Living donor liver hilar variations: surgical approaches and implications
2011-07-03OnurYaprakTolgaDemirbasCihanDuranMuratDayangacMuratAkyildizYamanTokatandYildirayYuzer
Onur Yaprak, Tolga Demirbas, Cihan Duran, Murat Dayangac, Murat Akyildiz, Yaman Tokat and Yildiray Yuzer
Istanbul, Turkey
Original Article / Transplantation
Living donor liver hilar variations: surgical approaches and implications
Onur Yaprak, Tolga Demirbas, Cihan Duran, Murat Dayangac, Murat Akyildiz, Yaman Tokat and Yildiray Yuzer
Istanbul, Turkey
BACKGROUND:Varied vascular and biliary anatomies are common in the liver. Living donor hepatectomy requires precise recognition of the hilar anatomy. This study was undertaken to study donor vascular and biliary tract variations, surgical approaches and implications in living liver transplant patients.
METHODS:Two hundred living donor liver transplantations were performed at our institution between 2004 and 2009. All donors were evaluated by volumetric computerized tomography (CT), CT angiography and magnetic resonance cholangiography in the preoperative period. Intraoperative ultrasonography and cholangiography were carried out. Arterial, portal and biliary anatomies were classified according to the Michels, Cheng and Huang criteria.
RESULTS:Classical hepatic arterial anatomy was observed in 129 (64.5%) of the 200 donors. Fifteen percent of the donors had variation in the portal vein. Normal biliary anatomy was found in 126 (63%) donors, and biliary tract variation in 70% of donors with portal vein variations. In recipients with single duct biliary anastomosis, 16 (14.4%) developed biliary leak, and 9 (8.1%) developed biliary stricture; however more than one biliary anastomosis increased recipient biliary complications. Donor vascular variations did not increase recipient vascular complications. Variant anatomy was not associated with an increase in donor morbidity.
CONCLUSIONS:Living donor liver transplantation provides information about variant hilar anatomy. The success of the procedure depends on a careful approach to anatomical variations. When the deceased donor supply is inadequate, living donor transplantation is a life-saving alternative and is safe for the donor and recipient, even if the donor has variant hilar anatomy.
(Hepatobiliary Pancreat Dis Int 2011; 10: 474-479)
living donor; liver transplantation; anatomical variation
Introduction
Living donor liver transplantation (LDLT) is a lifesaving procedure for cirrhotic patients, especially in countries where there is shortage of deceased organ donors. Donor operation safety is directly related to the precise recognition of liver anatomy. Anatomic variations of the vascular and biliary system in the liver are common. Biliary tract variations are found in 24%-57% of cases[1-5]while portal vein and hepatic arterial variations are usually seen in 16%-26% and 31%-33%, respectively.[4]Therefore, in this study, the prevalence of hepatic arterial, portal venous and biliary tract variations in a donor hepatectomy case series, as well as the surgical approaches and their outcomes, were retrospectively analyzed.
Methods
During the period between July 2004 and December 2009, 200 right lobe LDLTs and donor hepatectomies were performed in the Hepatobiliary Surgery and Transplantation Center of the Florence Nightingale Hospital in Istanbul. During this period, only two donor candidates were found unsuitable for living donation due to anatomical variations in hilar structures. In one of these, congenital left portal vein agenesis was found. MRCP of the other revealed that three bile ducts of the right lobe were separately draining into the left hepatic duct. The donors consisted of 118 men and 82 women with a mean age of 37.4 (range 18-63) years. The recipients were 152 men and 48 women [mean age 50.7 (range 16-72) years], and their mean model for end-stage liver disease (MELD) score was 17.2 (range 4-47). Donor evaluations were done by volumetriccomputerized tomography (CT), CT angiography, and magnetic resonance cholangiography in the preoperative period and ultrasonography (US) and cholangiography during the operation. CT angiography was performed with a 16-detector CT (Sensation 16-Siemens, Erlangen, Germany). All patients were imaged on a 1.5-T scanner (Magnetom Sonata, Siemens, Erlangen, Germany) using a phased-array torso coil. Arterial variations were classified as described by Michels[6]who defined arterial variations in 10 groups. An additional group (type 11) was used for those we could not place in any of the predefined groups. Portal venous variations were classified as described in the arterioportographic study by Cheng et al.[7]Biliary variations were grouped according to Huang et al,[8]with an additional type, A6, for those who did not fulfill the predefined criteria.
