Corticosteroids or non-corticosteroids: a fresh perspective on alcoholic hepatitis treatment
2011-07-03FeiWangandBingYuanWang
Fei Wang and Bing-Yuan Wang
Shenyang, China
Review Article
Corticosteroids or non-corticosteroids: a fresh perspective on alcoholic hepatitis treatment
Fei Wang and Bing-Yuan Wang
Shenyang, China
BACKGROUND:Alcoholic hepatitis (AH) is a necrotizing inflammatory process caused by alcoholic liver injury. It carries a significant short-term mortality. The management of AH is challenging. Although corticosteroids have been demonstrated to exert anti-inflammatory and antifibrotic effects, their efficacy for the treatment of AH remains debatable.
DATA SOURCES:A literature search was performed of MEDLINE, ScienceDirect, SpringerLink and Wiley InterScience using the key words "alcoholic hepatitis", "alcoholic liver disease", and "corticosteroids". The available data reported in the relevant literature were analyzed.
RESULTS:More than 17 controlled trials and at least 13 meta-analyses have reported the efficacy of corticosteroids in the treatment of AH in the past 40 years. Many were poorly designed and used different inclusion/exclusion criteria, making it difficult to reach a consensus. In this review, we summarized all the controversial data in the past decade and analyzed the potential causes for the varying therapeutic effects of corticosteroids in AH. The focus of the controversy has changed from "whether steroids are beneficial or harmful for AH patients" to "how to accurately identify responders to steroids early and rationalize corticosteroid treatment". An early response to glucocorticoids, as determined by calculating the Lille score after 7 days of treatment, has been shown to be a clinically useful indicator. Moreover, down-regulation of steroid sensitivity, risk of infection, and a rational therapeutic strategy of corticosteroids in AH patients are all crucial for therapeutic effect.
CONCLUSIONS:An early and accurate determination of steroid sensitivity is important. Besides, we need to overcome the down-regulation of steroid sensitivity, reduce the infection risk and rationalize the therapeutic strategy of corticosteroids. A fresh perspective is needed on the use of corticosteroids in AH patients.
(Hepatobiliary Pancreat Dis Int 2011; 10: 458-464)
corticosteroids; alcoholic liver disease; alcoholic hepatitis; steroid sensitivity; scoring system
Introduction
The causal association between alcohol intake and the development of chronic liver disease has been well demonstrated.[1]According to the World Health Organization, there are about 2 billion alcohol consumers worldwide and 76.3 million people may have alcohol-related disorders.[2]Most of them are moderate drinkers, while among heavy drinkers, more than 90% show evidence of alcoholic steatosis. Alcoholic steatosis is an initial pathology found in the early stages of alcoholic liver disease (ALD), characterized by accumulation of triglyceride in hepatocytes. Patients in this stage rarely manifest symptoms and they may reverse completely within several weeks after discontinuation of alcohol intake. If alcohol abuse continues, 35% of the patients will develop inflammatory changes in the liver (alcoholic hepatitis, AH), which triggers fibrogenesis and collagen deposition in perivenular and pericellular spaces.[3]The progression to AH is a key step in the development of ALD. Approximately 40% of the patients with this lesion will develop cirrhosis within 5 years, characterized by significant hardening of the liver, decreased hepatocyte regeneration and significant loss of liver function.[4]In reality, these entities overlap, and may present simultaneously in a given individual.
The correct diagnosis of AH is generally based on alcohol ingestion history, clinical features and laboratorydata. A liver biopsy is desirable to confirm the diagnosis and define the histopathological stage, but has mostly been replaced by less invasive scoring systems. The treatment of severe AH constitutes one of the main challenges to clinicians. Abstinence remains paramount in the management of all forms of ALD, and nutritional therapy is the first line of therapeutic intervention.[5,6]Other potential treatments include general supportive and symptomatic care.
An extensively studied intervention in AH is the use of corticosteroids. Previous studies have shown that corticosteroids inhibit important pro-inflammatory transcription factors, reduce cytokine production, suppress the formation of acetaldehyde adducts, and improve the short-term histology in AH.[7]However, because of the potential side-effects (including antianabolism, muscle breakdown, immunosuppression, increased susceptibility to infection and the risk of gastrointestinal bleeding) as well as conflicting data from clinical trials and meta-analysis, it is difficult to draw meaningful conclusions regarding the efficacy of corticosteroids in the treatment of AH.
