Effects of partial portal vein arterialization on the hilar bile duct in a rat model
2011-07-03ShaoHuaGuoChongHuiLiYongLiangChenJianNingSongAiQunZhangandChengZhou
Shao-Hua Guo, Chong-Hui Li, Yong-Liang Chen, Jian-Ning Song, Ai-Qun Zhang and Cheng Zhou
Beijing, China
Effects of partial portal vein arterialization on the hilar bile duct in a rat model
Shao-Hua Guo, Chong-Hui Li, Yong-Liang Chen, Jian-Ning Song, Ai-Qun Zhang and Cheng Zhou
Beijing, China
BACKGROUND:Liver revascularization is frequently required during the enlarged radical operation for hilar cholangiocarcinoma involving the hepatic artery. Researchers have carried out a number of experiments applying partial portal vein arterialization (PVA) in clinical practice. In this study we aimed to establish a theoretical basis for clinical application of partial PVA and to investigate the effects of partial PVA on rat hilar bile duct and hepatic functions.
METHODS:Thirty rats were randomly and equally assigned into 3 groups: control (group A), hepatic artery ligation+bile duct recanalization (group B), and partial PVA+bile duct recanalization (group C). Proliferation and apoptosis of rat hilar bile duct epithelial cells, arteriolar counts of the peribiliary plexus (PBP) of the bile duct wall, changes in serum biochemistry, and pathologic changes in the bile duct were assessed 1 month after operation.
RESULTS:The proliferation of hilar bile duct epithelial cells in group B was greater than in groups A and C (P<0.01). No apoptotic hilar bile duct epithelial cells were detected in any of the groups. The PBP arteriolar counts of the hilar bile duct wall were similar in groups A and C (P>0.05), but the count was lower in group B than in group A (P<0.01). No statistically significant differences in alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and albumin were found in the 3 groups. The gamma-glutamyltransferase value was higher in group B than in groups A and C (P<0.01). The hepatic tissues of groups A and C showed no significant abnormality. Chronic inflammatory changes in the hilar bile duct walls were observed only in group B.
CONCLUSION:Partial PVA can restore the arterial blood supply of the hilar bile duct and significantly extenuate the injury to hilar bile duct epithelial cells resulting from hepatic artery ligation.
(Hepatobiliary Pancreat Dis Int 2011; 10: 533-538)
portal vein arterialization; biliary epithelial cells; peribiliary plexus
Introduction
Bile duct epithelial cells are a group of highly active cytokine-producing cells characterized by high oxygen consumption, high metabolism and low tolerance to anoxia. In clinical practice, ischemic changes of the bile duct and injury to bile duct epithelial cells occur frequently if the blood supply of the hepatic artery is not restored promptly after liver transplantation or radical resection of hepatohilar cholangiocarcinoma.[1]In recent years, advances of diagnostic and therapeutic technology in hepatobiliary surgery have made radical resection of hepatohilar cholangiocarcinoma possible. However, the hepatic artery is frequently invaded due to the intrinsic biological properties of hepatohilar cholangiocarcinoma and is usually excised in an extended radical resection. When it is impossible or difficult to repair the hepatic artery, some researchers[2-4]performed experiments with reconstruction of the hepatic and biliary blood supply using the partial portal vein arterialization (PVA) technique and obtained satisfactory therapeutic results. In order to further determine the feasibility of partial PVA, we designed an experimental model of hepatic artery ligation combined with bile duct recanalization based on clinical evidence to assess the effects of partial PVA on the hilar bile duct.
Methods
Animal model and grouping
Thirty healthy and clean adult male Sprague-Dawley rats weighing 250-300 g were supplied by the Animal Center of the Academy of Military Medical Sciences. All rats were kept in specific pathogen-free conditions. A BX51TF system microscope was provided by Olympus Co..