The ethical aspect of this study was approved by the Ethics Committee of our hospital. All donors and recipients provided written informed consent prior to the operation.
Results
Arterial variations (Table 1)
Classical hepatic arterial anatomy was observed in 129 (64.5%) donors. The remaining 71 (35.5%) had several arterial variations. The most common variation was type 3 which was found in 22 donors (11%). Variations were not compatible with the descriptions in the Michelsclassification in nine (4.5%). In three of these, the right hepatic artery (RHA) originated from the celiac trunk as a separate branch (replaced RHA with celiac trunk origin). Trifurcation was detected in two donors, and two others had an accessory RHA with a gastroduodenal artery (GDA) origin (Fig. 1). In another, RHA with an aortic origin and an accessory left hepatic artery with a left gastric artery (LGA) origin were determined. In one donor, the celiac trunk bifurcated into different branches accompanied by a replaced RHA with a superior mesenteric artery (SMA) origin.
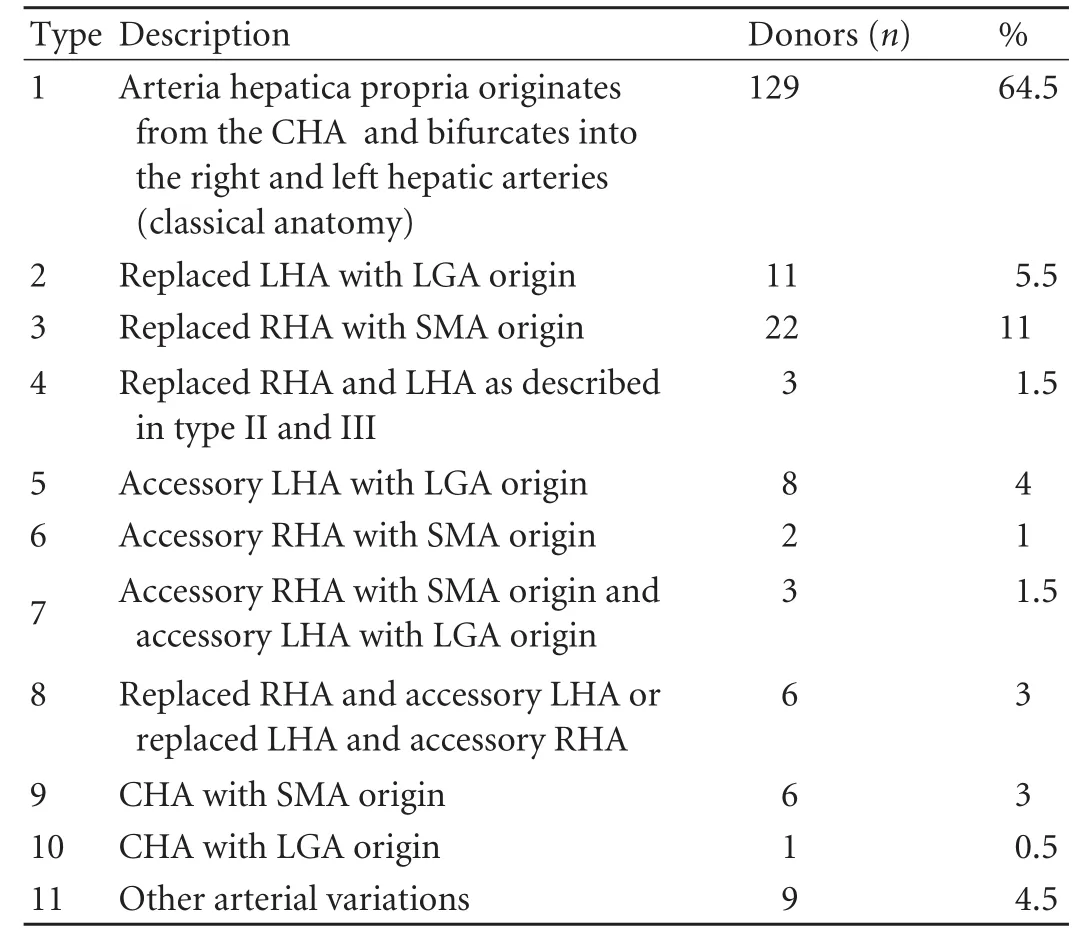
Table 1. Hepatic arterial variations according to the Michels classification
The segment 4 artery originated from the left hepatic artery in 106 (53%) donors, from the RHA in 66 (33%), from both hepatic arteries in eight (4%), and from the GDA in eight (4%) (Fig. 2). Other uncommon variants were as follows: from the arteria hepatica propria (HP) in 10 donors (5%), from the pancreaticoduodenal artery in one (0.5%), and from the LGA in one (0.5%).
