Fulminant liver failure models with subsequent encephalopathy in the mouse
2011-07-03AnnMarieBaineTomohideHoriFengChenLindsayGardnerShinjiUemotoandJustinNguyen
Ann-Marie T Baine, Tomohide Hori, Feng Chen, Lindsay B Gardner, Shinji Uemoto and Justin H Nguyen
Jacksonville, Florida, USA
Fulminant liver failure models with subsequent encephalopathy in the mouse
Ann-Marie T Baine, Tomohide Hori, Feng Chen, Lindsay B Gardner, Shinji Uemoto and Justin H Nguyen
Jacksonville, Florida, USA
BACKGROUND:A reliable model of fulminant liver failure (FLF) is urgently required in this research field. This study aimed to develop a murine FLF model.
METHODS:We used three groups of male C57BL/6 mice: control, with azoxymethane treatment (AOM group), and with galactosamine and tumor necrosis factor-alpha treatment (Gal+TNF-αgroup). The effects of body temperature (BT) control on survival in all three groups were investigated. Using BT control, we compared the survival, histopathological findings and biochemical/coagulation profiles between the two experimental groups. The effects of hydration on international normalized ratios of prothrombin time (PTINRs) were also checked. Dose-dependent survival curves were constructed for both experimental groups. Neurological behavior was assessed using a coma scale.
RESULTS:No unexpected BT effects were seen in the control group. The AOM group, but not the Gal+TNF-αgroup, showed a significant difference in survival curves between those with and without BT care. Histopathological assessment showed consistent FLF findings in both experimental groups with BT care. There were significant differences between the experimental groups in aspartate aminotransferase levels and PT-INRs, and significant differences in PT-INRs between the sufficiently and insufficiently hydrated groups. There were significant differences between FLF models in the duration of each coma stage, with significant differences in stages 1 and 3 as percentages of the disease state (stages 1-4). The two FLF models with BT care showed different survival curves in the dose-dependent survival study.
CONCLUSIONS:AOM provides a good FLF model, but requires a specialized environment and careful BT control. Other FLF models may also be useful, depending on the research purpose. Thoughtful attention to caregiving and close observation are indispensable for successful FLF models.
(Hepatobiliary Pancreat Dis Int 2011; 10: 611-619)
animal model; acute liver failure; azoxymethane; galactosamine; tumor necrosis factor-alpha
Introduction
Fulminant liver failure (FLF) has a mortality rate of 50%-90% if liver transplantation is unavailable; it is responsible for about 5% of liver-related deaths.[1-3]Although liver transplantation has improved FLF patients' outcomes over the last two decades,[4,5]this option is limited by shortages of deceased donors and safety of living donors, although auxiliary partial orthotopic liver transplantation is currently available.[6,7]Therefore, the development of effective alternative therapies is needed to improve the survival of FLF patients. When liver grafts are not immediately available, therapies to assist liver function until an allograft becomes available might also be used clinically in maintaining FLF patients with a suitable status as liver transplantation candidates. The development of such therapeutic strategies is indispensable for the improvement of FLF outcomes. Such research urgently requires appropriate models, but this field lacks a suitable animal model for FLF.[8-12]Ideally, an animal FLF model would offer reversibility, reproducibility, a therapeutic window, liver-related deaths, suitably-sized subject animals, and minimal hazard to research personnel.[8,9]Although researchers have exposed several species to many toxins [e.g., azoxymethane (AOM), galactosamine (Gal), tumor necrosis factoralpha (TNF-α), acetaminophen, carbon tetrachloride,paracetamol and thioacetamide],[8-10,13-22]all are far from ideal. Although an experimental FLF model is needed, each toxin has its advantages and disadvantages.[8-10,13-22]Therefore, evidence for any novel findings should be verified in several models. Here, we introduce mouse FLF models, with AOM, and with Gal and TNF-α, and discuss the advantages and disadvantages of each, with a review of the earlier literature.
Methods
Animals
Inbred C57BL/6 mice (male, 8-12 weeks of age, about 25 g) (C57BL/6NHsd; Harlan Laboratories, Indianapolis, IN, USA) were maintained under specific pathogenfree conditions. Body weight (bw) was measured before experiments. Mice were kept well-hydrated prior to drug injection. All experimental protocols were approved by the Ethics Committee of the Mayo Clinic (IACUC 33307 and 24907). Mice were kept in a 12-hour light/dark cycle in a temperature-controlled, air-conditioned room, and received food and water. Animal handling and care met the requirements of our institutional animal welfare guidelines.
AOM
Azoxymethane (A5486, Sigma-Aldrich Co., St. Louis, MO) was given by intraperitoneal injection (i.p.), 0.25 mL/mouse, using fine needles (30-gauge, 0.5-inch, BD Precision Single-use Needles; Becton Dickson and Co., Franklin Lakes, NJ, USA). AOM is carcinogenic and hepatotoxic to humans; its fumes are toxic after volatilization in room air. Experiments with AOM require prepared environments. To prevent the spread of vaporized AOM into room air, it was kept in a bottle with a tight rubber cap. All handling was performed in biohazard rooms in which negative atmospheric pressure prevented diffusion of vaporized AOM to outside areas (Fig. 1A). Detailed experimental procedures and closed observation after AOM injection were performed under a fume hood with an evacuation system (Fig. 1B). Unless otherwise noted (as in the dose-dependence study), we used the dose of 100 μg/g bw AOM.
Gal followed by TNF-α
A total of 1.0 mg of Gal (D-(+)-galactosamine hydrochloride, G1639; Sigma-Aldrich Co.) was initially given by i.p. injection, 0.50 mL/mouse, using 28-gauge needles. Mice then received second i.p. injections, 0.50 mL/mouse, of TNF-α (recombinant mouse TNF-α aa80235, 410MT; R&D Systems, Inc., Minneapolis, MN, USA), 2 hours after the initial Gal injection. Unless otherwise noted (as in the dose-dependent study), the dose of 0.10 μg/g bw of TNF-α was used.