The 30 rats were randomly and equally assignedinto 3 groups. The rats were fasted and deprived of water overnight prior to the operation. A median abdominal incision was made after anesthesia induced by an intraperitoneal injection of 10% chloral hydrate (0.3 mL/100 g) and sterilization using 0.5% iodophors. In group A (control), the hepatic artery, portal vein and bile duct were merely isolated. In group B, the portal vein was isolated first. The common hepatic artery, proper hepatic artery and gastroduodenal artery were isolated and ligated with 0 silk sutures before being excised in the middle. All tissues on the same plane as the anastomotic stoma and bile duct recanalization other than the portal vein were isolated and removed. Therefore, the blood supply to the hilar bile duct and the liver from collateral circulation was blocked. Bile duct cannulas made from stretched disposable anesthesia, epidural catheters were inserted into the bile duct through the upper and lower severed ends and immobilized with 0 silk suture cerclage and ligature of the sutures of the 2 ends to prevent cannula detachment. In group C, the portal vein was isolated first. The common hepatic artery, proper hepatic artery and gastroduodenal artery were isolated on the left side of the portal vein and ligated with 0 silk sutures before being excised in the middle. A piece of silk suture retained at the proximal end of the common hepatic artery was guided out from the posterior portion of the portal vein and attached to a small tapercut suture needle for later use. Two sutures were made close to the base of the dorsal common hepatic artery and close to the right edge of the portal vein bifurcation using 8-0 prolene sutures prior to occlusion of the portal vein. The reserved tapercut needle was guided between the 2 sutures just made into the lumen of the portal vein and out from the opposite side, passing through the common hepatic artery. Two more sutures were made close to the base of the ventral common hepatic artery and at the wound edge on the right side of the portal vein for further reinforcement. The redundant common hepatic artery on the left side of the portal vein was excised using microscissors after clamping of the abdominal aorta. Once the common hepatic artery stump retreated into the portal vein, wounds on the left side of the portal vein were sutured and tremor of the portal vein could be felt when the artery clamp was released. Clamps on the portal vein were released to restore portal vein blood flow (occlusion for approximately 7 minutes). All tissues other than the portal vein were isolated and excised at the anastomotic level. The bile duct reconstruction method was identical to that described in group B. In all groups, perihepatic ligaments were isolated by liver transplantation standards to block the blood supply of the hilar bile duct from collateral vessels (Fig. 1). Rats in all groups received intraperitoneal perfusion with 5 mL warm saline before closing the abdominal wall using whole-layer interrupted sutures.
Histological and immunohistochemical analysis
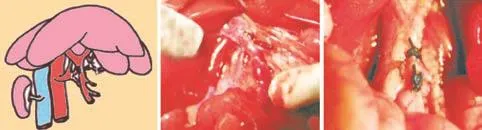
Fig. 1. A is a diagram of partial PVA. B was taken after partial PVA. The arrow shows the stoma of the hepatic artery and portal vein. C shows bile duct recanalization. The arrow shows the catheter. 1: gastroduodenal arteria; 2: proper hepatic artery; 3: portal vein; 4: splenic arteria; 5: common hepatic artery; 6: left gastric arteria; 7: common bile duct.
The hilar bile duct and liver were removed from sacrificed animals and fixed in 10% neutral formalin for at least 72 hours, followed by dehydration, paraffin impregnation, embedding, and serial sectioning at 5 μm. The sections of the hilar bile duct tissue and liver were stained with hematoxylin-eosin (HE), and proliferating cell nuclear antigen (PCNA) immunolabeling was performed using a mouse monclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) diluted at a ratio of 1/100 and an avidin-biotin-peroxidase technique. The terminal deoxynucleotidyl transferase mediated deoxyuridine-5'-triphosphate (dUTP) nick end labeling technique (TUNEL method) was used to measure apoptosis in rat hilar bile duct epithelial cells following the instructions with the TUNEL test kit (ABGAB Biotechnology, ABGAB, Germany). Smooth muscle actin immunolabeling was performed to obtainperibiliary plexus (PBP) arteriolar counts of the bile duct wall, using a mouse monoclonal antibody (Dako Biotechnology, Dako, Denmark). All analyses were made by a pathologist blinded to the experimental group under the study. PCNA-positive cells were identified by brownish staining of the biliary epithelial cell nuclei at various intensities that were clearly darker than the background. In each slide, 5 visual fields were selected under low power and biliary epithelial cells in each visual field were counted under a higher power (original magnification ×400). The PCNA index (PI) of biliary epithelial cells was presented as the percentage of positive cells over total cells. By TUNEL-positive determination, cell nuclei showed dark brownish staining. In each slide, 5 visual fields were selected under a low power and biliary epithelial cells in each field were counted under a higher power (original magnification ×400). The apoptosis index (AI) was presented as the percentage of positive cells over total cells. In PBP arteriolar count criteria, each brownish tubular structure was counted as one artery. In each slide, 5 visual fields were selected under a low power and counts were done under a higher power (original magnification ×400). PBP arteriolar counts were presented as the mean per high power field in each slide.