Portal venous variations (Table 2)
One hundred and seventy donors (85%) had classical bifurcation of the main portal vein, 11 (5.5%) had a right anterior sectoral branch originating from the left portal vein, nine (4.5%) had a trifurcation of the mainportal vein, and 10 (5%) had a right posterior sectoral branch arising from the main portal vein (the right anterior sectoral vein and left portal vein as a common trunk) (Fig. 3).
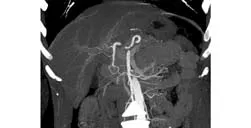
Fig. 1. Spiral CT reveals the accessory right hepatic artery originating from the gastroduodenal artery.

Fig. 2. Spiral CT shows segment 4 artery originating from the gastroduodenal artery.
Biliary tract variations (Table 3)
Normal biliary anatomy (type A1) was found in 126 (63%) of the donors. The most common anatomic variant was type A3 which was seen in 18.5%. Eight (4%) donors had variants different from the Huang classification. Five had an accessory right hepatic duct draining directly into the common hepatic duct (Fig. 4). Two had a segment 5 hepatic duct draining into the common hepatic duct. The right posterior sectoral biliary duct was joined to the segment 2 biliary duct and the right anterior sectoral duct and the segment 3 duct opened to the common hepatic duct separately in one donor (Fig. 5).
Biliary reconstruction techniques and outcomes (Table 4)
A single duct was achieved in 117 donors, while therewere multiple bile ducts in 83. In nine donors who had normal (type A1) anatomy, procurement was done at the incorrect point; thus very adjacent double orifices were achieved. One hundred and eleven of 117 grafts which had a single bile duct were anastomosed duct-to-duct. In six recipients, Roux-en-Y hepaticojejunostomy was done. In 77 donors, double orifice grafts were procured. In 45 of the 77 grafts, double bile duct orifices were sutured by using the back-to-back technique and the bile ducts were anastomosed as a single duct. In nine grafts, single anastomosis was made without a necessity for back wall suture. Double duct-to-duct anastomoses were made in 18 of the 77 patients and Roux-en-Y hepaticojejunostomy was performed in the remaining five. Three bile duct orifices were found in six donors. In three of these, anastomosis was made as a double duct-to-duct. In the remaining three, one single duct-to-duct, one triple duct-to-duct (one to the cystic duct), and one Roux-en-Y anastomosis was made.
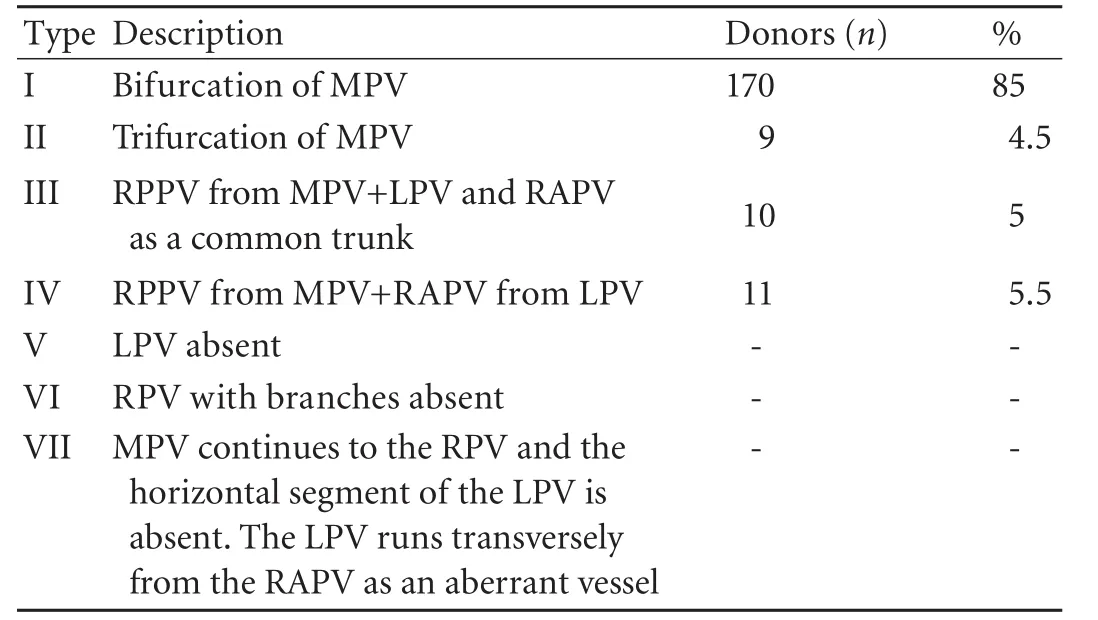
Table 2. Portal vein variations according to the Cheng classification

Table 3. Variations of biliary tract according to the Huang classification

Fig. 3. Spiral CT reveals left portal vein and the right anterior portal vein originating from a common trunk.