In this review, we summarized all the controversial data in the past decade and analyzed the potential causes of the different therapeutic effects of corticosteroids in AH, in order to answer several important questions such as (i) whether steroids are beneficial or harmful for AH patients, (ii) how steroid responders can be accurately identified early and how to rationalize corticosteroid treatment, (iii) why an early response to corticosteroids is important for AH patients, and (iv) how to calculate and determine early response. Moreover, from this review, we believe that other factors are also crucial for the therapeutic effect of corticosteroids in AH patients.
Alcohol-related liver injury and the role of corticosteroids
In the last several decades, significant progress has been made in understanding the cellular and molecular mechanisms contributing to the pathogenesis of ALDs. Alcohol-induced liver damage involves many biochemical reactions and the intercalation or interaction of different molecular pathways. Major players in the development of alcoholic liver injury include enzymatic reactions, reactive oxygen species, endotoxins, cytokines, innate immunity mediated by factors such as Kupffer cells, lipopolysaccharide/toll-like receptor 4 signaling, and the complement system, and genetic predisposition.[8,9]Among these, the immune-mediated inflammatory reaction, as represented by elevated circulating levels of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-8 (IL-8), plays a key role in liver damage.[10]Corticosteroids have been shown to reduce cytokine production, suppress the formation of acetaldehyde adducts, and inhibit the production of collagen.[11]Consequently, corticosteroids possess antiinflammatory and antifibrotic effects and have thus been used in the treatment of AH.
Adhesion molecules and neutrophils play a central role in the pathogenesis of AH. The high circulating levels of pro-inflammatory cytokines (autocrine loop) or endotoxin in AH result in circulating primed polymorphonuclear neutrophils, which may infiltrate the areas surrounding necrotic hepatocytes and strongly enhance AH-associated liver damage.[12]A study investigating blood polymorphonuclear neutrophil functions in three groups of people (AH, alcohol liver cirrhosis and healthy volunteers) demonstrated an important role of corticosteroids in the normalization of polymorphonuclear neutrophil parameters and cytokine levels.[12]Serial cytokine assays between days 0 and 21 showed that plasma levels of the pro-inflammatory cytokine IL-8 fell by more than 50% within 14 days of steroid administration, while levels of the anti-inflammatory cytokine IL-10 increased on day 21 of steroid therapy.
Cellular adhesion molecules are cell surface glycoproteins. Intercellular adhesion molecule-1 (ICAM-1) mediates firm endothelial adhesion and facilitates leukocyte transmigration in the liver. Increased expression of ICAM-1 has been observed in sinusoidal cells and hepatocytes in AH. In addition, a soluble form of ICAM-1 (sICAM-1) has been reported in AH, and it correlates with the histological severity. Researchers[13]found a rapid decrease in circulating inflammatory cytokines such as TNF-α, ICAM-1 and hepatic vein sICAM-1 expression during corticosteroid therapy, and these changes were associated with a shortterm histological improvement in AH. Corticosteroids also inhibit the production of neoantigens brought about by acetaldehyde adducts, including liver-specific lipoprotein, liver membrane antigen, Mallory bodies and epitopes of protein/aldehyde adducts in the liver.[13]
Controversy of corticosteroids in the treatment of AH
More than 17 controlled trials and at least 13 metaanalyses have reported the effects of corticosteroids in the treatment of ALD in the past 40 years.[14]Parts of the initial trials were small and poorly designed with limited statistical power. Others, although showingtherapeutic effects to various degrees, differed in the inclusion/exclusion criteria, corticosteroids regimens, scoring systems, and spectrum of clinical disorders. Therefore, it is difficult to reach a consensus on the efficacy of corticosteroids in the treatment of AH.