Liver functions in rats one month after partial PVA
Two milliliters of blood were collected from the inferior vena cava one month after the operation and centrifuged at 3000 rpm for 10 minutes to collect serum for the determination of liver functions.
Statistical analysis
Data were presented as mean±SD. SPSS 17.0 statistical software was used for analysis. One-way ANOVA analysis was used for inter-group comparison. The SNK method was used in a statistical test of pairwise comparisons. P values of less than 0.05 were considered statistically significant.
Results
General status of rats
The rats were able to move around freely after the operation, drink water (containing 10 mg/100 mL motilium) freely in 3-4 hours and eat freely in 6 hours. Body weights of the rats were recovered to preoperative levels in about 5 days.
PI and PBP arteriolar counts in the hilar bile duct
PI was used to indicate the proliferation of hilar bile duct epithelial cells. The PI value was higher in group B than in groups A and C (F=40.24, P<0.01) (Table 1). PBP arteriolar counts of the hilar bile duct were lower in group B than in group A (F=4.75, P<0.05), and there was no significant difference between group C and group B.
Postoperative apoptosis of biliary epithelial cells
The lack of clear apoptosis-positive cells in any group suggested that no apoptosis of biliary epithelial cells was induced in the 3 groups.
Liver functions
There were no statistically significant differences in the values of serum alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and albumin in the 3 groups (Table 2). The highest γ-glutamyltransferase (GGT) value was found in group B while the lowest value was found in group A. The GGT values of groups B and C were different (F=3.75, P<0.01).
Histology of the hilar bile duct and liver
In group A, epithelia of the hilar bile duct exhibited normal morphology and a regular arrangement without cell loss or wall thickening. In group B, the hilar bile duct wall thickened significantly and showed chronic inflammatory changes, and the alignment of biliary epithelial cells was in disarray with local cell loss and cytoplasmic vacuolation accompanied by infiltration of inflammatory cells and remarkable proliferation of goblet cells. In group C, cells of the hilar bile duct exhibited normal morphology and regular arrangement with slight wall thickening without clear glandular proliferation (Fig. 2). HE staining of hepatic tissuesfrom the 3 groups exhibited normal structure of hepatic lobules and regular arrangement of hepatocytes in all groups. But proliferation in the bile duct in the portal area was evident in group B (Fig. 3).