Fig. 4. Right accessory biliary duct joining to the common hepatic duct demonstrated by MRCP.
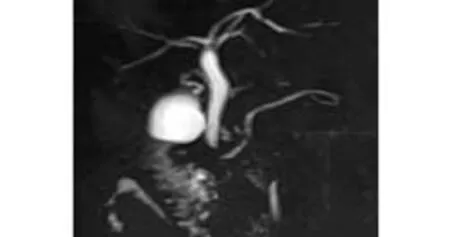
Fig. 5. MRCP demonstrates the right posterior segment biliary duct joining with segment 2 bile duct, right anterior segment duct and segment 3 duct opening separately to the common hepatic duct .
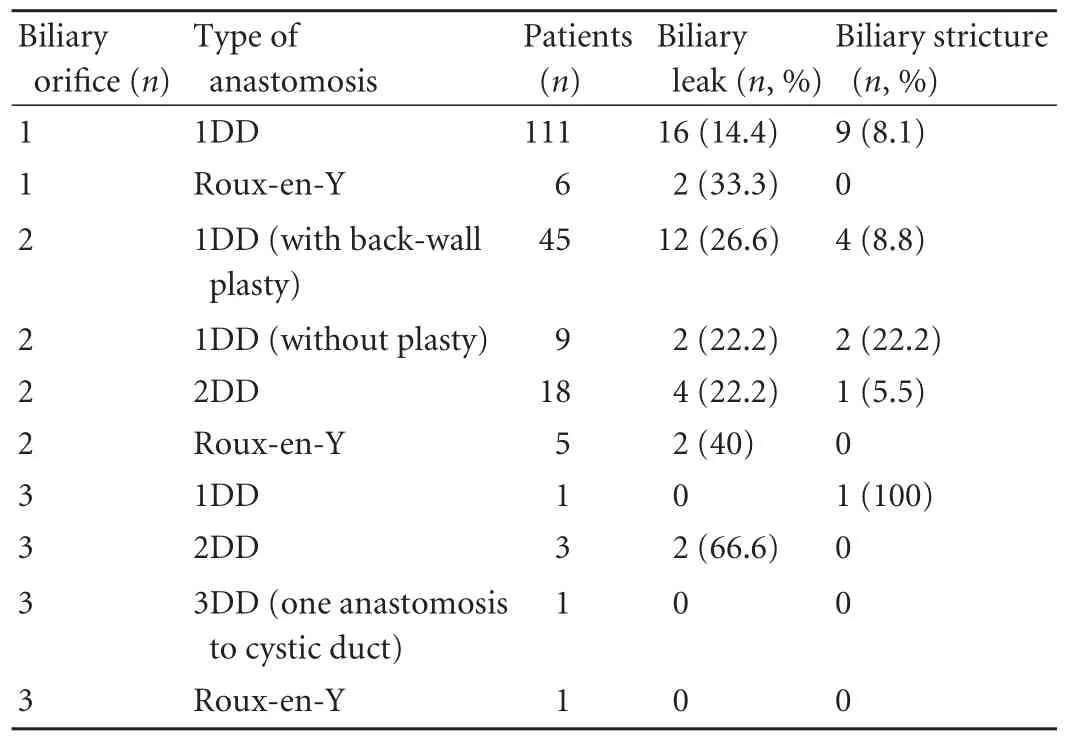
Table 4. Types of biliary reconstruction and outcomes
Forty-nine recipients developed 57 biliary problems: 40 of biliary leak and 17 of biliary stricture. Biliary reconstruction techniques and their results are shown in Table 4. Among 111 recipients who had single duct biliary anastomosis, 16 (14.4%) developed biliary leak, and 9 (8.1%) developed biliary stricture.