The first meta-analysis on corticosteroids as a therapy for AH was conducted in 1990.[15]It suggested that corticosteroids reduce short-term mortality, especially in patients with acute AH who have hepatic encephalopathy but without active gastrointestinal bleeding. Another analysis, involving 12 controlled trials, found that all large sample trials (those with a statistical weight of 2.5 or higher) showed no therapeutic effect, whereas all the trials that did show a therapeutic effect had smaller numbers of samples. The study also indicated an association between the therapeutic efficacy and the quality score, type and daily dose of corticosteroids, and the duration of therapy. Thus, these studies did not support the routine use of glucocorticoids in patients with AH, including those with encephalopathy.[16]
Despite these negative results, corticosteroids might be effective in selected patients. By using individual patient data across clinical trials, an approach considered to be the "gold standard" for meta-analysis,[17]another study showed the survival benefit of corticosteroids in the subgroup of patients with hepatic encephalopathy and/or those with a Maddrey discriminant function score (MDF) of ≥32. All combined original data were retrieved from three large randomized controlled trials (RCTs) of prednisolone vs placebo. A total of 215 patients including 102 in the placebo arm and 113 in the corticosteroid arm were studied. The results confirmed that acute AH is a severe illness with high in-hospital mortality. The 1-month survival rate was 85% for patients in the treatment group and 65% in the placebo group (P<0.001). Based on this study, for every five patients receiving treatment, one death was averted. Also, survival at 6 months was greater in patients receiving prednisolone than in those receiving placebo (65% vs 50%,P<0.01). This benefit was consistent during the first year of treatment, but disappeared at the second year of followup. In univariate analysis, corticosteroids treatment, age, MDF, albumin, creatinine and encephalopathy were prognostic factors. Multivariate analysis showed age, serum creatinine and corticosteroid treatment were independent prognostic variables.[18]
In order to develop a simple and powerful criterion for early identification of patients who would not benefit from corticosteroids, so-called "nonresponders", researchers in France selected a total of 238 patients from three hospitals between 1990 and 2001, with severe (MDF≥32) biopsy-proven AH in their subsequent study. Overall survival at 1 month was 85±2.3% and at 6 months was 64.3±3.3%. An early change in bilirubin levels at 7 days (defined as bilirubin level at 7 days lower than that on the first day of treatment) was found in 73% of patients.[19]Ninety-five percent of patients with early changes in bilirubin level continued to have improved liver function during treatment. At 6 months, the survival of patients with an early change in bilirubin levels was markedly higher than that of patients without this early change, 82.8±3.3% vs 23± 5.8% (P<0.0001). On multivariate analysis, early change in bilirubin levels, MDF and creatinine were independent prognostic variables, and early change in bilirubin levels had the most important prognostic value. They suggested that corticosteroid therapy should be interrupted in nonresponders after 7 days of treatment.[20]Another study compared an antioxidant regimen with prednisolone or methylprednisolone in AH. The anti-oxidants were β-carotene, vitamin C, vitamin E, selenium, methionine, allopurinol, desferrioxamine, and N-acetylcysteine. Although this trial was terminated prematurely, interim analysis showed a significant survival benefit in the corticosteroids arm. The odds of death after 30 days of antioxidant treatment were 2.4 fold greater than that of the corticosteroid group.[21]
However, some researchers[22]questioned the above conclusion and made a more comprehensive Cochrane review of 15 trials in 2008 with a total of 721 randomized patients. They found that 12 of the trials were at a risk of bias, and corticosteroids significantly reduced mortality only in the subgroup of patients with MDF≥32 or in those with encephalopathy. In all analyses, heterogeneity was significant and substantial. Trial sequential analyses (with heterogeneity-adjusted information size demonstrated) and weighted logistic regression analyses demonstrated no significant effect of corticosteroids on mortality. In addition to the limited efficacy found by these analyses, there is also a propensity for side-effects with even relatively short-term use of corticosteroids.[23]