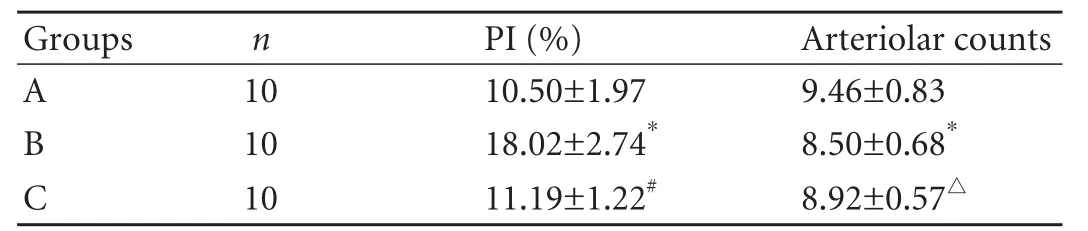
Table 1. PI and PBP arteriolar counts in hilar bile duct (mean±SD)
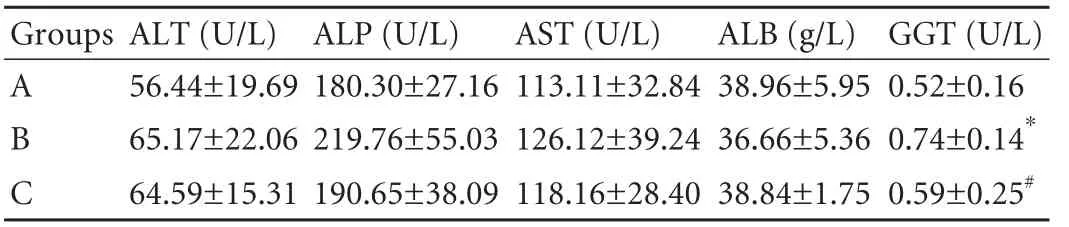
Table 2. Liver functions in rats 1 month after partial PVA (n=10, mean±SD)
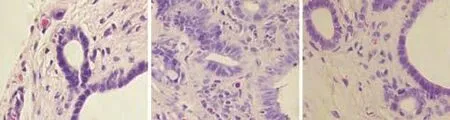
Fig. 2. Histological changes of rat hilar bile duct (HE staining, original magnification ×400). A: Normal hilar bile duct; B: hilar bile duct walls thickened significantly and showed chronic inflammatory changes; goblet cells showed remarkable proliferation; C: hilar bile duct epithelial cells exhibited normal morphology and regular arrangement with slight wall thickening without clear gland proliferation.
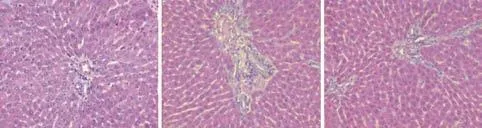
Fig. 3. HE staining of liver tissue (original magnification ×200). A, C: In groups A and C, the structure of the hepatic lobules was normal. Hepatocytes did not show any injury or a degeneration. B: In group B, the structure of hepatic lobules and hepatocytes was basically normal, but proliferating bile duct in the portal area was evident.
Discussion
Blood is supplied to the hilar bile duct by the hepatic artery-derived PBP. It was demonstrated that hepatic arterial blood was important in maintaining the normal physiological functions of biliary epithelial cells.[5]Considering the rich collateral circulation related to the blood supply network of the hilar bile duct,[6,7]we not only ligated the common hepatic artery and its branches, but also excised the bile duct for recanalization in designing our model. Meanwhile, all perihepatic ligaments were severed to ensure that the liver received its blood supply only from the portal vein in group B and that blood was only supplied from the arterialized portal vein in group C.
Kan et al[8]reported that lipiodol enters the portal vein through the PBP after infusion into the hepatic artery and confirmed the existence of communicating branches between the portal vein and the hepatic artery. Li et al[9]reconstructed the blood circulation of the bile duct in rats using surgically established complete PVA and demonstrated that arterialized portal vein blood can retrogradely perfuse the PBP to support normal physiological functions of the bile duct. Because a layer of continuous smooth muscle is present in the walls of small arteries and arterioles but is absent in the walls of venules and capillaries, subintimal arteries of the bile duct are stained using immunohistochemical staining of smooth muscle actin antigen.
We found a reduction of arteriole counts in both groups B and C after hepatic artery ligation, although there was significant difference in group B. Possibly a shortage of arterial blood supply resulted in occlusion of subintimal arterioles in group B, whereas arteriolar occlusion was relatively alleviated in group C by retrograde perfusion of a high flow volume of arterialized portal vein blood into the PBP.