Among 77 recipients who had double bile ducts in their grafts, 45 whose double bile ducts were reconstructed by back wall plasty developed 12 biliary leaks (26.6%) and 4 biliary strictures (8.8%). In 18 recipients anastomosed by the double duct-to-duct technique, 4 (22.2%) developed biliary leak and one developed biliary stricture. Nine recipients whose grafts had two ducts and anastomosis without reconstruction developed two biliary leaks (22.2%) and two biliary strictures (22.2%). Roux-en-Y hepaticojejunostomy was performed in 12 recipients; four of these (33.3%) developed biliary leak.
The strongest correlation between anatomical variations was detected between portal vein and bile duct variations with a rate of 70%. Twenty-one of 30 donors who had portal vein variation also had biliary variations. Sixteen (22.5%) of 71 donors who had a hepatic artery anomaly also had bile duct variations in magnetic resonance cholangiopancreatography (MRCP).
In 14 of the 30 patients who had a graft portal vein anomaly, double orifice single anastomoses were performed. Nine anastomoses were performed by the portal back wall plasty technique, three by deceased iliac vein conduit, and two by deceased arterial conduit. In sixteen patients, separate double anastomosis was done. Portal vein thrombosis was found in three of the 200 patients. None of these patients had a portal vein anomaly in their grafts.
Double hepatic arteries were observed in 23 grafts. Of these, reconstruction as a single artery was carried out in 10 and dual reconstruction in 13. Hepatic artery thrombosis occurred in only two patients but these patients did not have artery variation in their graft.
There was no mortality or life-threatening complication in our donors. None of the donors developed hepatic failure or signs of hepatic failure. Even if donors had arterial or portal vein variations, none of them developed arterial or portal vein complications. Biliary complication rates were similar in donors with and without biliary anatomical variation. While 74 donors with biliary variation developed five (6%) biliary leaks and one (1%) biliary stricture, 126 donors without biliary variation developed seven (6%) biliary leaks and one (1%) biliary stricture. Six biliary leaks resolved spontaneously. Biliary complications requiring intervention were seen in 8 donors (4%). One biliary leak and 1 biliary stricture required reoperation. Four biliary complications (three biliary leaks and one biliary stricture) were treated with sphincterotomy or stent placement under endoscopic retrograde cholangiography. Two donors with abdominal collection due to biliary leak underwent percutaneous drainage.
Discussion
Liver hilar anatomy is quite variable, and the variants may complicate transplantation. Moreover, they may require careful dissection and different anastomotic techniques. Preoperative mapping and identification of the hilar anatomy are critical for donor selection and surgical planning. Also, identification of the hilar variations presents critical information to all surgeons doing hepatobiliary surgery. Variations in the hepatic artery, portal vein and bile ducts are found at the rates of 25%, 11% and 28% of liver donor candidates, respectively.[9]
After Michels[6]defined normal hepatic arterial anatomy in 55% of the 200 cadaveric donors, several studies have reported common and rare variants. One of these studies demonstrating hepatic arterial anatomy is that of Hiatt et al.[10]Anatomic variations in thehepatic arteries were studied in donor livers which were used for orthotopic liver transplantation. This study revealed that the normal hepatic arterial pattern was found in 75.7% of 1000 patients.[10]We found normal hepatic arterial anatomy in 64.5% of donors. The most important anatomical issues related to the hepatic artery are the number, diameter, and stump length in the graft.[11]Selection of the arterial anastomosis site and reconstruction technique plays an important role in vascularization of the graft. Insufficient arterial perfusion may result in biliary stricture, cholangitis, and eventual graft failure because the biliary ducts are vascularized only by the hepatic arteries.[12]To prevent ischemia in the recipient, all the arteries supplying the graft must be preserved. Replaced arteries must always be anastomosed; accessory arteries are not necessarily reconstructed if there is adequate back-flow after the anastomosis of the other branch or if arterial flow in all the segments is demonstrated by intraoperative Doppler US.[13]
Preservation of the segment 4 artery is important to ensure sufficient regeneration of the remnant lobe. If the segment 4 artery originates from the RHA, division of the RHA must be made distal to the segment 4 artery. As a very uncommon anomaly, the segment 4 artery originated from the GDA in 4% of our donors.