At the same time, combining two subsequent RCTs with the three previous ones, the researchers in France mentioned above confined the beneficial effect of corticosteroids in the subgroup of "responders" according to the Lille model. In contrast, the "non-responders" had no significant benefit from corticosteroid therapy. The 28-day survival was significantly higher in corticosteroidtreated patients than in non-corticosteroid-treated patients (79.97±2.8% vs 65.7±3.4%, P=0.0005).[24]As to this result, there were still some different views: the Lille model may only apply to corticosteroid-treated patients, but not to the non-steroid-treated patients. Most likely, the coefficients in the model would be different in thecontrol patients. There could be an interaction between the therapy (corticosteroids/control) and the variables included in the model, which need a comprehensive analysis.[25]In response to this problem, the researchers in France performed a subgroup analysis according to the percentile distribution of the Lille score in a subsequent meta-analysis. Patients obtained from five RCTs evaluating corticosteroids in severe AH were classified into three groups using two new cut-offs of the Lille score: complete responders (Lille score ≤0.16; ≤35th percentile), partial responders (Lille score 0.16-0.56; 35th-70th percentile) and null responders (Lille score ≥0.56; ≥70th percentile). It was concluded that corticosteroids significantly improve the 28-day survival in complete responders and partial responders but not in null responders. In multivariate analysis, corticosteroids, MDF, leukocytes, Lille score and encephalopathy were independently predictive of the 28-day survival. This study proposed a new response-guided therapy based on the Lille model and may resolve the long-time controversy on the short-term efficacy of corticosteroids in severe AH.[24]
Potential reasons for different therapeutic effects
Prognostic indicators of AH
Appropriate pharmacologic management of patients with AH relies heavily on the estimation of a given patient's prognosis. Lack of a scientific clinical assessment system limits the power of clinical trials and meta-analysis, and thus may contribute to the conflicting results on the efficacy of corticosteroids.[26]Early experiences with corticosteroids in AH were disappointing, probably because the initial eligibility criteria for treatment were broad, resulting in the inclusion of many subjects with mild disease who would have had a good outcome even if they received placebo. As for the meta-analysis, which cannot pool the results restricted to patients with MDF≥32, one of the reasons is that most of the previous RCTs did not supply the specific survival data of this subgroup.[27]Once treatment was restricted to patients with severe AH, the efficacy of corticosteroid treatment began to emerge.
The initial scoring system was derived in the context of clinical trials of AH, and later modified to the MDF, which is defined as follows: MDF=total bilirubin (in mg/dL)+ 4.6×prothrombin time (in seconds prolonged). This scoring system remains the most commonly used tool for evaluating the severity of AH. It can be calculated from routine blood tests and facilitates the assessment of response to corticosteroids.[28]A value of more than 32, associated with a 28-day survival of 50%-80% in placebo-treated patients, is the threshold for initiating corticosteroid treatment,[29]while those with a score less than 32 have a 28-day survival of 93%.[18]Although relatively easy to use, several drawbacks of the MDF have been noted. The prothrombin time in this test is poorly standardized across different laboratories and may be difficult to score when only the international normalized ratio but not the prothrombin time (actual and control) is available. Another disadvantage of the MDF is that patients with a MDF<32 may also have a remarkable risk of mortality, approaching 17%. Besides, the sensitivity and specificity of the MDF in predicting mortality is 66.7% and 61.5%, respectively. Therefore, by relying solely on the MDF, the death risk may be underestimated in AH patients.[30]
The Glasgow alcoholic hepatitis score (GAHS) was developed in 2005. It was derived from a retrospective analysis of 241 AH patients, using a stepwise logistic regression to identify variables associated with mortality at days 28 and 84. The factors included in the GAHS are serum bilirubin, blood urea nitrogen, prothrombin time and peripheral blood white-cell count.[31]Patients with an MDF≥32 and a GAHS≥9 have a significant improvement in survival after corticosteroid therapy. Patients with GAHS<9, however, do not appear to benefit from corticosteroids. Compared with the MDF, the GAHS has a much higher specificity and overall accuracy, and thus has been considered a validated scoring system for evaluating the prognosis of patients with AH.[32]
The Lille model includes the most informative and reproducible variables for early prediction of mortality. It improves the current management of severe AH and proposes new management of AH. The six incorporated variables are age, renal insufficiency (serum creatinine >1.3 mg/dL or creatinine clearance <40 mL/min), albumin, prothrombin time, bilirubin, and change in bilirubin over 7 days. The Lille model seems to be more accurate than the MDF, MELD and Glasgow scores in predicting short-term mortality in patients with MDF≥32. A score higher than 0.45, which occurs in 40% of cases, identifies patients who will not benefit from corticosteroids. It allows clinicians to better stratify the response to treatment and improve prediction of survival.[33]
