Harmine induces apoptosis in HepG2 cells via mitochondrial signaling pathway
2011-07-03MingRongCaoQiangLiZhiLongLiuHuiHuiLiuWeiWangXiaoLiLiaoYunLongPanandJianWeiJiang
Ming-Rong Cao, Qiang Li, Zhi-Long Liu, Hui-Hui Liu, Wei Wang, Xiao-Li Liao, Yun-Long Pan and Jian-Wei Jiang
Guangzhou, China
Original Article / Liver
Harmine induces apoptosis in HepG2 cells via mitochondrial signaling pathway
Ming-Rong Cao, Qiang Li, Zhi-Long Liu, Hui-Hui Liu, Wei Wang, Xiao-Li Liao, Yun-Long Pan and Jian-Wei Jiang
Guangzhou, China
BACKGROUND:Harmine has antitumor and antinociceptive effects, and inhibits human DNA topoisomerase. However, no detailed data are available on the mechanisms of action of harmine in hepatocellular carcinoma. This study aimed to investigate the effects of harmine on proliferation and apoptosis, and the underlying mechanisms in the human hepatocellular carcinoma cell line HepG2.
METHODS:The proliferation of HepG2 cells was determined by the cell counting kit-8 (CCK-8) assay and the clone formation test. The morphology of HepG2 cells was examined using fluorescence microscopy after Hoechst 33258 staining. Annexin V/propidium iodide (PI) was used to analyze apoptosis and PI to analyze the cell cycle. Western blotting was used to assess expression of the apoptosis-regulated genes Bcl-2, Bax, Bcl-xl, Mcl-1, caspase-3, and caspase-9. Mitochondrial transmembrane potential (Ψm) was determined using JC-1.
RESULTS:Harmine inhibited the proliferation of HepG2 cells in a dose-dependent manner. Hoechst 33258 staining revealed nuclear fragmentation and chromosomal condensation, cell shrinkage, and attachment loss in HepG2 cells treated with harmine. The percentage of the sub/G1 fraction was increased in a concentration-dependent manner, indicating apoptotic cell death. PI staining showed that harmine changed the cell cycle distribution, by decreasing the proportion of cells inG0/G1 and increasing the proportion in S and G2/M. Harmine induced apoptosis in a concentration-dependent manner, with rates of 20.0%, 32.7% and 64.9%, respectively. JC-1 revealed a decrease inΨm. Apoptosis of HepG2 cells was associated with caspase-3 and caspase-9 activation, down-regulation of Bcl-2, Mcl-1, and Bcl-xl, and no change in Bax.
CONCLUSIONS:Harmine had an anti-proliferative effect in HepG2 cells by inducing apoptosis. Mitochondrial signal pathways were involved in the apoptosis. The cancer-specific selectivity shown in this study suggested that harmine is a promising novel drug for human hepatocellular carcinoma.
(Hepatobiliary Pancreat Dis Int 2011; 10: 599-604)
hepatocellular carcinoma; harmine; Bcl-2 protein; caspase-3; apoptosis
Introduction
Hepatocellular carcinoma (HCC) is a common and aggressive malignant tumor worldwide. Primary liver cancer, which consists predominantly of HCC, is the fifth most common cancer worldwide and the third most common cause of cancer mortality. In China, HCC accounts for 90% of primary liver cancer, which is the second most common cause of death.[1]Recent data indicate that the mortality of HCC in China is tending to increase, severely threatening the health and life of people. Currently, the treatments for HCC are mainly surgery and chemotherapy, but the curative effect of existing chemotherapeutic drugs is not good enough and they have numerous side-effects, including myelosuppression, neutropenia, and thrombocytopenia.[2,3]Therefore, it has become a focus to search for drugs which are capable of preventing and treating HCC and other malignancies. One possible way to increase the efficacy of anticancer drugs and decrease toxicity or side-effects is to develop traditional medicines, especially from medicinal plants.[4-7]Herbalmedicines are an important source of novel agents with pharmaceutical potential. Harmine is a major component isolated from Peganum harmala L. (Zygophyllaceae) seed extract. The pharmacological characterization shows that harmine has antitumor[8,9]and antinociceptive effects,[10]and inhibits human DNA topoisomerase.[11]However, no detailed data are available on the mechanisms of action of harmine in HCC. We investigated its effect on the growth of human HepG2 cells and the underlying intracellular signal transduction pathways involved in regulating apoptosis.
