Reconstruction of the middle hepatic vein tributary in adult right lobe living donor liver transplantation
2011-07-03XiaoMinShiYiFengTaoZhiRenFuGuoShanDingZhengXinWangandLiangXiao
Xiao-Min Shi, Yi-Feng Tao, Zhi-Ren Fu, Guo-Shan Ding, Zheng-Xin Wang and Liang Xiao
Shanghai, China
Original Article / Transplantation
Reconstruction of the middle hepatic vein tributary in adult right lobe living donor liver transplantation
Xiao-Min Shi, Yi-Feng Tao, Zhi-Ren Fu, Guo-Shan Ding, Zheng-Xin Wang and Liang Xiao
Shanghai, China
BACKGROUND:In adult-to-adult living donor liver transplantation (LDLT), the use of a right lobe graft without the middle hepatic vein (MHV) can cause hepatic congestion and disturbance of venous drainage. To solve this problem, we successfully used cadaveric venous allografts preserved in 4℃University of Wisconsin (UW) solution within 10 days as interposition veins for drainage of the paramedian portion of the right lobe in adult LDLT.
METHODS:From June 2007 to January 2008, 11 adult LDLT patients received modified right liver grafts. The major MHV tributaries (greater than 5 mm in diameter) of 9 cases were preserved and reconstructed using cadaveric interposition vein allografts that had been stored for 1 to 10 days in 4℃UW solution. The regeneration of the paramedian sector of the grafts and the patency of the interposition vein allografts were examined by Doppler ultrasonography after the operation.
RESULTS:MHV tributaries were reconstructed in 9 recipients. Only 1 recipient died of renal failure and severe pulmonary infection on day 9 after transplantation without any hemiliver venous outflow obstruction. The other 8 recipients achieved long-term survival with a median follow-up of 30 months. The cumulative patency rates of the 8 recipients were 63.63% (7/11), 45.45% (5/11), 45.45% (5/11) and 36.36% (4/11) at 3, 6, 12 and 24 months, respectively. Regeneration of the paramedian sectors was equivalent.
CONCLUSION:The cadaveric venous allograft preserved in 4℃UW solution within 10 days serves as a useful alternative for interposition veins in facilitating implantation of a right lobe graft and guarantees outflow of the MHV.
(Hepatobiliary Pancreat Dis Int 2011; 10: 581-586)
adult-to-adult; living donor; liver transplantation; middle hepatic vein; venous allograft; reconstruction
Introduction
Since the first successful adult living donor liver transplantation (LDLT) reported in 1994,[1]the number of adult patients has increased rapidly. The use of a right lobe graft in adult-to-adult LDLT is a solution to the situation when a left lobe graft from a donor does not fulfill the metabolic demands of a larger recipient. In recent years, right lobe LDLT has become a routine procedure for adults with acute or chronic end-stage liver disease.[2,3]However, the viability and immediate function of the graft and the safety of the donor are major issues of this approach. An extended right lobe including venous drainage by the middle hepatic vein (MHV) would guarantee optimal graft function.[4]As donor safety is of prime importance, the majority of liver transplantation teams currently advocate standard right hemihepatectomy (Couinaud segments 5-8) in right lobe LDLT, preserving the intact MHV for the donor.[5-8]But a right liver graft without the MHV (modified right liver graft) can sometimes cause severe congestion of the right paramedian sector (corresponding to Couinaud segments 5 and 8). Such congestion can lead to severe graft dysfunction and septic complications because hepatic venous outflow of the right paramedian sector is drained mostly intothe MHV.[9]Many centers agree to preserve sizable (>5 mm in diameter) MHV tributaries during donor hepatectomy followed by vascular reconstruction.[10,11]When venous reconstruction is indicated, mostly the recipient's own great saphenous vein, and in a few cases the superficial femoral vein, ovarian vein, umbilical vein, inferior mesenteric vein, as well as the left portal vein from the removed liver, have been used so far.[12,13]These vessels, however, despite their availability, are burdened with the disadvantage of inadequate caliber and length. We therefore decided to use the cadaveric iliac vein and femoral vein as the interposition vein allografts, stored for 1-10 days in 4 ℃ University of Wisconsin (UW) solution, for outflow reconstruction of the MHV when needed.
