression of translation initiation factor 3. J Infect Dis 2004;190:819-825.
39 Fillebeen C, Pantopoulos K. Iron inhibits replication of infectious hepatitis C virus in permissive Huh7.5.1 cells. J Hepatol 2010;53:995-999.
Received November 13, 2010
Accepted after revision January 28, 2011
One should not be arrogant and haughty, but he should have a self-respecting and unyielding character.
—Xu Beihong
Author Affiliations: Department of Infectious Diseases, Medical University of Gdansk, ul. Smoluchowskiego 18, 80-218 Gdansk, Poland (Sikorska K, Stalke P); Department of Biotechnology, Intercollegiate Faculty of Biotechnology, University of Gdansk and Medical University of Gdansk, ul. Kładki 24, 80-822 Gdańsk, Poland (Romanowski T and Bielawski KP); Department of Pathology, Medical University of Gdansk, ul. Dębinki 7, 80-952 Gdansk, Poland (Iżycka-Świeszewska E)
Katarzyna Sikorska, MD, PhD, Department of Infectious Diseases, Medical University of Gdansk, ul. Smoluchowskiego 18, 80-218 Gdansk, Poland (Tel: 48-58-3412887; Fax: 48-58-3412887; Email: ksikorska@gumed.edu.pl)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Emergency re-routing of anterior sector venous outflow for right lobe living donor liver transplantation including the middle hepatic vein
- Cytokine and apoptosis gene polymorphisms influence the outcome of hepatitis C virus infection
- Persistent port-site sinus in a patient after laparoscopic cholecystectomy: watch out for gallbladder tuberculosis
- Long-term survival of a patient after resection of a gastrointestinal stromal tumor arising from the pancreas
- Predictive value and main determinants of abnormal features of intraoperative cholangiography during cholecystectomy