Methods
Materials
Harmine was from Xi'an Feida Bio-Tech Co., Ltd. The following were all from Cell Signaling (Danvers, Massachusetts, USA): antibodies for immunoblots anticaspase-3 (#9662), anti-caspase-9 (#9502), anti-Bcl-2 (#2870), anti-Mcl-1 (#4572), anti-Bcl-xl (#2764), anti-Bax (#2772), and GAPDH (#3683); and cell lysis buffer.
Cell culture and treatment with harmine
Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum at 37 ℃in a humidified atmosphere containing 5% CO2. The harmine was dissolved in dimethyl sulfoxide (DMSO), and diluted to appropriate concentrations with culture medium. The final concentration of DMSO in the culture medium did not exceed 0.1%.
CCK-8 assay
The CCK-8 test was used to monitor cell proliferation. Cells were plated at a density of 5000 cells/well in 96-well plates. After 24 hours in culture, the cells were treated with harmine at the final concentrations 0, 0.625, 1.25, 2.5, 5, 10, or 20 μg/mL for 48 hours. Control cells were treated with DMSO. After addition of test compounds, 10 μL CCK-8 was added to each well. Absorbance was detected with an enzyme calibrator at 570 nm and then optical density (OD) values were measured. Inhibition of cell growth was computed by the percentage of viable cells compared with control. Percentage (%)=(ODCODT)/ODC×100%. ODT is the OD value of the treated sample, and ODC is the OD value of the control sample. Experiments were done in triplicate.
Clone formation assay
A clone formation assay was used to evaluate the effects of harmine on the proliferation of HepG2 cells.[12]Cells were first cultured in 12-well microplates (300 cells/well) in 2.5 mL of complete RPMI-1640 for 24 hours. Then the cells were treated with the indicated concentrations harmine for 7 days. Finally, the cells were stained in crystal violet for 20 minutes. Images of the colonies were captured by a digital camera.
Fluorescence microscopy assay
Harmine-induced apoptosis in HepG2 cells was assessed by Hoechst 33258 staining. Treated with 5 μg/mL harmine for 48 hours, the cells were harvested and smeared on slides. The slides were air-dried, fixed in methanol-acetone (3/1, v/v), and stained with Hoechst 33258 (5 μg/mL) at 37 ℃ for 20 minutes. Nuclear morphology was examined under fluorescence microscopy (DFC480; Leica Microsystems, Wetzlar, Germany) to identify cells undergoing apoptosis.
Flow cytometry
HepG2 cells at a density of 20 000 cells/well were incubated in 6-well plates for 48 hours with DMSO, 0, 5, 10, or 20 μg/mL harmine. After incubation, the cells were collected and analyzed by flow cytometry. Then the cells were harvested, washed with phosphate buffer solution (PBS), and fixed in 70% ice-cold ethanol overnight. The fixed cells were incubated with 20 U/mL RNaseiand 50 μg/mL propidium iodide (PI) for 30 minutes. The DNA content was determined by flow cytometry (Beckman Coulter, Fullerton, CA, USA). Apoptotic cells were identified by the sub-G1 phase in the cellcycle distribution. For assessment of the apoptotic rate, Annexin V-FITC/PI (Becton Dickinson, USA) staining was performed according to the manufacturer's protocol. The cells stained with Annexin V but not with PI were defined as apoptotic. The apoptotic rate was measured by flow cytometry (FCM, Becton Dickinson, USA) using CellQuest software.
Western blotting
Total proteins were extracted by incubation of cell pellets with lysis buffer. The protein concentration of the extracts was determined by bicinchoninic acid (Sigma) according to the manufacturer's instructions. Cell lysates (50 μg/well) were electrophoresed in 12% SDS-PAGE and then transferred onto nitrocellulose membranes. After blotting in 5% non-fat dry milk in TBST buffer, the membranes were incubated with primary antibodies in 5% non-fat milk overnight at 4 ℃, and then secondary antibodies conjugated with horseradish peroxidase for 1 hour at room temperature. Immunoreactive bands were visualized by enhanced chemiluminescence regents (Amersham, UK). To assessthe presence of comparable amounts of proteins in each lane, the membranes were stripped to assess GAPDH.