Biochemical assays and coagulation profiles in blood samples
Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin (TBIL) were assessed by quantitative determination kits (Biotron, Hemet, CA, USA). The microplate reader (Spectra Max M5e; Molecular Devices, Sunnyvale, CA, USA) was set at 540 nm for measurement. Plasma and serum were taken in separator tubes (BD Microtainer; Becton Dickson). Plasma was used to measure international normalized ratios of prothrombin time (PT-INRs), using a blood analyzer (i-STAT System, Abbott, Princeton, NJ, USA).
Caregiving after injections

Fig. 1. Safe environment for experiments with AOM. A: Biohazard room. AOM is very hazardous for both mice and humans. All handling should therefore be performed in a biohazard room where controlled ambient pressure restricts diffusion of vaporized AOM to outside areas. B: Fume hood with an evacuation system. Detailed experimental procedures and close observation after AOM injection should be performed at a fume hood with an evacuation system. C: Heating pads for BT care after AOM injection. Heating pads were used to maintain BT after AOM injection. Surface temperature of the cage bottom was maintained at 37.1-37.3 ℃. D: Bushed cage with food and water. Solid food was placed in the cage. Food gel for rodents was also prepared with water in feeding dishes. AOM: azoxymethane; BT: body temperature.
Changes in body temperature (BT) offer a means of assessing the health of the mice, especially after AOM injections;[14]BT was periodically measured with rectal probes (Diqi-Sense, Type T Thermocouple Thermometer; Eutech Instruments Pte Ltd., Singapore).The surface temperature of cage bottoms was maintained at 37.1-37.3 ℃ using heating pads (RTHS-SM; Kent Scientific Co., Torrington, CT, USA) (Fig. 1C);[14]BT was kept at about 36 ℃. After AOM injection, BT was measured every 2 hours for the first 12 hours; thereafter BT was checked every hour. For Gal+TNF-α-treated mice, BT was checked every hour after TNF-α injection.
Accurate survival curves for an FLF model require close follow-up. After AOM injection, mice were checked visually every 2 hours for the first 12 hours, and once an hour thereafter. Mice treated with Gal+TNF-α were checked once an hour after the TNF-α injection.
The coma scale for mice, which was slightly modified from the previous scale, is summarized in Table.[8,23,24]To obtain a reliable coma stage, close and/or continuous observation of behavior was required. Detailed evaluation of the coma status of the mice was performed after injections of AOM or Gal+TNF-α.
Bushed cages were used. Solid food was placed in the cage. Food gel for rodents was also prepared in feeding dishes with water (Fig. 1D).
Hydration supplements
Sufficient hydration is important for a successful FLF model because spontaneous drinking activity decreases. We used supplements with 10% dextrose (10% dextrose injection USP, 505 mOsmol/L, pH 4.0; Baxter, Deerfield, IL, USA), dextrose (5% dextrose injection USP, 252 mOsmol/L, pH 4.0; Baxter), Ringer's solution (Ringer's solution USP, 310 mOsmol/L, pH 5.8; B. Braun Medical, Inc., Irvine, CA, USA), isotonic saline (0.9% sodium chloride injection USP, 308 mOsmol/L, pH 5.6; Hospira, Inc., Lake Forest, IL, USA), and lactated Ringer's solution (lactated Ringer's solution USP, 275mOsmo/L, pH 6.2; B. Braun Medical, Inc.). The lactated Ringer's solution was warmed to about 37 ℃ using warm water. In AOM-treated mice, 0.5 mL was given i.p. every 1-2 hours, starting 6 hours after AOM injection. In Gal+TNF-α-treated mice, 0.5 mL was given i.p. every hour, starting 2 hours after TNF-α injection.
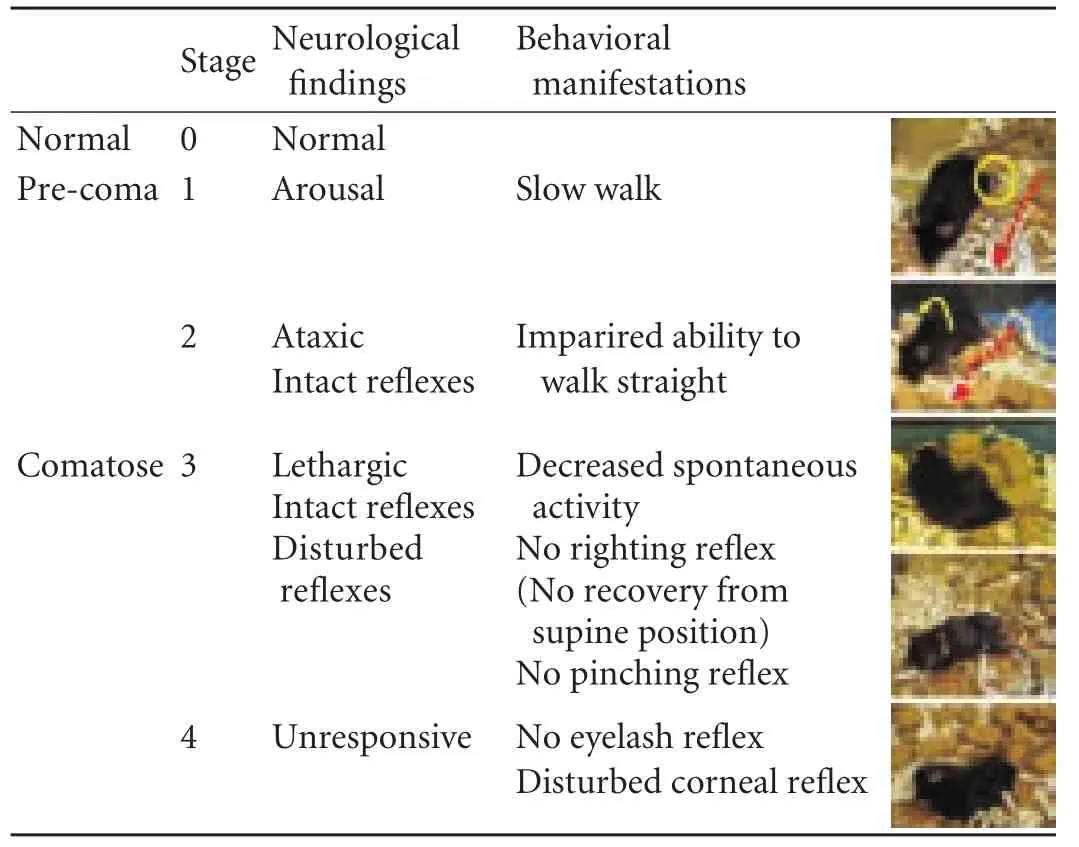
Table. Coma scale for mice
Histopathological assessment
Liver tissue was fixed in 10% neutral-buffered formalin (Fisher Scientific, Inc., Kalamazoo, MI, USA), embedded in paraffin, and sliced into 4-μm sections. Morphological characteristics were evaluated after standard hematoxylin-eosin staining.