Down-regulation of steroid sensitivity
Just as in such conditions as chronic obstructive pulmonary disease, inflammatory skin diseases, rheumatoid arthritis and ulcerative colitis, impaired steroid responsiveness is prevalent in AH patients, especially in the acute severe form.[34,35]A recent study[36]investigated the sensitivity to steroids of lymphocytes from patients with clinically severe acute AH and found it to be significantly lower than in age- and sexmatched controls. The reduced steroid sensitivity in acute AH was mainly shown in differences in the maximum inhibition of proliferation (Imax), a measure of steroid efficacy, rather than in the concentration of dexamethasone required to achieve a 50% maximal inhibition (IC50), which measures steroid potency. This implies that the poor response to the antiinflammatory actions of corticosteroids is unlikely to be overcome simply by increasing the dose. The molecular mechanism of steroid resistance in AH is still unclear. By deacetylating the hyperacetylated histones through the recruitment of histone deacetylase 2 (HDAC2) to the actively transcribing gene, corticosteroids reverse the coordinated expression of multiple inflammatory genes in chronic inflammation. It is now proposed that ethanol metabolism, as a potent source of reactive oxygen species, may impair the recruiting function of glucocorticoids, lessen the action of HDACs to switch off inflammatory gene transcription, and finally result in steroid resistance. However, whether steroid resistance is an intrinsic feature or a consequence of earlier events in the pathogenesis of acute AH is still not confirmed.[37]
Theophylline, a traditional bronchodilator, has been shown to protect against advanced ALD. A recent study proposed that theophylline exerts an anti-inflammatory effect by improving HDAC recruitment to silence proinflammatory genes and therefore improves steroid sensitivity in acute AH.[37]
Infection
The development/aggravation of life-threatening infections is the most worrisome of complications in patients undergoing corticosteroid treatment for severe AH. A greater incidence of fungal infections among patients receiving glucocorticoids has been reported. This was associated with a higher mortality in the glucocorticoid group than in the placebo group.[38,39]That study clearly shows that infection occurs in nearly 50% of cases during the short-term evolution of severe AH. Besides, most patients with AH have a neutrophil leucocytosis and/or low-grade pyrexia.[20]An active infection is generally regarded as a contraindication to steroid therapy. Repeated cultures and possibly a trial of empirical antibiotics may be necessary to exclude sepsis in this patient group. A prospective study showed that patients infected before using corticosteroids had a 2-month survival rate similar to that of others who were not infected: 70.9±6.1% vs 71.6±3.4%, respectively. Corticosteroid treatment is not associated with a higher risk of infection, and its use should not be precluded in already infected patients after appropriate and effective antibiotic therapy. In multivariate analysis, only the Lille model was able to predict the probability of being infected after use of corticosteroids, and showed that this probability was drastically lower in responders (Lille model <0.45) than in non-responders. The authors concluded that infection is not an independent prognostic factor, and routine screening is warranted but should not contraindicate corticosteroids. Rather, non-responsiveness to corticosteroids was a key factor contributing to the development of infection and prediction of survival. They proposed that the early improvement of liver function induced by corticosteroids may decrease the development of infection.[40,41]
Difference in clinical strategies with corticosteroids
The efficacy of pharmaceutical therapy is closely related to practical strategy. In Western countries, the standard practical dosage of corticosteroids in AH patients is 35-80 mg/day with durations varying between 28 days and 1 year.[23]ALD practice guidelines approved by the American Association for the Study of Liver Diseases and the American College of Gastroenterology recommend that patients with severe disease (MDF≥32, with or without hepatic encephalopathy) should be considered for a 4-week course of prednisolone: 40 mg/ day for 28 days, typically followed by discontinuation or a 2-week tapering.[6]Owing to potential adverse effects, corticosteroids are generally avoided in patients with upper gastrointestinal bleeding, chronic hepatitis B virus infection, evidence of active infection, and probable renal failure.[42]The Lille score should be evaluated on the 7th day of corticosteroid therapy, and the "responders" and "non-responders" should be identified. For the responders,therapy should continue, whereas for the non-responders, therapy should be discontinued and other therapies may be considered (Fig.).
