Emergency re-routing of anterior sector venous outflow for right lobe living donor liver transplantation including the middle hepatic vein
2011-07-03KennethSHChokSeeChingChanChungMauLoandSheungTatFan
Kenneth SH Chok, See Ching Chan, Chung Mau Lo and Sheung Tat Fan
Hong Kong, China
Case Report
Emergency re-routing of anterior sector venous outflow for right lobe living donor liver transplantation including the middle hepatic vein
Kenneth SH Chok, See Ching Chan, Chung Mau Lo and Sheung Tat Fan
Hong Kong, China
BACKGROUND:Controversy remains over whether the middle hepatic vein should be included in the liver graft in right liver living donor liver transplantation. Congestion in the anterior sector of a right liver graft can cause graft malfunction, which is especially devastating in the case of a graft with marginal size in relation to recipient body size on top of poor pre-transplant recipient status. The case we report here highlighted the importance of the middle hepatic vein in right liver living donor liver transplantation.
METHODS:We illustrated the rectification of outflow obstruction of the middle hepatic vein in the anterior sector of right liver graft caused by technical error during transplantation. The rectification was performed with emergency re-routing using an artificial conduit.
RESULT:Congestion in the anterior sector of the graft improved immediately and the patient's postoperative liver function test results improved gradually.
CONCLUSIONS:The middle hepatic vein is important for effective drainage of the anterior sector of a right liver graft. The re-routing technique described in the report can also be applied to cases in which the middle hepatic vein is injured during hepatectomy requiring immediate reconstruction.
(Hepatobiliary Pancreat Dis Int 2011; 10: 325-327)
Gore-Tex graft; vein graft; liver graft; cirrhosis; hepatitis B infection
Introduction
Congestion of the poorly drained right anterior sector of a liver graft in living donor liver transplantation (LDLT) has been well described. Despite that, inclusion of the middle hepatic vein (MHV) in a right liver graft in adult LDLT remains controversial.[1]We believe adequate venous outflow of the MHV territories, which definitely favors regeneration of the graft, is particularly crucial in case of a graft with marginal size in relation to recipient body size on top of poor pre-transplant recipient status.[2,3]
We report a patient with decompensated cirrhosis who underwent right liver LDLT which was complicated by MHV outflow obstruction as a sequela of technical error. This circumstance nevertheless allowed illustration of the importance of venous outflow provided by the MHV in the anterior sector of a right liver graft. The technique to restore the continuity of the outflow was also described.
Case report
The patient was a 47-year-old Chinese man (weight 111.5 kg, height 183 cm) with chronic hepatitis B infection and acute decompensation from hepatitis B. His score in the model of end-stage liver disease was 32 and his estimated standard liver volume as calculated by the formula devised by Chan et al[4]was 1667.2 mL (weight 111.5 kg, height 183 cm). His 18-year-old son (weight 82 kg, height 174 cm) volunteered to be the liver donor to save his father. Computed tomography and volumetry revealed that the son's right liver volume was 982.5 ml, which was 59% of his father's estimated standard liver volume. His remnant liver would be 35% of his total liver volume.
LDLT was performed using the right liver with the MHV included[5,6](Fig. 1). Following clamp release, there was bleeding from the anastomosis connecting the inferior vena cava (IVC) and the right and middlehepatic venoplasty. The defect on the MHV was further weakened by suturing during vascular anastomosis with plication with 5/0 prolene. This resulted in outflow occlusion in the right anterior sector, manifested by engorgement of the MHV, loss of the triphasic flow pattern of the MHV on intraoperative ultrasonography, and congestion of the right anterior sector (Fig. 2A). To re-establish venous outflow of the MHV territories, rerouting of the venous drainage from the MHV to the IVC was decided. The middle portion of the MHV was hence exposed further with the aid of a Cavitron ultrasonic surgical aspirator. Under a 19-minute total vascular occlusion, a 6-cm long, 10-mm wide ringed Gore-Tex polytetrafluoroethylene graft (W. L. Gore & Associates, Newark, Delaware, USA) was used to anastomose the MHV to the IVC (Fig. 2B). On release of the clamps, the right anterior sector congestion was resolved and the triphasic flow pattern of the MHV became evident on intraoperative ultrasonography (Fig. 3A).