Statistical analysis
Data are presented as mean±standard deviation. Univariate and multivariate analyses were used for between-group comparisons as follows: Student's t test and the Chi-square test for unpaired continuous or discontinuous variables; repeated-measures ANOVA for changes over time; the Kaplan-Meier method for survival curves; and the log-rank test for survival rates. Statistical calculations were performed using SPSS16.0 (SPSS Inc., Chicago, IL., USA). P values of less than 0.05 were considered statistically significant.
Results
Effects of BT care in controls
First, the effect of BT care itself was investigated in mice receiving saline injections (0.50 mL/mouse, i.p.; n=10). There were two groups of mice: those with BT care , and those without such care (n=10). Hydration supplements were given every 6 hours after saline injection. Survival and BT were checked every 2 hours. Liver and blood samples were taken 30 hours after the initial injection. There were no significant differences between the two control groups in survival curves, changes in BT over time, or levels of ALT, AST, TBIL and PT-INR (P>0.05 for all categories). Histopathological assessment showed normal findings in mice with BT care. Hence, BT care had no effect on control mice.
Effects of BT care on survival in FLF models
As described above, BT care had no relevant effects on control mice. However, hypothermia in mice after AOM injection has been described.[14,25]We therefore investigated the effects of BT care and changes in BT in mice injected with AOM (n=10) or with Gal+TNF-α (n=10). Hydration supplements were given.
The AOM-treated mice that received BT care hadsurvival curves and BT changes over time different from AOM-treated mice that received no such care (P=0.0002 and P=0.0003, respectively). In contrast, among mice treated with Gal+TNF-α, there were no differences between groups with or without BT care in survival curves and BT changes over time after TNF-α injection (P>0.05 for both).
Histopathological findings, biochemical parameters and coagulation profiles in FLF models with BT care
Histopathological findings, biochemical parameters and coagulation profiles were investigated in mice after AOM treatment (n=10) or Gal+TNF-α treatment (n=10), with both groups receiving BT care. Mice in both treatment groups were given hydration supplements. Liver and blood samples were taken at death or at 30 hours after the initial injection.
In both FLF models given BT care, histopathological assessment showed consistent FLF findings, such as massive necrosis, intrahepatic bleeding, vacuolization and inflammatory cell infiltration (Fig. 2). While there were no significant differences in ALT and TBIL between the FLF models, there were significant differences in AST and PT-INR (Fig. 3).
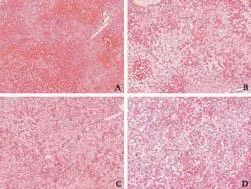
Fig. 2. Histopathological findings for liver-related deaths in both FLF models with BT care. Consistent findings of FLF, such as massive necrosis, intrahepatic bleeding, vacuolization and inflammatory cell infiltration, were confirmed. A: Histopathological findings in mice treated with AOM with BT care (HE, original magnification ×100). B: Histopathological findings in mice treated with AOM with BT care (HE, original magnification ×200). C: Histopathological findings in mice treated with Gal+TNF-α with BT care (HE, original magnification ×100). D: Histopathological findings in mice treated with Gal+TNF-α with BT care (HE, original magnification ×200). AOM: azoxymethane; BT: body temperature; FLF: fulminant liver failure; Gal: galactosamine; HE: hematoxylin-eosin; TNF-α: tumor necrosis factor-alpha.
Effects of hydration on coagulation profiles and coma-stage intervals in FLF models with BT care
The survival curves of mice given BT care after AOM (n=10) or Gal+TNF-α (n=10) injections are shown in Fig. 4A. Mice treated with AOM clearly showed liverrelated death in the necropsy findings.
Sufficient hydration was also found to be important, as were close observation and BT care, because spontaneous activity decreased after AOM and Gal+TNF-α treatment.[8]Coagulation profiles were investigated in mice with BT care after AOM or Gal+TNF-α injections. The mice were classified into two groups with or without sufficient hydration (n=10 for both groups). Sufficient hydration was hydration supplements (lactated Ringer's solution, 0.5 mL/mouse, i.p.) given every hour, starting 6 hours after AOM injection or 2 hours after TNF-α injection. Insufficient hydration consisted of hydration supplements given every 4 hours after AOM or Gal+TNF-α injection. Blood samples were taken at death or 30 hours after the initial injection. There were significant differences in PT-INRs between sufficiently and insufficiently hydrated mice after AOM injection and Gal+TNF-α injection (Fig. 4B). Hydration had clear effects on PT-INR in both FLF models.