Membrane potential of mitochondria (Ψm)
Changes of Ψmwere monitored by determination of JC-1. HepG2 cells were treated with 5 μg/mL harmine for 48 hours, then the cells were harvested, washed with PBS, and fixed in JC-1 at 37 ℃ in the dark for 30 minutes, when they were harvested and smeared on slides. Changes in Ψmwere measured after staining under fluorescence microscopy.
Statistical analysis
Data were analyzed by ANOVA and Student's t test. These analyses were performed using SPSS 13.0 software. Differences withP<0.05 were considered significant.
Results
Harmine inhibits HepG2 cell proliferation
Cell viability was decreased remarkably by harmine treatment (Fig. 1A). Harmine inhibited the growth of HepG2 cells in a dose-dependent manner (P<0.05 vs control), with an IC50of 9.80 μg/mL. To assess the effect of harmine on proliferation, the cells were treated with the indicated concentrations for 7 days. The result showed that harmine strongly inhibited their proliferation (Fig. 1B).

Fig. 1. A: Effects of harmine on viability of HepG2 cells. The indicated concentrations of harmine were added, and the cells were incubated for 48 hours. Viability was measured by CCK-8 assay. Results represented 3 independent experiments. B: Clone formation assay was used to evaluate the effects of harmine on the proliferation of HepG2 cells. HepG2 cells were treated with 0, 0.625, 1.25, 2.5, or 5 μg/mL harmine for 7 days and stained in crystal violet for 20 minutes.
Harmine induces apoptosis in HepG2 cells
Apoptotic nuclear morphology was observed after Hoechst 33258 staining using fluorescence microscopy. After treatment with 5 μg/mL harmine for 48 hours, HepG2 cells began to exhibit apoptotic characteristics, such as cell shrinkage, nuclear condensation, and fragmentation. In the control group, the cells were regular in morphology and grew fully in patches and were confluent, rarely sloughing off (Fig. 2).
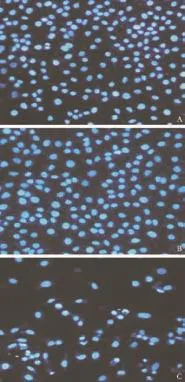
Fig. 2. Harmine-induced apoptosis in HepG2 cells was assessed by Hoechst 33258 staining. Morphology of HepG2 cells exposed to harmine at different concentrations photographed under a fluorescence microscope (original magnification ×200). A: DMSO; B: 0 μg/mL; C: 5 μg/mL.
Apoptosis was also detected through fluorescein Annexin V-FITC/PI double labeling.[13]The staining of cells with Annexin V-FITC and PI was used todistinguish and quantitatively determine the percentage of apoptotic cells. HepG2 cells underwent apoptosis after exposure to harmine at 5, 10, and 20 μg/mL for 48 hours. The percentage of apoptotic cells stained by Annexin V-FITC is shown in Fig. 3A. PI staining and flow cytometry were used to investigate whether harmine inhibited the growth of HepG2 cells by initiating the apoptotic pathway. HepG2 cells underwent apoptosis after being exposed to harmine at 5, 10, and 20 μg/mL for 48 hours (Fig. 3B). The percentage of apoptotic cells in the sub-G1 phase of the cell cycle increased. The percentage of the sub/G1 fraction, which was indicative of apoptotic cell death in harmine-treated cells, increased in a dose-dependent manner.
Harmine changes the cell cycle in HepG2 cells
Flow cytometry with only PI staining showed that treatment of HepG2 cells with 5, 10, and 20 μg/mL harmine for 48 hours resulted in a higher number of cells in S phase and G2/M phase compared with the control group (Fig. 4). This increase was coupled with the decreased percentage of cells in G0/G1 phase.