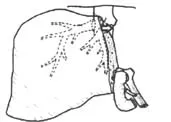
Fig. 1. Schematic diagram of right lobe living donor liver transplantation including the MHV. MHV: middle hepatic vein; RHV: right hepatic vein; LHV: left hepatic vein; RPV: right portal vein; RHD: right hepatic duct; RHA: right hepatic artery; MPV: main portal vein; IVC: inferior vena cava.
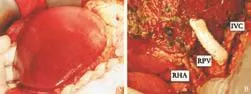
Fig. 2. A: Congestion of the anterior sector of the right liver graft. B: Gore-Tex vein graft connecting the MHV to the IVC. IVC: inferior vena cava; RPV: right portal vein; RHA: right hepatic artery.
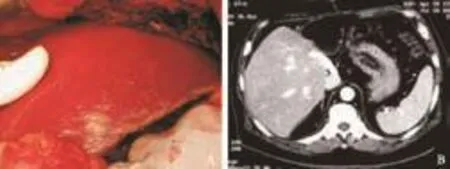
Fig. 3. A: Disappearance of congestion after vein grafting. B: Computed tomography showing patent vein graft and normal liver perfusion.
The patient's postoperative liver function improved gradually. Daily bedside Doppler ultrasonography showed that the Gore-Tex graft and all inflow and outflow vessels were patent. Pathologically the explanted liver showed hepatitis-B-related cirrhosis. The patient was discharged on postoperative day 14. Reassessment by computed tomography four months later showed a patent Gore-Tex graft and normal perfusion of the liver (Fig. 3B).
Discussion
The importance of effective drainage of the anterior sector of a right liver graft is well recognized. There is clear evidence that the inclusion of the MHV in the right liver graft improves the drainage of the right anterior sector and improves the ratio of anterior sector volume to graft volume.[3,7]Some centers assess the need for reconstruction of the MHV tributaries by clamping the right hepatic artery after parenchymal transection in donor hepatectomy, and if the discolored area is large, reconstruction of the MHV tributaries is indicated.[8]
In the present case, the graft volume (920 g) was only 58.2% of the recipient's estimated standard liver volume. If the anterior sector was congested and hence malfunctioned, the remaining functional graft volume would probably be less than 30%. In order to avoid this, venous reconstruction was deemed necessary. Allogenous cryopreserved veins and arteries have been used for this purpose.[9-14]
The use of allogenous cryopreserved vessel can obviate time-consuming dissection for an autologous conduit in the recipient, who is usually in poor general condition at the time of transplantation. The long-term patency of the interpositional, cryopreserved vein graft for anterior sector drainage may not be satisfactory as the vein graft undergoes sclerosis. However, graft patency during the first two weeks can normally be sustained and is enough for achievement of adequate graft function. Damaged sinusoids in the congested liver would recover because intra-hepatic venous collaterals can be expected to develop by day 7 after transplantation.[9]
Nevertheless, cryopreserved vein graft is not available in all transplant centers, and its use requiresthawing, which may take 30-40 minutes. In case of urgency, such as the present case, a delay of 30-40 minutes may result in irreversible damage to the right anterior sector. Thus, synthetic Gore-Tex graft, which was immediately available, was used, and graft function was restored immediately. The only disadvantage of using Gore-Tex graft is the need for aspirin to prevent graft thrombosis. Graft patency can be maintained for a long time by this means. The rings in Gore-Tex graft also contribute to graft patency by preventing graft collapse secondary to raised intra-abdominal pressure. In the design of synthetic graft for venous anastomosis, a short and wide graft has a distinct advantage. The ringed Gore-Tex graft can be trimmed to fit the lumen and orientation of the MHV orifice. By bridging the graft down to the IVC immediately near it instead of anastomosing it to the recipient's own MHV, a short and direct conduit is obtained.
The importance of the MHV for right liver LDLT was highlighted by the potentially fatal complication in this case. The technique used in this case can be applied to situations in which MHV injury is encountered during hepatectomy and vascular reconstruction is immediately required. It is apparent that ringed Gore-Tex graft is a good alternative to cryopreserved vessel for venous reconstruction in the future.
Funding:None.
Ethical approval: Not needed.
Contributors: CKSH performed data analysis and interpretation and drafted the article. CSC initiated the study and revised the article. LCM was responsible for approval of the article. FST was responsible for critical revision and approval of the article. CSC is the guarantor.