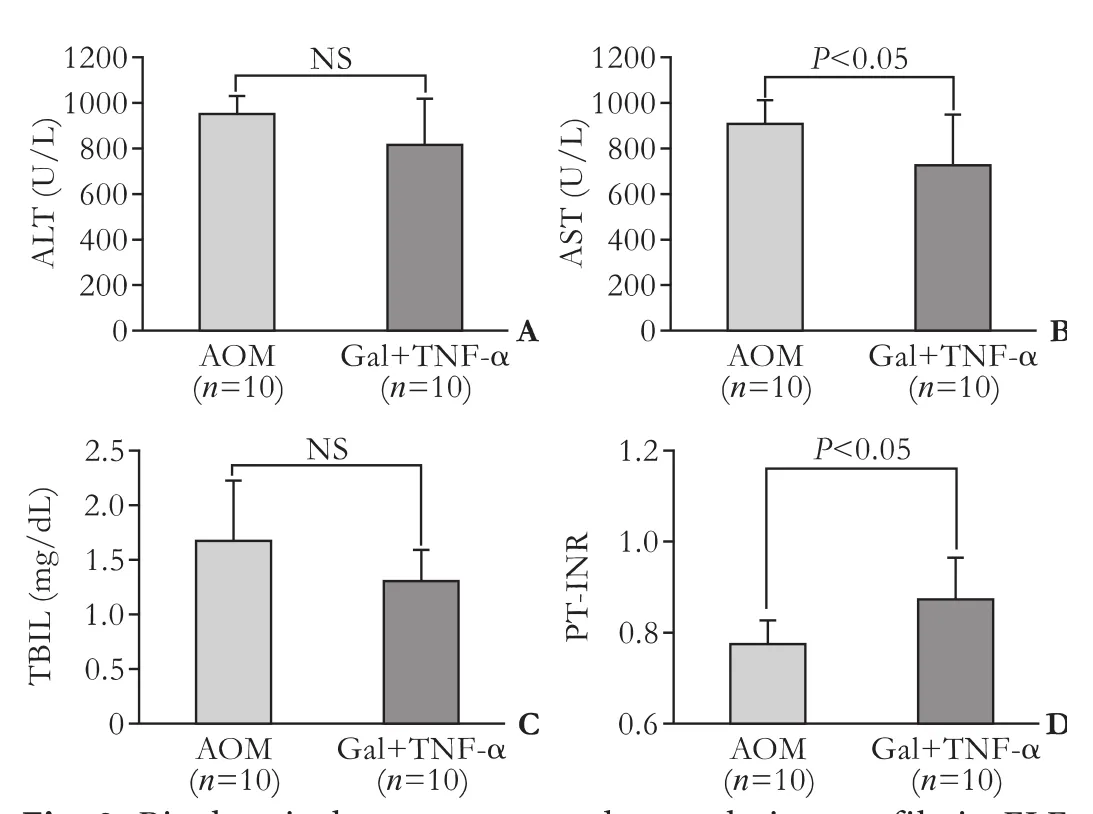
Fig. 3. Biochemical parameters and coagulation profile in FLF models. Levels of ALT (A), AST (B), TBIL (C) and PT-INR (D) in mice after AOM or Gal+TNF-α injection with BT care (n=10 for both groups). AOM: azoxymethane; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BT: body temperature; FLF: fulminant liver failure; HE: hematoxylin-eosin; i.p.: intraperitoneal; NS: not significant; PT-INR: international normalized ratio of prothrombin time; TBIL: total bilirubin.
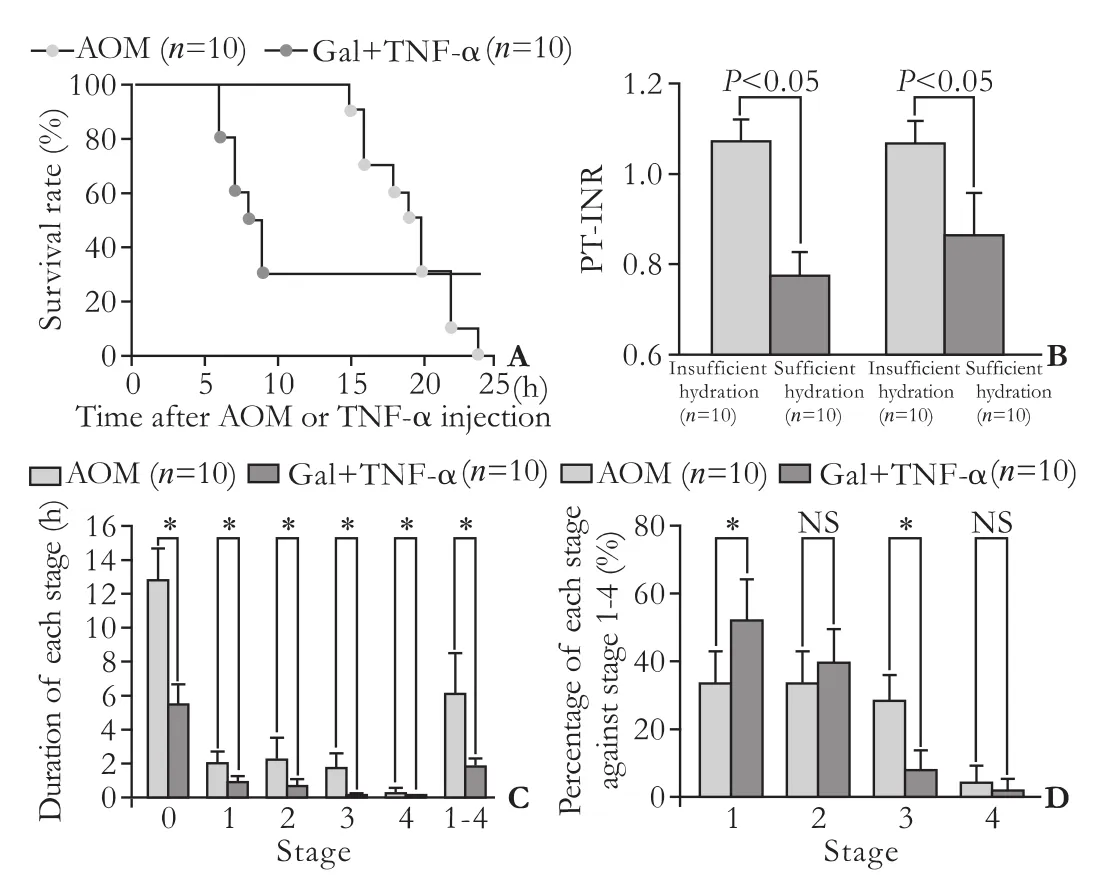
Fig. 4. Survival curves, effects of hydration on coagulation profiles and differences in the intervals of coma stages between FLF models. A: Survival curves after AOM or Gal+TNF-α injection with BT care. We used doses of 100 μg/g bw for AOM and 0.10 μg/g bw for TNF-α, with routine BT care (n=10 for both groups). B: Effects of hydration on coagulation profile. Mouse PT-INR was assessed after AOM or Gal+TNF-α injection with BT care. Mice were classified into two groups with and without sufficient hydration (n=10 for both groups). Sufficient hydration was defined as hydration supplements (lactated Ringer's, 0.5 mL/mouse, i.p.) once an hour from 6 hours after AOM injection and from 2 hours after TNF-α injection. Insufficient hydration was defined as hydration supplements given every 4 hours after AOM or Gal+TNF-α injection. Blood samples were taken at death or 30 hours after initial injection. C: Durations of each coma stage in FLF models. The durations of each coma stage were measured in mice after AOM or Gal+TNF-α injections with BT care (n=10 for both groups). D: Length of each stage as a percentage of total stage-1-4 time. The period between stages 1 and 4 was considered to be the disease state; lengths of each stage as a percentage of the diseased state were calculated in the mice after AOM or Gal+TNF-α injection with BT care (n=10 for both groups). AOM: azoxymethane; BT: body temperature; bw: body weight; FLF: fulminant liver failure; Gal: galactosamine; NS: not significant; TNF-α: tumor necrosis factor-alpha. *: P<0.05.