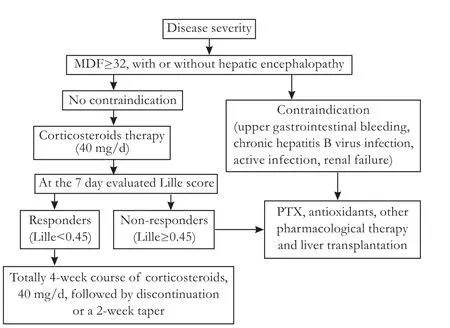
Fig. Treatment algorithm of corticosteroids for AH.
The management of non-responders remains a subject of caution. Pentoxifylline, a nonselective phosphodiesterase inhibitor, is an alternative treatment for AH. Several clinical trials both support[43,44]and refute[45,46]the benefit of pentoxifylline in the treatment of AH, and cirrhosis was reported. A two-step strategy consisting of an early withdrawal of corticosteroids and a switch to pentoxifylline for 28 additional days was recommended as a reasonable alternative in non-responders, but this regimen failed to show a survival benefit in a recent study.[45]Corticosteroids plus aggressive enteral nutrition is another reasonable approach to patients with AH.[47]
Conclusion
Despite our best efforts, ALD remains one of the leading causes of liver diseases and liver-related death in the general population throughout the world. Glucocorticosteroids represent the most widely accepted but yet the most debatable therapy in patients with severe AH. Early experiments with corticosteroids in AH were disappointing, probably because of the varied initial eligibility criteria for treatment and the inaccurate severity indicators of AH. Once treatment was restricted to patients with severe AH (MDF≥32), the efficacy of corticosteroid treatment began to emerge. The most pertinent discovery regarding AH and corticosteroids is of importance in early and accurate determination of steroid sensitivity. Significant benefit of corticosteroids can be achieved in complete responders (MDF≥32 and Lille score ≤0.16).
Besides an accurate clinical assessment system, how to overcome the down-regulation of steroid sensitivity in AH patients is a crucial issue in corticosteroid therapy. The molecular mechanism of the resistance is yet to be defined. Infection may be a potential reason for the different therapeutic effects. However, steroids should not be precluded in infected patients after appropriate and effective antibiotic therapy. The practical dosage and duration of corticosteroid treatment should be standardized. Further studies are required to rationalize corticosteroid treatment to minimize unwanted effects and maximize clinical benefit.
Funding:This study was supported by a grant from the General Project Foundation of the Education Department of Liaoning Province (2009A809).
Ethical approval:Not needed.
Contributors:WF wrote the main body of the article under the supervision of WBY. WBY is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Becker U, Deis A, Sorensen TI, Grønbaek M, Borch-Johnsen K, Müller CF, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology 1996;23:1025-1029.
2 WHO. Global Status Report on Alcohol 2004. Available from: http://www.faslink.org/WHO_global_alcohol_status_report_ 2004.pdf
3 Breitkopf K, Nagy LE, Beier JI, Mueller S, Weng H, Dooley S. Current experimental perspectives on the clinical progression of alcoholic liver disease. Alcohol Clin Exp Res 2009;33:1647-1655.
4 Lefkowitch JH. Morphology of alcoholic liver disease. Clin Liver Dis 2005;9:37-53.
5 Amini M, Runyon BA. Alcoholic hepatitis 2010: a clinician's guide to diagnosis and therapy. World J Gastroenterol 2010; 16:4905-4912.
6 O'Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol 2010;105:14-32.
7 Mathurin P, Louvet A, Dharancy S. Treatment of severe forms of alcoholic hepatitis: where are we going? J Gastroenterol Hepatol 2008;23:S60-62.
8 Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 2009;50:638-644.
9 Crabb DW, Liangpunsakul S. Acetaldehyde generating enzyme systems: roles of alcohol dehydrogenase, CYP2E1 and catalase, and speculations on the role of other enzymes and processes. Novartis Found Symp 2007;285:4-16.