Methods
Patients
Between June 2007 and January 2008, 11 adult patients underwent LDLT using the modified right liver graft at Changzheng Hospital, Second Military Medical University, Shanghai, China. MHV tributaries were reconstructed in 9 recipients, who were all male [median age 46 (range 39-56) years, mean body weight 65.22± 5.67 (range 56-74) kg]. The indications for adult-toadult LDLT were hepatitis B virus cirrhosis (3), hepatitis B virus cirrhosis associated with small hepatocellular carcinoma (3), chronic fulminant hepatic failure (1), chronic fulminant hepatic failure associated with small hepatocellular carcinoma (1), and cryptogenic cirrhosis (1). The demographic data of the patients are listed in Table 1.
The 9 donors were all males [median age 40 (range 23-45) years; mean body weight 75.44±4.75 (range 70-85) kg], who were the sons (3) or brothers (6) ofthe recipients. The clinical data of the donors are listed in Table 2. All cases were approved by the Ethics Committee of Changzheng Hospital, Second Military Medical University, Shanghai, China.

Table 1. Clinical data of 9 patients receiving right lobe LDLT without the MHV
Preparation of cadaveric venous allografts
The iliac vein and femoral veins as interposition allografts were obtained by the cadaveric liver harvest procedure. After harvesting, venous allografts were selected and prepared under a cold light source (examination of the quality of vein wall, seeking the vein valve, ligation of collaterals, repair of minor tears, and end-to-end suturing of healthy segments) (Fig. 1). The length and diameter were measured with a set of bougies. Bacteriological and serological tests of blood samples (for HIV, hepatitis B virus, hepatitis C virus, syphilis and cytomegalovirus) were performed in compliance with regulatory requirements after vein selection. These vein grafts were then immediately stored in 4 ℃ UW solution containing 40 mg gentamicin for use within 10days. Before implantation, the vein grafts were rinsed with 4 ℃ saline containing heparin.
Table 2. Clinical data of 9 donors and liver grafts
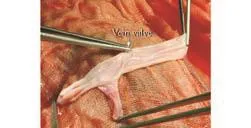
Fig. 1. Vein valve of the cadaveric iliac vein.
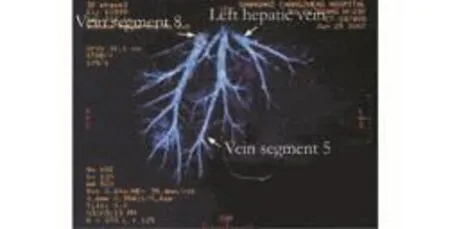
Fig. 2. MHV trunk with two significant tributaries draining segments 5 and 8.
Preoperative assessment of donor liver
The liver of the donor was estimated by computed tomography (CT). If the estimated right hemiliver volume was less than 45% of the estimated standard liver mass of the recipient by CT volumetry, or if a relatively small-sized right hepatic vein or a prominent hepatic vein from segment 5 and a hepatic vein from segment 8 were identified on CT scan in the donor (Fig. 2), segment 5 or 8 of greater than 5 mm was clipped intraoperatively during transection of the liver parenchyma. After transection of the parenchyma and before transection of the portal vein and hepatic artery, the right hepatic artery was clamped to access the congested area. During perfusion with UW solution at the back table, the congested area was evaluated again before the clips of the hepatic vein tributaries were removed and the decision of whether to carry out an interposition was made. If the donor had a moderate or severe fatty liver or if the recipient's condition was poor, an interposition graft was also considered. Anastomosis of segment 5 (Fig. 3) or 8 to vein allografts was made using continuous 6-0 prolene suture. Normally, it took 15 minutes to reconstruct one middle hepatic vein tributary on the back table.
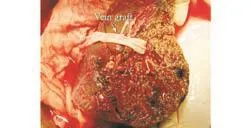
Fig. 3. Vein graft at the major vein of segment 5.

Fig. 4. Liver graft after completion of venous drainage of segment 5.