Effect of harmine on the protein expression level
Loss of Ψmis a crucial step in the apoptotic process and is lethal to cells because it leads to the release of diverse pro-apoptotic factors from the mitochondria into the cytoplasm.[14]In this study, we used the unique cationic dye JC-1 to determine the status of mitochondria. In non-apoptotic cells, JC-1 enters the negatively-charged mitochondria where it aggregates and turns red. However, in cells undergoing apoptosis, where Ψmhas collapsed, JC-1 exists as monomers in the cytosol and turns green. Our results showed that harmine induced a depletion of Ψmin HepG2 cells (Fig. 5A).
The Bcl-2 family of proteins are key regulators of mitochondrial permeability.[15]Therefore, we investigated whether the mitochondria-mediated apoptosis in HepG2 cells induced by harmine was modulated by Bcl-2 family members. Harmine suppressed the expression of Bcl-2, Mcl-1, and Bcl-xl, and did not change the expression of Bax (Fig. 5B). As a result of these changes, the ratios of Bcl-2/Bax, Mcl-1/Bax, and Bcl-xl/Bax were reduced significantly during apoptosis. To delineate the possible signaling pathways by which harmine induced HepG2 cellapoptosis, we determined the changes in the expression levels of various apoptosis-regulating proteins.
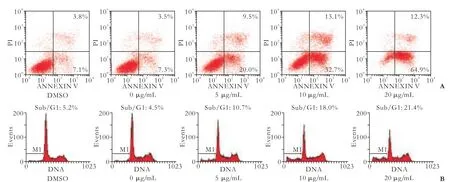
Fig. 3. Staining cells with Annexin V-FITC and PI was used to distinguish and quantitatively determine the percentage of apoptotic cells. The rate of apoptosis increased in a dose-dependent manner.

Fig. 4. PI staining showed characteristic features of apoptosis in harmine-exposed cells. The number of cells in S phase and G2/M phase increased and the percentage of cells in G0/G1 phase decreased compared with the control group.

Fig. 5. Harmine induced apoptosis of HepG2 cells through a mitochondrial signaling pathway. A: Change of Ψmwas evaluated by fluorescence microscopy. HepG2 cells were treated with 5 μg/mL harmine for 48 hours. B, C: Harmine activated Bcl-2 family members, caspase-3 and caspase-9. HepG2 cells were treated with 0, 1.25, 2.5, and 5 μg/mL harmine for 48 hours. The effect of harmine on the protein expression level was evaluated by immunoblotting. Immunoblotting data were quantitated from at least three separate experiments.
Caspase, a family of cysteine acid proteases, is known to act as an important mediator of apoptosis and contributes to the overall apoptotic morphology by cleavage of various cellular substrates. Exposure of HepG2 cells to harmine resulted in cleavage of caspase-3 and caspase-9 (Fig. 5C).
Discussion
Although contemporary therapeutic strategies have shown remarkable anticancer ability, severe sideeffects are unavoidable. The search for new antitumor agents that are more effective but less toxic has kindled great interest. Since apoptosis plays an important role in developmental processes, homeostasis, and the elimination of damaged cells, accumulating data indicate that many anticancer drugs cause the death of tumor cells through the induction of apoptosis.[16,17]Although harmine shows effective antitumor activity, its effects on human hepatocytes are still unclear. Therefore, the purpose of the present study was to reveal the underlying molecular mechanism by which harmine induces apoptosis in the human HCC cell line HepG2.
We demonstrated that harmine dose-dependently inhibited the proliferation of HepG2 cells, and moreover, it also induced apoptosis and arrested the cell cycle. Morphological changes in apoptotic characteristics, such as cellular shrinkage, rounding, poor adherence, and round floating shapes in harmine-treated cells were also observed by fluorescence microscopy. Flow cytometry analysis revealed that harmine treatment resulted in an increase of apoptotic cells. These results suggested that the growth inhibition of HepG2 cells by harmine is due to its ability to induce apoptosis.