To evaluate the therapeutic window in each coma stage, the duration of each stage was measured. Using the murine coma scale (Table), the intervals in each coma stage were measured after treatment with AOM (n=10) or Gal+TNF-α (n=10), with BT care. The AOM and the Gal+TNF-α models showed significant differences in the durations of stages 0, 1, 2, 3 and 4 (Fig. 4C). Mice treated with AOM had longer intervals in each stage than did those treated with Gal+TNF-α. The period during stages 1-4 was considered to be a disease state; there were significant differences between the FLF models in the duration of this state. The percentage of each stage against the entire disease state was also calculated. Although stages 2 and 4 did not show significant differences, there were significant differences in stages 1 and 3 (Fig. 4D).
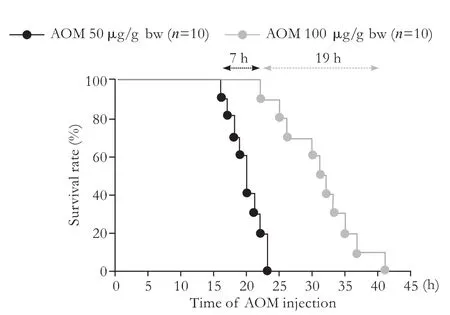
Fig. 5. Dose-dependent survival curves after AOM injections, with BT care. AOM: azoxymethane.
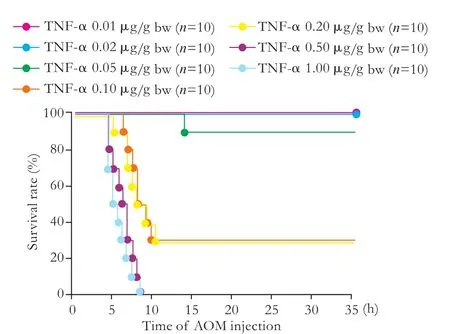
Fig. 6. Dose-dependent survival curves after Gal+TNF-α injection, with BT care. BT: body temperature; Gal:galactosamine; TNF-α: tumor necrosis factor-alpha.
Dose-dependent survival in BT-treated mice given AOM or Gal+TNF-α
While mice were usually given 100 μg/g bw AOM or 0.10 μg/g bw TNF-α, with routine BT care, dosedependent survival studies in both FLF models with BT care were performed.
In many previous studies, 50 or 100 μg/g bw AOM have been used.[8,14,23,25-29]After use of these doses (n=10 for each), survival ranged from within 19 hours at 50 μg/g bw and within 7 hours at 100 μg/g bw, though all mice died from liver-related causes (Fig. 5).
For TNF-α, researchers have used doses ranging from 0.001 to 0.1 μg/g bw.[15,30-34]We used 0.01, 0.02, 0.05, 0.10, 0.20, 0.50 and 1.00 μg/g bw TNF-α, (n=10 for each) because all mice receiving TNF-α ≤0.02 μg/g bw in the preliminary study survived. Survival curves for mice after Gal+TNF-α injections with BT care are shown in Fig. 6.

Fig. 7. Neurological findings for coma staging in FLF models. To obtain a reliable coma stage, close and/or continuous observation of murine behavior was required. Detailed evaluation of coma status of the mice was performed by close observation every 30 minutes until stage 2 and continuous monitoring after stage 2. A: Mouse at stage 0 stands straight and runs fast. B: Mouse at stage 1 walks slowly but straight (red arrow). The movement of the feet is slightly impaired (blue arrow). C: At stage 2, ataxia is enhanced, but reflexes are retained. Tottering is observed (blue arrow), and the mouse cannot walk straight (red arrow). D: Mouse at stage 3 is lethargic, and all spontaneous activity is lost. Subsequently, the mouse cannot recover from the supine position. E: The pinch reflex is disturbed at the late phase of stage 3. F: Mouse becomes flaccid at stage 4 (blue arrow). Corneal reflex is disturbed.
Coma scale based on behavior
To obtain a reliable coma stage, close and/or continuous observation of behavior was required. Detailed evaluation of coma status was performed by close observation every 30 minutes until stage 2 and continuous monitoring thereafter (Fig. 7). Note that continuous observation is crucial for a reliable staging, especially after stage 2, because periodic observations never provide an accurate assessment of disease progression and reliable staging for encephalopathy.
Discussion
AOM is the active metabolite of cycasin, found in cycad palm nuts, only on the island of Guam, USA.[35]In 1963, Laqueur et al[36]discovered that cycad palm nuts induce various cancers of the gastrointestinal and colorectal tracts. Interestingly, they described anecdotally that AOM also causes liver injury.[36]Thereafter, AOM was widely used for research in oncology and hepatology. Though an AOM dose of 20 μg/g bw, i.p. is not hepatotoxic in mice,[8]doses of 50-200 μg/g bw i.p. clearly result in liver failure with identical histopathological findings.[8]
Mild hypothermic changes during disease progression have been documented in FLF models using AOM or acetaminophen.[14,25,37]Mild hypothermia is effective in reducing the liver injury caused by a hepatotoxin; this protective effect is mediated by mechanisms involving improved antioxidant status, together with modulation of inflammation.[14]Reduction of specific proinflammatory cytokines in plasma and the brain results in the improvement of FLF prognosis, including neurological complications.[14]Mild hypothermia results in reduced hepatic damage, improvement in neurological function, normalization of glutathione levels, and selective attenuation in the expression of circulating proinflammatory cytokines.[14,29]In our own results, the hypothermic mice showed prolonged survival, although this hypothermia was more drastic than mild hypothermia, around 35 ℃.[14,29]Possible explanations are differences in age, experimental conditions and intentional hydration. Our results suggest that the AOM FLF model is also useful for investigation of the beneficial effects of hypothermic antioxidant and antiinflammatory mechanisms in FLF prognosis.