Miyachi et al[10]first identified PCNA in the serum of systemic lupus erythematosus patients in 1978and named it because of its presence only in normal proliferative cells and tumor cells. Subsequent studies revealed the close relationship between PCNA and cellular DNA synthesis, the critical role of PCNA in initiation of cell proliferation, and its use as a fine indicator of cell proliferation status. Animal studies demonstrated that biliary epithelial cell proliferation can be induced by ligation of the bile duct, feeding animals with α-naphthylamine hydrochloride, or ischemia of the bile duct.[1,11,12]In this study, PI elevated remarkably in group B when hilar bile duct epithelial cells were completely deprived of an arterial blood supply. The biliary epithelial cells displayed an apparent proliferative response to ischemia and anoxemia after the hilar bile duct was deprived of its oxygen-rich arterial blood supply. In group C undergoing partial PVA, however, the proliferative response of biliary epithelial cells was significantly lower than that in group B. We consider that the PVA surgical procedure can significantly improve the anoxic condition of the hilar bile duct through the reverse current in the branches of the PBP.
Energy charge can objectively reflect the energy level of cells, and the normal functioning of mitochondria is essential to the maintenance of stable energy charge. Production of sufficient ATP by mitochondria depends on oxygen and energy substrates. Yamazoe et al[13]demonstrated in animal studies that PVA is a feasible surgical technique that can significantly improve the hepatic blood oxygen supply and increase hepatocyte oxygenation capacity and hepatocyte energy charge. Also shown in their study, partial PVA can maintain the stability of liver functions by supplying the liver and bile duct with sufficient oxygen and energy substrates. The statistically significant difference in serum GGT between group B and groups A and C in the current study suggested that injury of biliary epithelial cells after hepatic artery ligation results in elevation of serum GGT although notable alleviation occurred in group C. We speculate that oxygen-rich portal vein blood is able to reflow to supply the hilar bile duct after partial PVA, alleviate ischemia and anoxemia of biliary epithelial cells, and compensate for injury to biliary epithelial cells resulting from hepatic artery ligation.
Apoptosis is a basic biological phenomenon in multicellular organisms; it is gene-controlled autonomous sequenced death in contrast to necrocytosis. Under normal circumstances, apoptosis of biliary epithelial cells is rare in the rat hilar bile duct. The TUNEL technique was used in this study to detect apoptosis by labeled DNA breakage and did not identify any positive bile duct cells. Apoptosis of bile duct epithelial cells were supposed to occur within 1 or 2 days after operation in groups B and C due to the surgical procedure. One month after operation, however, all bile duct epithelial cells were normal or proliferative, and there was no apoptosis.
Our previous studies[14-16]suggested that the main mechanism of partial PVA is to increase the partial pressure of oxygen and perfusion pressure of portal vein blood in order to compensate for ischemia and anoxemia of the liver after hepatic artery ligation. The results in this study further demonstrate that PVA is important to reduce injury to the hilar bile duct after hepatic artery ligation.
Inoue et al[17]compared patients with and without hepatic artery reconstruction in extended radical resection of hilar cholangiocarcinoma. They found that the incidence rate of postoperative complications of the bile duct was 20% for patients receiving hepatic artery reconstruction and 100% for those not receiving this procedure. This result verifies the importance of the hepatic artery in biliary blood supply. Yamanaka et al[18]reported that the incidence of postoperative complications of the bile duct decreased significantly in patients receiving hepatic artery reconstruction when compared to those not receiving the procedure. Kondo et al[19]compared the two surgical procedures, after hepatic artery resection and retention of the hepatic artery in patients with cholangiocarcinoma. They found no statistical difference in the incidence of postoperative bile leakage and believed that PVA can safely replace artery reconstruction.
In summary, arterial blood with a certain flow rate and oxygen content plays a critical role in maintaining normal physiological functions of rat hilar bile ducts. Clinically, the partial PVA procedure can be used to replace artery reconstruction when it is impossible or difficult to reconstruct the hepatic artery during extended radical resection of hepatohilar cholangiocarcinoma. Short-term application of this technique is safe and effective.
Acknowledgements
We thank Dr. Wei Chen and Mr. Ben-Chu Gu from Department of Pathology, General Hospital of PLA for valuable comments on this study.
Funding:None.