Variations in intrahepatic portal venous anatomy have been described in 0.09%-29% of the patients. Akgul et al[14]reported, in a helical CT study, 86.2% of patients had a classical portal distribution and 13.8% had variations. Cheng et al[7]found that 29.1% of patients had variations in arterioportography while 15% of donors had variations in their portal veins. When the donor has portal variation, the portal vein should be divided carefully at a critical point. If the donor side remains small, the donor may develop portal hypertension. If the portal vein is divided very close to the recipient side, two orifices may be left for anastomosis. Grafts with double portal orifices may either be anastomosed separately to the recipient right and left portal veins, or be joined together to make 1 orifice, or connected to a Y-shaped vascular graft at the back table for a single anastomosis in the recipient.[13]
Right lobe biliary variations have been classified by Huang et al[8]into five types. While the incidence of classical bile duct bifurcation is 62.6%, variants of bile duct communication have been defined as follows: trifurcation (right posterior duct draining into the junction of the right anterior duct and the left duct) in 19%, right posterior duct draining into the left hepatic duct in 11%, right posterior duct draining into the common bile duct in 5.8% and into the cystic duct in 1.6%.[8]Normal biliary anatomy (type A1) was found in 126 (63%) of donors as in the Huang classification. The most common anatomic variant was type A3 (18.5%) in our study. Accessory right or left hepatic ducts, which are not described in the Huang classification, have been reported to occur in 2% of individuals,[1]while 2.5% of our donors had an accessory right hepatic duct draining directly into the common hepatic duct.
MRCP has a sensitivity as low as 70% for aberrant biliary anatomy in the living related donor population.[15]Compared MRCP findings with intraoperative findings, we found that the sensitivity, specificity, positive predictive and negative predictive value of MRCP were 66.1%, 94.4%, 85.7% and 78.1%, respectively. Interestingly, donors with portal variations had a rate of biliary tract variation for 70%. Because the sensitivity of MRCP is low according to our results, careful examination of the biliary tract is very important in donors with portal venous variations. Liver grafts that have aberrant bile ducts require anastomosis of all ducts to prevent postoperative biliary leak and segmental atrophy in the recipient graft. The presence of multiple biliary orifices is a problem almost specific for right lobe grafts. Surgical options for the management of double bile duct include double reconstruction and reconstruction as a single orifice with or without ductoplasty. According to the Kyoto group, double anastomosis and a single anastomosis without ductoplasty have less leakage.[5]
If the distance between biliary orifices is larger than 3 mm in grafts including double bile ducts, uniting orifices to yield a single duct via the "back-wall plasty" method has not been recommended due to the possible risk of ischemia and fibrosis secondary to septotomy and plasty performed during this procedure.[16,17]The Rouxen-Y anastomosis technique has been recommended in such patients. Based on our experience in reoperated patients, the highest risk of complication occurred in patients who underwent single anastomosis of double duct via plasty. In these patients, regeneration of the liver resulted in a further increase in the distance between orifices and even complicated the success of Roux-en-Y anastomosis in the reoperation due to the development of hilar and perihepatic fibrosis. For this reason, in grafts with double or triple ducts, provided that the ducts do not share a common bile sheath, it is more convenient to perform either a separate anastomosis of each duct or the application of Roux-en-Y anastomosis in the first operation.
In our series, none of the recipients of grafts from donors with variant anatomy developed arterial or portal vein complications, and there was no donorvascular complication. The donor biliary complication rate did not differ between those with variant anatomy and normal anatomy.
In conclusion, living donor liver transplantation is a procedure requiring detailed evaluation of the liver vascular and biliary systems. To ensure successful results, all variations must be carefully identified. Countries with an inadequate deceased donor rate tend to perform living donor transplantation more frequently. In this process, use of donors with variant hilar anatomy is sometimes inevitable. Our results indicate that with a careful approach to identifying and reconstructing anomalous vascular and biliary structures, variant hilar anatomy does not threaten donor safety and only marginally increases recipient biliary complications.
Acknowledgement
We thank our coordinator Bade Vatan for registrating the data.
Funding:None.
Ethical approval:Not needed.
Contributors:YO proposed the study. YO and DC wrote the first draft. DT, DM, AM, TY and YY analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. YO is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Puente SG, Bannura GC. Radiological anatomy of the biliary tract: variations and congenital abnormalities. World J Surg 1983;7:271-276.