Mitochondria play an essential role in apoptosis, since both the intrinsic and extrinsic apoptosis pathways converge at the mitochondrial level and trigger mitochondrial membrane permeabilization.[15]Mitochondria-mediated apoptosis is precisely regulated by Bcl-2 family proteins, which can be divided into two subfamilies:[15,18,19]one is anti-apoptotic such as Bcl-2 and Bcl-xl, the other is pro-apoptotic like Bax, Bad, and Bid.[20]The expression of Bcl-2 and Bax is important in the balance of pro-apoptotic and anti-apoptotic signals at the mitochondrial level.[21]In our experiments, harmine had no effect on expression of the pro-apoptotic Bax protein but decreased the expression levels of anti-apoptotic Bcl-2, Bcl-xl, and Mcl-1, leading to up-regulation of the Bax/Bcl-2, Bax/Bcl-xl, and Bax/Mcl-1 ratios. This might be responsible for the concomitant execution phase of apoptosis, which included disruption of Ψm.
Caspase, a family of cysteine proteases, is an integral part of the apoptotic pathway. Caspase-9 is the apical caspase in the intrinsic or mitochondriainitiated apoptosis pathway and requires the release of cytochrome C from the mitochondria. Activation of caspase-3 correlates with activation of caspase-9. Caspase-3 in particular, when activated, has many cellular targets.[22]During apoptosis, caspase-3 is one of the key executioners of apoptosis in response to various stimuli.[23]In many studies, it has been determined that a variety of chemotherapeutic agents induce apoptosis through the activation of caspase.[16]Consistent with an increase in the ratio of Bax/Bcl-2 and disruption of Ψm, this study showed that harmine resulted in dosedependent activation of caspase-9 and caspase-3.
In conclusion, we found that harmine-induced apoptosis was accompanied by modulation of the Bcl-2 family, mitochondrial dysfunction and activation of caspase in HepG2 cells, implying that harmine induced apoptosis via the mitochondrial death pathway. Therefore, we believe that harmine might be a promising molecule in cancer chemoprevention or chemotherapy and further efforts to explore this therapeutic strategy are needed.
Funding:This study was supported by grants from the Sci-Tech Project Foundation of Guangdong Province, China (2010B031600248) and the National Natural Science Foundation of China (30772131).Ethical approval:Not needed.
Contributors:CMR, LQ and JJW proposed the study and wrote the first draft. LQ analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. JJW is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Chen JA, Shi M, Li JQ, Qian CN. Angiogenesis: multiple masks in hepatocellular carcinoma and liver regeneration. Hepatol Int 2010;4:537-547.
2 Chau GY, Lui WY, Tsay SH, Chao Y, King KL, Wu CW. Postresectional adjuvant intraportal chemotherapy in patients with hepatocellular carcinoma: a case-control study. Ann Surg Oncol 2006;13:1329-1337.
3 Lo CM, Liu CL, Chan SC, Lam CM, Poon RT, Ng IO, et al. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg 2007;245:831-842.
4 Dai ZJ, Wang XJ, Li ZF, Ji ZZ, Ren HT, Tang W, et al. Scutellaria barbate extract induces apoptosis of hepatoma H22 cells via the mitochondrial pathway involving caspase-3. World J Gastroenterol 2008;14:7321-7328.
5 El Gendy MA, Somayaji V, El-Kadi AO. Peganum harmala L. is a candidate herbal plant for preventing dioxin mediated effects. Planta Med 2010;76:671-677.
6 Astulla A, Zaima K, Matsuno Y, Hirasawa Y, Ekasari W, Widyawaruyanti A, et al. Alkaloids from the seeds of Peganum harmala showing antiplasmodial and vasorelaxant activities. J Nat Med 2008;62:470-472.
7 Li H, Wang LJ, Qiu GF, Yu JQ, Liang SC, Hu XM. Apoptosis of Hela cells induced by extract from Cremanthodium humile. Food Chem Toxicol 2007;45:2040-2046.
8 El Gendy MA, El-Kadi AO. Peganum harmala L. differentially modulates cytochrome P450 gene expression in human hepatoma HepG2 cells. Drug Metab Lett 2009;3:212-216.
9 Farouk L, Laroubi A, Aboufatima R, Benharref A, Chait A. Evaluation of the analgesic effect of alkaloid extract of Peganum harmala L: possible mechanisms involved. J Ethnopharmacol 2008;115:449-454.