Spontaneous activity and intake of food/water start to decrease from 6 hours after AOM injection.[8]As reported in the literature[8]and confirmed by our own observations, at the dose of 100 μg/g bw, about 50% of spontaneous behaviors were lost by 6 hours after AOM injection, and almost all spontaneous activities disappeared at 12 hours. Initially, we tried to feed the mice orally, using a pipette, but did not maintain this because of the risk of aspiration. Supplements via i.p. injection are a reasonable and reliable form of caregiving. Decreased serum glucose occurs from 4 hours after AOM injection in the FLF model.[8]However, administration of exogenous glucose and maintenance of euglycemia did not improve the coma status in the AOM FLF model. This is similar to previous reports in other species, where hypoglycemia is commonly observed to complicate FLF in humans[38-40]and in other animal models of FLF.[41-44]In no instance does correction of hypoglycemia have any impact on neurological status in humans or other species suffering from FLF. Finally, edema complicates the progression of FLF,[3]with up to 25% of patients reported to suffer from edema as a late complication.[45]Previously, we found that FLF mice at stage 3 died immediately after i.p. injection of supplement with 10% dextrose. A possible explanation is the rapid change of osmolarity and subsequent hemodynamic disorder due to the injection of a high osmolarity solution under severe hydration. If so, the disease course and cause of death may be affected by unexpected issues.
Encephalopathy and brain edema are also important complications in the FLF model. Matkowskyj et al[8]described murine behavior in detail. They classified progressive encephalopathy after the prodromal phase due to liver failure into four stages based on neurological behaviors. We also found progressive encephalopathy in the FLF model, and suggested that continuous observation is crucial for a reliable staging, especially after stage 2. Note that periodic observations never provide an accurate assessment of disease progression and a reliable staging for encephalopathy, even in the wide window of FLF mediated by AOM.
Hepatic encephalopathy is a syndrome characterized by altered neurological status that may rapidly progress to stupor and coma.[29]Brain edema resulting in increased intracranial pressure is a second major complication of FLF.[29]Brain edema leads to intracranial hypertension and herniation. This is a common cause of mortality in FLF.[29]A cytotoxic mechanism and changes in the permeability of the blood-brain barrier cause brain edema in FLF;[46,47]many researchers have focused on the subtle blood-brain barrier disruption in FLF.[23,26,27,47]Osmolarity is an important factor as the disease progresses to organ failure and brain edema. Overall, to obtain relevant findings regarding subtle blood-brain barrier disruption we gave sufficient hydration every 1-2 hours from 6 hours after AOM injection with lactated Ringer's solution, which has the lowest osmolarity and highest pH, though our previous protocol was every 8-12 hours after AOM injection (50 μg/g bw) with 10% dextrose solution.[23,28]
An ideal animal FLF model should fulfill the requirements of reversibility, reproducibility, liver-related death, a therapeutic window, suitably-sized animals, and minimal hazard to personnel.[8,11]AOM is the first toxin to satisfy all these criteria, and is also associated with the development of hepatic encephalopathy;[8,11]mice treated with AOM provide the best model for FLF, except for the possible biohazard to personnel. Although work with this model requires a specialized environment to prevent toxic effects to personnel, and intensive caregiving with regard to BT and hydration to avoid confounding influences, our experience shows that many researchers can cope with these conditions. The murine FLF model with AOM is highly reproducible, causes death from liver failure, has a long therapeutic window, and generates coagulopathy, liver-associated encephalopathy and brain edema in end-stage liver disease.
Injection of TNF-α in animals causes severe hepatocyte toxicity, especially when Gal is co-administered.[30]TNF-α is a dominant and terminal mediator of specific failure in Gal-sensitized mice. Some researchers have used an FLF model based on Gal followed by TNF-α,[13,15]or a TNF-α-dependent FLF model with Gal followed by lipopolysaccharide.[13,48]However, researchers used various doses of these toxins and intervals between injections.[13,15]Although many cytokines are involved in inflammatory organ failure, TNF-α is sufficient to induce hepatic apoptosis in Gal-sensitized mice.[13]We performed a preliminary dose-dependence study, because FLF models with Gal and/or TNF-α have low reversibility, poor reproducibility and low rates of liverrelated death compared to the AOM FLF.[8,14]Depending on the purpose of an investigation, FLF models using Gal followed by TNF-α (0.10 μg/g bw) are useable as a second FLF model.[26]
Our results have shown that AOM-induced FLF is highly reproducible, without evidence of lot-to-lot variability. AOM-induced FLF is the best murine model for research in the fields of liver failure and hepatic encephalopathy, although a specialized environment and caregiving are required. One putative concern is the extrahepatic toxicity of AOM. Therefore, as described above, any novel findings in experiments should be verified by several FLF models. In comparison with AOM, our results suggested that Gal followed by TNF-α has less systemic damage, including BT, and lower extrahepatic toxicity. A second FLF model is also needed for investigations requiring negation of some of AOM's unexpected effects (i.e., extrahepatic toxicity). From this viewpoint, FLF with Gal followed by TNF-α has a reasonable advantage as the second FLF model, though FLF with Gal followed by TNF-α showed low reversibility, poor reproducibility, a narrow window and low rates of liver-related death compared to FLF with AOM. In any case, thoughtful attention to caregiving and close observation are indispensable for reliable data with successful FLF models. Only these well-considered FLF models will provide relevant data and novel insights for FLF patients.
Acknowledgments
We are grateful to Dennis W Dickson, Monica Castanedes-Casey, Virginia R Phillips, Linda G Rousseau and Melissa E Murray (Department of Neuroscience, Mayo Clinic in Florida, Jacksonville, fl32224, USA) for their technical support in histopathological evaluation, and to Satoshi Yamamoto (Division of Digestive and General Surgery, Niigata University Hospital, Niigata, Japan), Naoki Shimojima (Department of Pediatric Surgery, Keio University Hospital, Tokyo, Japan) and Norifumi Ohashi (Department of Surgery II, Nagoya University Hospital, Aichi, Japan) for their support.