Recipient operation
Briefly, we first anastomosed the right hepatic vein of the hemiliver graft to the side of the recipient's inferior vena cava in an end-to-side fashion with 4-0 prolene sutures, followed by portal vein anastomosis. After portal vein blood flow was re-opened, the middle hepatic vein tributaries were reconstructed with 6-0 prolene sutures. When segments 5 and 8 were reconstructed to ensure adequate hepatic venous outflow and to avoid hepatic compression and reconstructive vascular kinking after reperfusion, it was necessary to obtain a wide orifice and the vessel graft anastomosis should be as short as possible (Fig. 4). Finally, hepatic artery and bile duct anastomoses were made. Usually, it took 20 minutes to reconstruct one middle hepatic vein tributary in the recipient operation.
Postoperative evaluation
Doppler ultrasonography was performed daily until discharge, revealing the patency of the vascular flow in the graft or interposition vein. Enhanced CT wasperformed 1 and 3 months after LDLT. The volumes of the paramedian sectors were calculated. Alanine aminotransferase and total bilirubin levels were measured postoperatively for 4 weeks.
Results
From June 2007 to January 2008, cadaveric interposition vein grafts were used for venous drainage of segments 5 and 8 in 9 (81.8%) of 11 recipients who received a modified right liver graft. Venous drainage patterns were as follows: segment 5 only (4); segment 8 (2); segments 5 and 8 (3). ABO-identical interposition vein grafts were used in 7 cases, whereas ABO-compatible interposition vein grafts were used in 2 (Table 3). The right liver grafts weighed 560-860 (mean 708) g, which corresponded to 0.91%-1.20% (mean 1.09%) of the graftrecipient weight ratio (Table 2). No complications were recognized in the reconstruction of the right hepatic vein and major MHV tributary. Intraoperative Doppler ultrasonography showed no evidence of congestion in the right paramedian sector. The alanine aminotransferaselevel peaked on postoperative day 1 and then decreased gradually. The total bilirubin level decreased rapidly after transplantation. One patient died 9 days after transplantation due to renal failure and severe pulmonary infection without any hemiliver venous outflow obstruction. The other 8 patients survived longer with a median follow-up of 30 months. In these 8 patients, Doppler ultrasonography showed no thrombosis and blood flow was smooth within the first postoperative 30 days (Fig. 5). The cumulative patency rates of these 8 patients were 63.63% (7/11), 45.45% (5/11), 45.45% (5/11) and 36.36% (4/11) at 3, 6, 12 and 24 months, respectively. Regeneration of paramedian sectors was equivalent. No mortality or severe postoperative complications were observed in the donors.
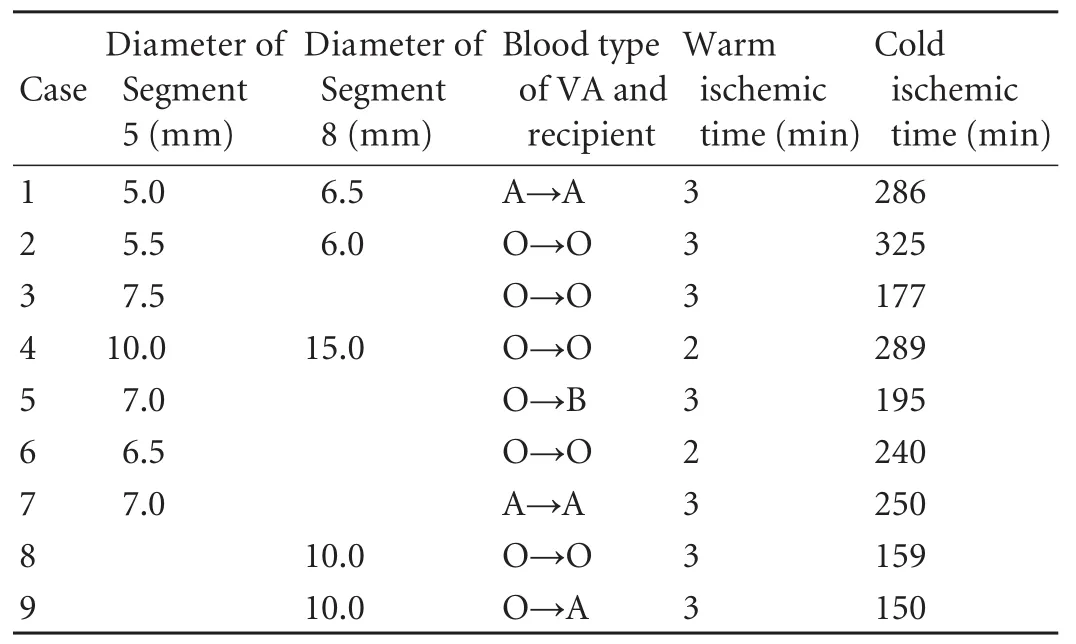
Table 3. Diameter of reconstructed MHV tributaries and warm/ cold ischemic time of 9 patients
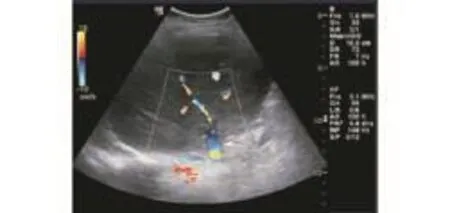
Fig. 5. Doppler ultrasonographic image of the segment 5 vein graft (arrow) draining into the inferior vena cava.