10 McClain CJ, Barve S, Deaciuc I, Kugelmas M, Hill D. Cytokines in alcoholic liver disease. Semin Liver Dis 1999;19: 205-219.
11 Tome S, Lucey MR. Review article: current management of alcoholic liver disease. Aliment Pharmacol Ther 2004;19:707-714.
12 Taïeb J, Mathurin P, Elbim C, Cluzel P, Arce-Vicioso M, Bernard B, et al. Blood neutrophil functions and cytokine release in severe alcoholic hepatitis: effect of corticosteroids. J Hepatol 2000;32:579-586.
13 Spahr L, Rubbia-Brandt L, Pugin J, Giostra E, Frossard JL, Borisch B, et al. Rapid changes in alcoholic hepatitis histology under steroids: correlation with soluble intercellular adhesion molecule-1 in hepatic venous blood. J Hepatol 2001;35:582-589.
14 Gluud C. Alcoholic hepatitis: no glucocorticosteroids? In: Leuschner U, James OFW, Dancygier H, editors. Steatohepatitis (NASH and ASH) - Falk Symposium 121. Lancaster: Kluwer Academic Publisher; 2001:322-342.
15 Imperiale TF, McCullough AJ. Do corticosteroids reduce mortality from alcoholic hepatitis? A meta-analysis of the randomized trials. Ann Intern Med 1990;113:299-307.
16 Christensen E, Gluud C. Glucocorticoids are ineffective in alcoholic hepatitis: a meta-analysis adjusting for confounding variables. Gut 1995;37:113-118.
17 Sutton AJ, Higgins JP. Recent developments in meta-analysis. Stat Med 2008;27:625-650.
18 Mathurin P, Mendenhall CL, Carithers RL Jr, Ramond MJ, Maddrey WC, Garstide P, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomized placebo controlled double blind trials of corticosteroids in severe AH. J Hepatol 2002;36:480-487.
19 Morris JM, Forrest EH. Bilirubin response to corticosteroids in severe alcoholic hepatitis. Eur J Gastroenterol Hepatol 2005;17:759-762.
20 Mathurin P, Abdelnour M, Ramond MJ, Carbonell N, Fartoux L, Serfaty L, et al. Early change in bilirubin levels is an important prognostic factor in severe alcoholic hepatitis treated with prednisolone. Hepatology 2003;38:1363-1369.
21 Phillips M, Curtis H, Portmann B, Donaldson N, Bomford A, O'Grady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis--a randomised clinical trial. J Hepatol 2006;44:784-790.
22 Christensen E. Alcoholic hepatitis--glucocorticosteroids or not? J Hepatol 2002;36:547-548.
23 Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis--a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther 2008;27:1167-1178.
24 Mathurin P, O'Grady J, Carithers RL, Phillips M, Louvet A, Mendenhall CL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: metaanalysis of individual patient data. Gut 2011;60:255-260.
25 Christensen E. Glucocorticosteroids in acute alcoholic hepatitis: the evidence of a beneficial effect is getting even weaker. J Hepatol 2010;53:390-391.
26 Tan HH, Virmani S, Martin P. Controversies in the management of alcoholic liver disease. Mt Sinai J Med 2009; 76:484-498.
27 Mathurin P. The use of corticosteroids in severe alcohol hepatitis: we need to look beyond this controversy. J Hepatol 2010;53:392-393.
28 Carithers RL Jr, Herlong HF, Diehl AM, Shaw EW, Combes B, Fallon HJ, et al. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Ann Intern Med 1989;110:685-690.
29 Mathurin P, Duchatelle V, Ramond MJ, Degott C, Bedossa P, Erlinger S, et al. Survival and prognostic factors in patients with severe alcoholic hepatitis treated with prednisolone. Gastroenterology 1996;110:1847-1853.
30 Kulkarni K, Tran T, Medrano M, Yoffe B, Goodgame R. The role of the discriminant factor in the assessment and treatment of alcoholic hepatitis. J Clin Gastroenterol 2004; 38:453-459.
31 Forrest EH, Evans CD, Stewart S, Phillips M, Oo YH, McAvoy NC, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut 2005;54:1174-1179.