Funding:This work was partly supported by grants from the Deason Foundation (Sandra and Eugene Davenport, Mayo Clinic CD CRT-II), the AHA (0655589B), the NIH (R01NS051646-01A2), and the Uehara Memorial Foundation (200940051).
Ethical approval:All experimental protocols were approved by the Ethics Committees of the Mayo Clinic (IACUC 33307 and 24907).
Contributors:NJH proposed the study. BAMT and HT performed the research. HT analyzed the data and wrote the paper. CF and GLB helped to collect data. All authors contributed to the design and interpretation of the study and to further drafts. US and NJH supervised the report. HT is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Hoofnagle JH, Carithers RL Jr, Shapiro C, Ascher N. Fulminant hepatic failure: summary of a workshop. Hepatology 1995;21:240-252.
2 Lee WM. Acute liver failure. N Engl J Med 1993;329:1862-1872.
3 Munoz SJ. Difficult management problems in fulminant hepatic failure. Semin Liver Dis 1993;13:395-413.
4 Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010;52:2065-2076.
5 Farmer DG, Anselmo DM, Ghobrial RM, Yersiz H, McDiarmid SV, Cao C, et al. Liver transplantation for fulminant hepatic failure: experience with more than 200 patients over a 17-year period. Ann Surg 2003;237:666-676.
6 Azoulay D, Samuel D, Ichai P, Castaing D, Saliba F, Adam R, et al. Auxiliary partial orthotopic versus standard orthotopic whole liver transplantation for acute liver failure: a reappraisal from a single center by a case-control study. Ann Surg 2001; 234:723-731.
7 Gubernatis G, Pichlmayr R, Kemnitz J, Gratz K. Auxiliary partial orthotopic liver transplantation (APOLT) for fulminant hepatic failure: first successful case report. World J Surg 1991; 15:660-666.
8 Matkowskyj KA, Marrero JA, Carroll RE, Danilkovich AV, Green RM, Benya RV. Azoxymethane-induced fulminant hepatic failure in C57BL/6J mice: characterization of a new animal model. Am J Physiol 1999;277:G455-462.
9 Terblanche J, Hickman R. Animal models of fulminant hepatic failure. Dig Dis Sci 1991;36:770-774.
10 Shito M, Balis UJ, Tompkins RG, Yarmush ML, Toner M. A fulminant hepatic failure model in the rat: involvement of interleukin-1beta and tumor necrosis factor-alpha. Dig Dis Sci 2001;46:1700-1708.
11 Maddison JE, Dodd PR, Johnston GA, Farrell GC. Brain gamma-aminobutyric acid receptor binding is normal in rats with thioacetamide-induced hepatic encephalopathy despite elevated plasma gamma-aminobutyric acid-like activity. Gastroenterology 1987;93:1062-1068.
12 Zimmermann C, Ferenci P, PiflC, Yurdaydin C, Ebner J, Lassmann H, et al. Hepatic encephalopathy in thioacetamideinduced acute liver failure in rats: characterization of an improved model and study of amino acid-ergic neurotransmission. Hepatology 1989;9:594-601.
13 Leist M, Gantner F, Bohlinger I, Tiegs G, Germann PG, Wendel A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am J Pathol 1995;146:1220-1234.
14 Bémeur C, Desjardins P, Butterworth RF. Antioxidant and anti-inflammatory effects of mild hypothermia in the attenuation of liver injury due to azoxymethane toxicity in the mouse. Metab Brain Dis 2010;25:23-29.
15 Wielockx B, Lannoy K, Shapiro SD, Itoh T, Itohara S, Vandekerckhove J, et al. Inhibition of matrix metalloproteinases blocks lethal hepatitis and apoptosis induced by tumor necrosis factor and allows safe antitumor therapy. Nat Med 2001;7:1202-1208.
16 Rahman TM, Selden AC, Hodgson HJ. A novel model of acetaminophen-induced acute hepatic failure in rabbits. J Surg Res 2002;106:264-272.
17 Newsome PN, Henderson NC, Nelson LJ, Dabos C, Filippi C, Bellamy C, et al. Development of an invasively monitored porcine model of acetaminophen-induced acute liver failure. BMC Gastroenterol 2010;10:34.
18 Mark AL, Sun Z, Warren DS, Lonze BE, Knabel MK, Melville Williams GM, et al. Stem cell mobilization is life saving in an animal model of acute liver failure. Ann Surg 2010;252:591-596.
19 Tunón MJ, Alvarez M, Culebras JM, González-Gallego J. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J Gastroenterol 2009;15:3086-3098.
20 Newsome PN, Plevris JN, Nelson LJ, Hayes PC. Animal models of fulminant hepatic failure: a critical evaluation. Liver Transpl 2000;6:21-31.
21 Francavilla A, Makowka L, Polimeno L, Barone M, Demetris J, Prelich J, et al. A dog model for acetaminophen-induced fulminant hepatic failure. Gastroenterology 1989;96:470-478.
22 Miranda AS, Rodrigues DH, Vieira LB, Lima CX, Rachid MA, Vidigal PV, et al. A thioacetamide-induced hepatic encephalopathy model in C57BL/6 mice: a behavioral and neurochemical study. Arq Neuropsiquiatr 2010;68:597-602.
23 Nguyen JH, Yamamoto S, Steers J, Sevlever D, Lin W, Shimojima N, et al. Matrix metalloproteinase-9 contributes to brain extravasation and edema in fulminant hepatic failure mice. J Hepatol 2006;44:1105-1114.
24 Yamamoto S, Nguyen JH. TIMP-1/MMP-9 imbalance in brain edema in rats with fulminant hepatic failure. J Surg Res 2006;134:307-314.
25 Bémeur C, Vaquero J, Desjardins P, Butterworth RF. N-acetylcysteine attenuates cerebral complications of non-acetaminophen-induced acute liver failure in mice: antioxidant and anti-inflammatory mechanisms. Metab Brain Dis 2010;25:241-249.