2 Gazelle GS, Lee MJ, Mueller PR. Cholangiographic segmental anatomy of the liver. Radiographics 1994;14:1005-1013.
3 Cheng YF, Huang TL, Chen CL, Chen YS, Lee TY. Variations of the intrahepatic bile ducts: application in living related liver transplantation and splitting liver transplantation. Clin Transplant 1997;11:337-340.
4 Kawarada Y, Das BC, Taoka H. Anatomy of the hepatic hilar area: the plate system. J Hepatobiliary Pancreat Surg 2000;7: 580-586.
5 Nakamura T, Tanaka K, Kiuchi T, Kasahara M, Oike F, Ueda M, et al. Anatomical variations and surgical strategies in right lobe living donor liver transplantation: lessons from 120 cases. Transplantation 2002;73:1896-1903.
6 Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 1966;112:337-347.
7 Cheng YF, Huang TL, Lee TY, Chen TY, Chen CL. Variation of the intrahepatic portal vein; angiographic demonstration and application in living-related hepatic transplantation. Transplant Proc 1996;28:1667-1668.
8 Huang TL, Cheng YF, Chen CL, Chen TY, Lee TY. Variants of the bile ducts: clinical application in the potential donor of living-related hepatic transplantation. Transplant Proc 1996; 28:1669-1670.
9 Lee VS, Morgan GR, Lin JC, Nazzaro CA, Chang JS, Teperman LW, et al. Liver transplant donor candidates: associations between vascular and biliary anatomic variants. Liver Transpl 2004;10:1049-1054.
10 Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg 1994;220:50-52.
11 Duran C, Uraz S, Kantarci M, Ozturk E, Doganay S, Dayangac M, et al. Hepatic arterial mapping by multidetector computed tomographic angiography in living donor liver transplantation. J Comput Assist Tomogr 2009;33:618-625.
12 Kamel IR, Raptopoulos V, Pomfret EA, Kruskal JB, Kane RA, Yam CS, et al. Living adult right lobe liver transplantation: imaging before surgery with multidetector multiphase CT. AJR Am J Roentgenol 2000;175:1141-1143.
13 Lee KK, Lee SK, Moon IS, Kim DG, Lee MD. Surgical techniques according to anatomic variations in living donor liver transplantation using the right lobe. Transplant Proc 2008;40:2517-2520.
14 Akgul E, Inal M, Soyupak S, Binokay F, Aksungur E, Oguz M. Portal venous variations. Prevalence with contrast-enhanced helical CT. Acta Radiol 2002;43:315-319.
15 Macdonald DB, Haider MA, Khalili K, Kim TK, O'Malley M, Greig PD, et al. Relationship between vascular and biliary anatomy in living liver donors. AJR Am J Roentgenol 2005; 185:247-252.
16 Chan SC, Fan ST. Biliary complications in liver transplantation. Hepatol Int 2008;2:399-404.
17 Hwang S, Lee SG, Sung KB, Park KM, Kim KH, Ahn CS, et al. Long-term incidence, risk factors, and management of biliary complications after adult living donor liver transplantation. Liver Transpl 2006;12:831-838.
Received April 1, 2011
Accepted after revision June 17, 2011
Author Affiliations: Hepatobiliary and Organ Transplant Center, Florence Nightingale Hospital, Istanbul, Turkey (Yaprak O, Demirbas T, Duran C, Dayangac M, Akyildiz M, Tokat Y and Yuzer Y)
Onur Yaprak, MD, Florence Nightingale Hastanesi, Organ Nakil Merkezi, Abide-i Hurriyet Cad. 34381, Sisli/Istanbul, Turkey (Tel: 90-212 2258398; Fax: 90-212-2240356; Email: onuryaprak@hotmail.com)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(11)60081-7
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Protective effect of clodronate-containing liposomes on intestinal mucosal injury in rats with severe acute pancreatitis
- Collagen proportionate area of liver tissue determined by digital image analysis in patients with HBV-related decompensated cirrhosis
- Evaluation outcomes of donors in living donor liver transplantation: a single-center analysis of 132 donors
- Salvianolic acid B modulates the expression of drug-metabolizing enzymes in HepG2 cells
- Protective effect of probiotics on intestinal barrier function in malnourished rats after liver transplantation