10 Wu C, Jiang XL, Shen HW, Yu AM. Effects of CYP2D6 status on harmaline metabolism, pharmacokinetics and pharmacodynamics, and a pharmacogenetics-based pharmacokinetic model. Biochem Pharmacol 2009;78:617-624.
11 Singh Y, Palombo M, Sinko PJ. Recent trends in targeted anticancer prodrug and conjugate design. Curr Med Chem 2008;15:1802-1826.
12 Shao XD, Wu KC, Guo XZ, Xie MJ, Zhang J, Fan DM. Expression and significance of HERG protein in gastric cancer. Cancer Biol Ther 2008;7:45-50.
13 Chen S, Cheng AC, Wang MS, Peng X. Detection of apoptosis induced by new type gosling viral enteritis virus in vitro through fluorescein annexin V-FITC/PI double labeling. World J Gastroenterol 2008;14:2174-2178.
14 Marzetti E, Wohlgemuth SE, Lees HA, Chung HY, Giovannini S, Leeuwenburgh C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech Ageing Dev 2008;129:542-549.
15 Wang JB, Qi LL, Zheng SD, Wu TX. Curcumin induces apoptosis through the mitochondria-mediated apoptotic pathway in HT-29 cells. J Zhejiang Univ Sci B 2009;10:93-102.
16 Lah JJ, Cui W, Hu KQ. Effects and mechanisms of silibinin on human hepatoma cell lines. World J Gastroenterol 2007; 13:5299-5305.
17 Edderkaoui M, Odinokova I, Ohno I, Gukovsky I, Go VL, Pandol SJ, et al. Ellagic acid induces apoptosis through inhibition of nuclear factor kappa B in pancreatic cancer cells. World J Gastroenterol 2008;14:3672-3680.
18 Suzuki S, Yokoyama U, Abe T, Kiyonari H, Yamashita N, Kato Y, et al. Differential roles of Epac in regulating cell death in neuronal and myocardial cells. J Biol Chem 2010;285:24248-24259.
19 Ghibelli L, Diederich M. Multistep and multitask Bax activation. Mitochondrion 2010;10:604-613.
20 Roos DH, Puntel RL, Lugokenski TH, Ineu RP, Bohrer D, Burger ME, et al. Complex methylmercury-cysteine alters mercury accumulation in different tissues of mice. Basic Clin Pharmacol Toxicol 2010;107:789-792.
21 Hetz CA. ER stress signaling and the BCL-2 family of proteins: from adaptation to irreversible cellular damage. Antioxid Redox Signal 2007;9:2345-2355.
22 Bialik S, Zalckvar E, Ber Y, Rubinstein AD, Kimchi A. Systems biology analysis of programmed cell death. Trends Biochem Sci 2010;35:556-564.
23 Lüthi AU, Martin SJ. The CASBAH: a searchable database of caspase substrates. Cell Death Differ 2007;14:641-650.
Received December 27, 2010
Accepted after revision May 19, 2011
Author Affiliations: Department of General Surgery, First Affiliated Hospital, Jinan University, Guangzhou 510632, China (Cao MR, Li Q, Liu ZL and Pan YL); Department of Anesthesiology, First Affiliated Hospital, Guangzhou University of TCM, Guangzhou 510632, China (Liu HH); Department of Biochemistry, Medical College of Jinan University, Guangzhou 510632, China (Wang W, Liao XL and Jiang JW)
Jian-Wei Jiang, MD, Department of Biochemistry, Medical College of Jinan University, Guangzhou 510632, China (Tel: 86-20-85220256; Email: jjw703@163.com)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(11)60102-1
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Risk factors of intrahepatic cholangiocarcinoma in patients with hepatolithiasis: a case-control study
- Fulminant liver failure models with subsequent encephalopathy in the mouse
- Relationship between pancreaticobiliary maljunction and gallbladder carcinoma: a meta-analysis
- Undifferentiated embryonal sarcoma of the liver presenting as a hemorrhagic cystic tumor in an adult
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Clinicopathological analysis of 14 patients with combined hepatocellular carcinoma and cholangiocarcinoma