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 de Villa VH, Chen CL, Chen YS, Wang CC, Lin CC, Cheng YF, et al. Right lobe living donor liver transplantation-addressing the middle hepatic vein controversy. Ann Surg 2003;238: 275-282.
2 Kim DG, Moon IS, Kim SJ, Lee YJ, Lee MD. Effect of middle hepatic vein reconstruction in living donor liver transplantation using right lobe. Transplant Proc 2006;38: 2099-2101.
3 Mizuno S, Iida T, Yagi S, Usui M, Sakurai H, Isaji S, et al. Impact of venous drainage on regeneration of the anterior segment of right living-related liver grafts. Clin Transplant 2006;20:509-516.
4 Chan SC, Liu CL, Lo CM, Lam BK, Lee EW, Wong Y, et al. Estimating liver weight of adults by body weight and gender. World J Gastroenterol 2006;12:2217-2222.
5 Chan SC, Fan ST, Lo CM, Liu CL, Wong J. Toward current standards of donor right hepatectomy for adult-to-adult live donor liver transplantation through the experience of 200 cases. Ann Surg 2007;245:110-117.
6 Fan ST. Living donor liver transplantation. Shenzhen, China: Takungpao;2007. Available from http://www.ldlt.hk/.
7 Cho EH, Suh KS, Lee HW, Shin WY, Yi NJ, Lee KU. Safety of modified extended right hepatectomy in living liver donors. Transpl Int 2007;20:779-783.
8 Sano K, Makuuchi M, Miki K, Maema A, Sugawara Y, Imamura H, et al. Evaluation of hepatic venous congestion: proposed indication criteria for hepatic vein reconstruction. Ann Surg 2002;236:241-247.
9 Gyu Lee S, Min Park K, Hwang S, Hun Kim K, Nak Choi D, Hyung Joo S, et al. Modified right liver graft from a living donor to prevent congestion. Transplantation 2002;74:54-59.
10 Cattral MS, Greig PD, Muradali D, Grant D. Reconstruction of middle hepatic vein of a living-donor right lobe liver graft with recipient left portal vein. Transplantation 2001;71:1864-1866.
11 Lee KW, Lee DS, Lee HH, Joh JW, Choi SH, Heo JS, et al. Interpostion vein graft in living donor liver transplantation. Transplant Proc 2004;36:2261-2262.
12 Dong G, Sankary HN, Malagò M, Oberholzer J, Panaro F, Knight PS, et al. Cadaver iliac vein outflow reconstruction in living donor right lobe liver transplantation. J Am Coll Surg 2004;199:504-507.
13 Sugawara Y, Makuuchi M, Akamatsu N, Kishi Y, Niiya T, Kaneko J, et al. Refinement of venous reconstruction using cryopreserved veins in right liver grafts. Liver Transpl 2004; 10:541-547.
14 Hwang S, Lee SG, Ahn CS, Park KM, Kim KH, Moon DB, et al. Cryopreserved iliac artery is indispensable interposition graft material for middle hepatic vein reconstruction of right liver grafts. Liver Transpl 2005;11:644-649.
Received October 27, 2010
Accepted after revision December 10, 2010
Author Affiliations: Department of Surgery, Queen Mary Hospital (Chok KSH, Chan SC, Lo CM and Fan ST); State Key Laboratory for Liver Research (Chan SC, Lo CM and Fan ST), The University of Hong Kong, 102 Pok Fu Lam Road, Hong Kong, China
See Ching Chan, MS, PhD, Department of Surgery, Queen Mary Hospital, The University of Hong Kong, 102 Pok Fu Lam Road, Hong Kong, China (Tel: 852-22553025; Fax: 852-28165284; Email: seechingchan@gmail.com)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Borderline resectable pancreatic tumors: Is there a need for further re fi nement of this stage?
- Dose requirement and complications of diluted and undiluted propofol for deep sedation in endoscopic retrograde cholangiopancreatography
- Clinical features and treatment of sump syndrome following hepaticojejunostomy
- Current surgical management of pancreatic endocrine tumor liver metastases
- Liver transplantation for hepatocellular carcinoma:an update
- Cytokine and apoptosis gene polymorphisms influence the outcome of hepatitis C virus infection