Discussion
In early reports regarding LDLT using a modified right liver graft, the MHV tributaries were not reconstructed.[14-17]Despite the impressive results of rightlobe LDLT, considerable debate persists concerning donor safety. Lee et al[18]used modified right lobe liver grafting with interposition vein grafts to drain the anterior segment in 1999. They noted that the graft could cause severe congestion of the right paramedian sector because hepatic venous outflow of this sector is drained mostly into the MHV. Such congestion can cause severe graft dysfunction and septic complications. We agree that drainage from the MHV tributaries is necessary when the congested area is dominant by the clamping test or when the diameter of the tributaries is greater than 5 mm.
Appropriate blood vessel grafts should be selected for reconstruction when needed. The ideal vascular graft should have a high graft patency rate and should be easily available in different diameters and lengths.
Some centers reported the use of autogenous vessels for interposition.[12,13]Size disparity between the recipient's great saphenous vein and the hepatic venous tributaries has often been found.[11]The use of the external iliac veins is hazardous, since harvesting them may lead to significant swelling of the limb.[19]The ovarian vein, umbilical vein, inferior mesenteric vein as well as the left portal vein from the removed liver may also be of inappropriate length for multiple jump grafts. In our study, we chose the cadaveric iliac vein and femoral vein for their sufficient length and variety of caliber as the interposition grafts. Obviously, such a harvest procedure for vessel grafts had no disadvantage for both recipients and donors.
The major concerns in venous reconstruction usingcryopreserved vein grafts are vein graft obstruction, possible thrombosis, and narrowing in the longterm observation period. Kuang et al[20]and Millis et al[21]reported that the incidence of complications, such as thrombosis and stenosis, was high when cryopreserved veins were used as interposition grafts. Moreover, compared with the cold storage method, cryopreservation is relatively complicated and requires higher costs and equipment. Some grafts were reported to be stored at 4 ℃ in a saline solution.[22-24]Preservation of saphenous allografts in saline at 4 ℃ was preferred over other preservation techniques (glycerol at 4 ℃ or freezing at -50 ℃ or -196 ℃) on the basis of the results from a study by Davies and Parums,[25]that these would cause the rapid disappearance of endothelial cells. Fahner et al[26]reported that glutaraldehyde preservation of human umbilical vein allografts was superior to other methods of vascular allograft preservation. Nevertheless, they also pointed out that in most studies, the glutaraldehyde preservation protocol itself was not described in detail. In our study, therefore, we attempted to preserve the cadaveric alloveins in 4 ℃ UW solution containing gentamicin for use within 10 days.
Before our clinical study, we used the venous allografts transplantation model established by anastomosis of the jugular vein in white New Zealand rabbits to determine the patency rates of venous grafts. The results showed that the destruction of structure was clearly aggravated when the vascular allograft was stored over 10 days, and those vein grafts tended to be more thrombosed after surgery. The postoperative patency rates of vascular allografts stored for 2, 10, 14 and 28 days were 76.92%, 64.29%, 38.46% and 5.12%, respectively (submitted for publication). The results indicated that the optimal storage duration for venous grafts in UW solution at 4 ℃ was within 10 days. Simultaneously, we examined the pathological changes of cadaveric alloveins stored in UW solution at 4 ℃ for clinical adult LDLT. After one-day preservation, part of the epithelial cells died, followed by slight decomposition of elastic fibers and vacuolar degeneration of smooth muscle. Over 10 days of storage, there were significant microscopic pathological changes in the venous grafts, the degree of endothelial detachment exceeded 50%, and the closer to the vessel lumen, the more serious structural damage took place.