26 Chen F, Hori T, Ohashi N, Baine AM, Eckman CB, Nguyen JH. Occludin is regulated by epidermal growth factor receptor activation in brain endothelial cells and brains of mice with acute liver failure. Hepatology 2011;53:1294-1305.
27 Chen F, Ohashi N, Li W, Eckman C, Nguyen JH. Disruptions of occludin and claudin-5 in brain endothelial cells in vitro and in brains of mice with acute liver failure. Hepatology 2009;50:1914-1923.
28 Shimojima N, Eckman CB, McKinney M, Sevlever D, Yamamoto S, Lin W, et al. Altered expression of zonula occludens-2 precedes increased blood-brain barrierpermeability in a murine model of fulminant hepatic failure. J Invest Surg 2008;21:101-108.
29 Bélanger M, Coté J, Butterworth RF. Neurobiological characterization of an azoxymethane mouse model of acute liver failure. Neurochem Int 2006;48:434-440.
30 Libert C, Wielockx B, Grijalba B, Van Molle W, Kremmer E, Colten HR, et al. The role of complement activation in tumour necrosis factor-induced lethal hepatitis. Cytokine 1999;11:617-625.
31 Song HL, Lv S, Liu P. The roles of tumor necrosis factor-alpha in colon tight junction protein expression and intestinal mucosa structure in a mouse model of acute liver failure. BMC Gastroenterol 2009;9:70.
32 Wullaert A, Wielockx B, Van Huffel S, Bogaert V, De Geest B, Papeleu P, et al. Adenoviral gene transfer of ABIN-1 protects mice from TNF/galactosamine-induced acute liver failure and lethality. Hepatology 2005;42:381-389.
33 Takenaka K, Sakaida I, Yasunaga M, Okita K. Ultrastructural study of development of hepatic necrosis induced by TNF-alpha and D-galactosamine. Dig Dis Sci 1998;43:887-892.
34 Van Lint P, Wielockx B, Puimège L, Noel A, López-Otin C, Libert C. Resistance of collagenase-2 (matrix metalloproteinase-8)-deficient mice to TNF-induced lethal hepatitis. J Immunol 2005;175:7642-7649.
35 Wright N, Alison M. Cell proliferation in gastrointestinal carcinogenesis. In: The Biology of Epithelial Cell Populations. Oxford, UK: Clarendon;1984:805-841.
36 Laqueur GL, Mickelsen O, Whiting MG, Kurland LT. Carcinogenic Properties of Nuts from Cycas Circinalis L. Indigenous to Guam. J Natl Cancer Inst 1963;31:919-951.
37 Vaquero J, Butterworth RF. Mild hypothermia for the treatment of acute liver failure--what are we waiting for? Nat Clin Pract Gastroenterol Hepatol 2007;4:528-529.
38 Chapman RW, Forman D, Peto R, Smallwood R. Liver transplantation for acute hepatic failure? Lancet 1990;335:32-35.
39 Emond JC, Aran PP, Whitington PF, Broelsch CE, Baker AL. Liver transplantation in the management of fulminant hepatic failure. Gastroenterology 1989;96:1583-1588.
40 O'Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989;97:439-445.
41 Blitzer BL, Waggoner JG, Jones EA, Gralnick HR, Towne D, Butler J, et al. A model of fulminant hepatic failure in the rabbit. Gastroenterology 1978;74:664-671.
42 Kelly JH, Koussayer T, He DE, Chong MG, Shang TA, Whisennand HH, et al. An improved model of acetaminopheninduced fulminant hepatic failure in dogs. Hepatology 1992; 15:329-335.
43 van Leenhoff JA, Hickman R, Saunders SJ, Terblanche J. Massive liver cell necrosis induced in the pig with carbon tetrachloride. S Afr Med J 1974;48:1201-1204.
44 Sielaff TD, Hu MY, Rollins MD, Bloomer JR, Amiot B, Hu WS, et al. An anesthetized model of lethal canine galactosamine fulminant hepatic failure. Hepatology 1995; 21:796-804.
45 Ellis A, Wendon J. Circulatory, respiratory, cerebral, and renal derangements in acute liver failure: pathophysiology and management. Semin Liver Dis 1996;16:379-388.
46 Traber P, DalCanto M, Ganger D, Blei AT. Effect of body temperature on brain edema and encephalopathy in the rat after hepatic devascularization. Gastroenterology 1989;96: 885-891.
47 Butterworth RF. Hepatic encephalopathy: a central neuroinflammatory disorder? Hepatology 2011;53:1372-1376.
48 Stuart WD, Kulkarni RM, Gray JK, Vasiliauskas J, Leonis MA, Waltz SE. Ron receptor regulates Kupffer cell-dependent cytokine production and hepatocyte survival following endotoxin exposure in mice. Hepatology 2011;53:1618-1628.
Received July 11, 2011
Accepted after revision October 25, 2011
Author Affiliations: Department of Neuroscience (Baine AMT, Hori T, Chen F and Gardner LB), and Division of Transplant Surgery, Department of Transplantation (Nguyen JH), Mayo Clinic in Florida, Jacksonville, fl32224, USA; Divisions of Hepato-Pancreato-Biliary, Transplant and Pediatric Surgery, Department of Surgery, Kyoto University Graduate School of Medicine, Kyoto 606-8507, Japan (Uemoto S)
Tomohide Hori, PhD, MD, Department of Neuroscience, Mayo Clinic in Florida, Birdsall Research Bldg., #323, 3rd floor, 4500 San Pablo Rd., Jacksonville, fl32224, USA (Tel: +1-904-953-2449; Fax: +1-904-953-7117; Email: hori.tomohide@mayo.edu)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(11)60104-5
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Risk factors of intrahepatic cholangiocarcinoma in patients with hepatolithiasis: a case-control study
- Relationship between pancreaticobiliary maljunction and gallbladder carcinoma: a meta-analysis
- Harmine induces apoptosis in HepG2 cells via mitochondrial signaling pathway
- Undifferentiated embryonal sarcoma of the liver presenting as a hemorrhagic cystic tumor in an adult
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Clinicopathological analysis of 14 patients with combined hepatocellular carcinoma and cholangiocarcinoma