32 Forrest EH, Morris AJ, Stewart S, Phillips M, Oo YH, Fisher NC, et al. The Glasgow alcoholic hepatitis score identifies patients who may benefit from corticosteroids. Gut 2007;56: 1743-1746.
33 Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007;45:1348-1354.
34 Hearing SD, Norman M, Probert CS, Haslam N, Dayan CM. Predicting therapeutic outcome in severe ulcerative colitis by measuring in vitro steroid sensitivity of proliferating peripheral blood lymphocytes. Gut 1999;45:382-388.
35 Barnes PJ, Ito K, Adcock IM. Corticosteroid resistance in chronic obstructive pulmonary disease: inactivation of histone deacetylase. Lancet 2004;363:731-733.
36 Kendrick SF, Henderson E, Palmer J, Jones DE, Day CP. Theophylline improves steroid sensitivity in acute alcoholic hepatitis. Hepatology 2010;52:126-131.
37 Kendrick SF, O'Boyle G, Mann J, Zeybel M, Palmer J, Jones DE, et al. Acetate, the key modulator of inflammatory responses in acute alcoholic hepatitis. Hepatology 2010;51:1988-1997.
38 Blitzer BL, Mutchnick MG, Joshi PH, Phillips MM, Fessel JM, Conn HO. Adrenocorticosteroid therapy in alcoholic hepatitis. A prospective, double-blind randomized study. Am J Dig Dis 1977;22:477-484.
39 Theodossi A, Eddleston AL, Williams R. Controlled trial of methylprednisolone therapy in severe acute alcoholic hepatitis. Gut 1982;23:75-79.
40 Cabré E, Rodríguez-Iglesias P, Caballería J, Quer JC, Sánchez-Lombraña JL, Parés A, et al. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology 2000;32:36-42.
41 Louvet A, Wartel F, Castel H, Dharancy S, Hollebecque A, Canva-Delcambre V, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology 2009;137:541-548.
42 Yu CH, Xu CF, Ye H, Li L, Li YM. Early mortality of alcoholic hepatitis: a review of data from placebo-controlled clinical trials. World J Gastroenterol 2010;16:2435-2439.
43 Sidhu S, Singla M, Bhatia K. Pentoxifylline reduces disease severity and prevents renal impairment in severe acute alcoholic hepatitis: a double blind, placebo controlled trial. Hepatology 2006;44:373A.
44 Macavoy NC, Forrest E, Hayes PC. The influence of Pentoxifylline on mortality in alcoholic hepatitis and benefit of the Glasgow Alcoholic Hepatitis Score (GAHS). Hepatology 2005;42:492A.
45 Louvet A, Diaz E, Dharancy S, Coevoet H, Texier F, Thévenot T, et al. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol 2008;48:465-470.
46 Lebrec D, Thabut D, Oberti F, Perarnau JM, Condat B, Barraud H, et al. Pentoxifylline does not decrease short-term mortality but does reduce complications in patients with advanced cirrhosis. Gastroenterology 2010;138:1755-1762.
47 Alvarez MA, Cabré E, Lorenzo-Zúñiga V, Montoliu S, Planas R, Gassull MA. Combining steroids with enteral nutrition: a better therapeutic strategy for severe alcoholic hepatitis? Results of a pilot study. Eur J Gastroenterol Hepatol 2004;16: 1375-1380.
Received May 10, 2011
Accepted after revision August 15, 2011
Author Affiliations: Department of Gastroenterology, First Affiliated Hospital, China Medical University, Shenyang 110001, China (Wang F and Wang BY)
Bing-Yuan Wang, MD, PhD, Department of Gastroenterology, First Affiliated Hospital, China Medical University, Shenyang 110001, China (Tel: 86-24-83282900; Fax: 86-24-83282997; Email: wangby@medmail.com.cn)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(11)60079-9
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Protective effect of probiotics on intestinal barrier function in malnourished rats after liver transplantation
- Naproxen-induced liver injury
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Salvianolic acid B modulates the expression of drug-metabolizing enzymes in HepG2 cells
- Evaluation outcomes of donors in living donor liver transplantation: a single-center analysis of 132 donors
- Collagen proportionate area of liver tissue determined by digital image analysis in patients with HBV-related decompensated cirrhosis