In this study, the cadaveric interposition vein grafts were used for venous drainage of segments 5 and 8 in 9 (81.8%) of 11 patients who received a modified right liver graft. We observed that all of the 9 patients had no impairment of graft function and the MHV tributary vessel grafts worked well before the regeneration of paramedian sectors and graft functional recovery.
In summary, this study indicate that cadaveric venous allografts preserved in 4 ℃ UW solution within 10 days provide a useful alternative for interposition veins when outflow reconstruction of the MHV is necessary. Further studies will be carried out by prolonging the preservation time and examining the pathological change of the vessel grafts to improve the middle-term and long-term patency rates.
Acknowledgement
We thank Gui-Hua Wang for data collection.
Ethical approval:The protocol of the study was approved by the Committee of Ethical Affairs of Shanghai Changzheng Hospital.
Contributors:SXM, FZR and DGS proposed the study. SXM and TYF wrote the first draft. WZX and XL analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. FZR is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Hashikura Y, Makuuchi M, Kawasaki S, Matsunami H, Ikegami T, Nakazawa Y, et al. Successful living-related partial liver transplantation to an adult patient. Lancet 1994;343: 1233-1234.
2 Liu CL, Fan ST. Adult-to-adult live-donor liver transplantation: the current status. J Hepatobiliary Pancreat Surg 2006;13:110-116.
3 Makuuchi M, Sugawara Y. Technical progress in living donor liver transplantation for adults. HPB (Oxford) 2004;6:95-98.
2017年1月25日,天成控股对于2016年度业绩预告是“预计2016年与上年同期相比将实现扭亏为盈,实现归属于上市公司股东的净利润在1000万元至1500万元之间”。但这份业绩预告,既未进行董事会决议,也未征求独立董事意见。
4 Fan ST, Lo CM, Liu CL, Wang WX, Wong J. Safety and necessity of including the middle hepatic vein in the right lobe graft in adult-to-adult live donor liver transplantation. Ann Surg 2003;238:137-148.
5 Wu J, Wang W, Zhang M, Shen Y, Liang T, Yu P, et al. Reconstruction of middle hepatic vein in living donor liver transplantation with modified right lobe graft: a single center experience. Transpl Int 2008;21:843-849.
6 Kamei H, Fujimoto Y, Nagai S, Suda R, Yamamoto H, Kiuchi T. Impact of non-congestive graft size in living donor liver transplantation: new indicator for additional vein reconstruction in right liver graft. Liver Transpl 2007;13:1295-1301.
7 Wu H, Yang JY, Yan LN, Li B, Zeng Y, Wen TF, et al. Hepatic venous outflow reconstruction in adult right lobe living donor liver transplantation without middle hepatic vein. Chin Med J (Engl) 2007;120:947-951.
8 De Carlis L, Lauterio A, Giacomoni A, Slim AO, Pirotta V, Mangoni J, et al. Adult living donor liver transplantation with right lobe graft: the venous outflow management in the Milan-Niguarda experience. Transplant Proc 2008;40:1944-1946.
9 Park KM, Lee SG, Lee YJ, Hwang S, Nam CW, Choi KM, et al. Adult-to-adult living donor liver transplantation at Asian Medical Center, Seoul, Korea. Transplant Proc 1999;31:456-458.
10 Kubota T, Togo S, Sekido H, Shizawa R, Takeda K, Morioka D, et al. Indications for hepatic vein reconstruction in living donor liver transplantation of right liver grafts. Transplant Proc 2004;36:2263-2266.
11 Gyu Lee S, Min Park K, Hwang S, Hun Kim K, Nak Choi D, Hyung Joo S, et al. Modified right liver graft from a living donor to prevent congestion. Transplantation 2002;74:54-59.
12 Lee KW, Lee DS, Lee HH, Joh JW, Choi SH, Heo JS, et al. Interpostion vein graft in living donor liver transplantation. Transplant Proc 2004;36:2261-2262.
13 Kornberg A, Heyne J, Schotte U, Hommann M, Scheele J. Hepatic venous outflow reconstruction in right lobe livingdonor liver graft using recipient's superficial femoral vein. Am J Transplant 2003;3:1444-1447.
14 Yamaoka Y, Washida M, Honda K, Tanaka K, Mori K, Shimahara Y, et al. Liver transplantation using a right lobe graft from a living related donor. Transplantation 1994;57: 1127-1130.
15 Wachs ME, Bak TE, Karrer FM, Everson GT, Shrestha R, Trouillot TE, et al. Adult living donor liver transplantation using a right hepatic lobe. Transplantation 1998;66:1313-1316.
16 Marcos A, Fisher RA, Ham JM, Shiffman ML, Sanyal AJ, Luketic VA, et al. Right lobe living donor liver transplantation. Transplantation 1999;68:798-803.
17 Marcos A, Ham JM, Fisher RA, Olzinski AT, Posner MP. Surgical management of anatomical variations of the right lobe in living donor liver transplantation. Ann Surg 2000;231: 824-831.
18 Lee S, Park K, Hwang S, Kim K, Ahn C, Moon D, et al. Anterior segment congestion of a right liver lobe graft in living-donor liver transplantation and strategy to prevent congestion. J Hepatobiliary Pancreat Surg 2003;10:16-25.
19 Kaneoka Y, Yamaguchi A, Isogai M, Hori A. Hepatic vein reconstruction by external iliac vein graft using vascular clips. World J Surg 2000;24:377-382.
20 Kuang AA, Renz JF, Ferrell LD, Ring EJ, Rosenthal P, Lim RC, et al. Failure patterns of cryopreserved vein grafts in liver transplantation. Transplantation 1996;62:742-747.
21 Millis JM, Seaman DS, Piper JB, Alonso EM, Kelly S, Hackworth CA, et al. Portal vein thrombosis and stenosis in pediatric liver transplantation. Transplantation 1996;62:748-754.
22 van Reedt Dortland RW, van Leeuwen MS, Steijling JJ, Theodorides T, van Vroonhoven TJ. Long-term results with vein homograft in femoro-distal arterial reconstructions. Eur J Vasc Surg 1991;5:557-564.
23 Rebane E, Tikko H, Tunder E, Lepner U, Helberg A, Pulges A, et al. Venous allografts for infrainguinal vascular bypass. Cardiovasc Surg 1997;5:21-25.
24 De Leersnijder D, Willocx P, Van Marck E, Vanmaele R. Venous homografts in infra-inguinal procedures: an eight years experience. J Cardiovasc Surg (Torino) 1992;33:633-640.
25 Davies AH, Parums DV. Storage of donor long saphenous vein. J Cardiovasc Surg (Torino) 1992;33:92-97.
26 Fahner PJ, Idu MM, van Gulik TM, Legemate DA. Systematic review of preservation methods and clinical outcome of infrainguinal vascular allografts. J Vasc Surg 2006;44:518-524.
Received December 20, 2010
Accepted after revision June 28, 2011
Author Affiliations: Division of Liver Transplantation, Department of Organ Transplantation, Shanghai Changzheng Hospital, Second Military Medical University, Shanghai 200003, China (Shi XM, Tao YF, Fu ZR, Ding GS, Wang ZX and Xiao L)
Zhi-Ren Fu, MD, PhD, Division of Liver Transplantation, Department of Organ Transplantation, Shanghai Changzheng Hospital, 415 Fengyang Rd., Shanghai 200003, China (Tel: 86-21-81885741; Fax: 86-21-63276788; Email: fuzhirenmail@163.com)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(11)60099-4
猜你喜欢
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Harmine induces apoptosis in HepG2 cells via mitochondrial signaling pathway
- Clinicopathological analysis of 14 patients with combined hepatocellular carcinoma and cholangiocarcinoma
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Undifferentiated embryonal sarcoma of the liver presenting as a hemorrhagic cystic tumor in an adult
- Fulminant liver failure models with subsequent encephalopathy in the mouse
- Risk factors of intrahepatic cholangiocarcinoma in patients with hepatolithiasis: a case-control study