Ethical approval:All study methods were approved by the Ethics Committee of General Hospital of PLA.
Contributors:CYL proposed the study. GSH and SJN performed the experiments. LCH, ZAQ and ZC were responsible for data collection. All authors contributed to the interpretation of the study and to further drafts. GSH analyzed the data and wrote the paper. CYL is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Beaussier M, Wendum D, Fouassier L, Rey C, Barbu V, Lasnier E, et al. Adaptative bile duct proliferative response in experimental bile duct ischemia. J Hepatol 2005;42:257-265.
2 Ozeki Y, Umemoto T, Tateyama K, Katagiri Y, Katayama M, Sugiyama A. Partial portal arterialization for dearterialized liver after hepatectomy. Br J Surg 1997;84:1011.
3 Young AL, Prasad KR, Adair R, Abu Hilal M, Guthrie JA, Lodge JP. Portal vein arterialization as a salvage procedure during left hepatic trisectionectomy for hilar cholangiocarcinoma. J Am Coll Surg 2008;207:e1-6.
4 Chen YL, Huang ZQ, Zhou NX, Huang XQ, Dong JH, Duan WD, et al. Radical resection of hilar cholangiocarcinoma with portal vein arteriolization, a report of two cases. Zhonghua Pu Tong Wai Ke Za Zhi 2007;22:404-406.
5 Gaudio E, Franchitto A, Pannarale L, Carpino G, Alpini G, Francis H, et al. Cholangiocytes and blood supply. World J Gastroenterol 2006;12:3546-3552.
6 Castaing D, Houssin D, Bismuth H. Anatomy of the liver and portal system. In: Castaing D, Houssin D, Bismuth H, ed. Hepatic and portal surgery in the rat. Paris: Masson;1980:27-45.
7 Itai Y, Matsui O. Blood flow and liver imaging. Radiology 1997;202:306-314.
8 Kan Z. Dynamic study of iodized oil in the liver and blood supply to hepatic tumors. An experimental investigation in several animal species. Acta Radiol Suppl 1996;408:1-25.
9 Li WG, Chen YL, Chen JX, Qu L, Xue BD, Peng ZH, et al. Portal venous arterialization resulting in increased portal inflow and portal vein wall thickness in rats. World J Gastroenterol 2008;14:6681-6688.
10 Miyachi K, Fritzler MJ, Tan EM. Autoantibody to a nuclear antigen in proliferating cells. J Immunol 1978;121:2228-2234.
11 Johnstone JM, Lee EG. A quantitative assessment of the structural changes the rat's liver following obstruction of the common bile duct. Br J Exp Pathol 1976;57:85-94.
12 Alpini G, Lenzi R, Zhai WR, Slott PA, Liu MH, Sarkozi L, et al. Bile secretory function of intrahepatic biliary epithelium in the rat. Am J Physiol 1989;257:G124-133.
13 Yamazoe K, Yanai T, Matsuki N, Bonkobara M, Ono K, Kudo T. Hepatic oxygen supply, energy charge, and histological findings in dogs with portal vein arterialization. J Vet Med Sci 1997;59:1057-1061.
14 Chen YL, Huang XQ, Huang ZQ, Duan WD, Wang YS. The effect of partial vein arterialization on changes of hepatic vascular framework in rats. Zhongguo Pu Tong Wai Ke Za Zhi 2007;16:223-226.
15 Chen YL, Li WG, Huang ZQ, Huang XQ, Chen MY, Duan WD. Effects of portal venous arterialization on acute occlusion of hepatic artery in rats. Chin Med J (Engl) 2008;121:1302-1306.
16 Chen YL, Huang ZQ, Huang J, Wang YS, Zhao JG. An experimental study on hilar en bloc resection and reconstruction of hepatic blood flow with arterialization of portal vein in rats with obstructive jaundice. Zhonghua Pu Tong Wai Ke Za Zhi 2001;16:94-96.
17 Inoue K, Makuuchi M, Takayama T, Torzilli G, Yamamoto J, Shimada K, et al. Long-term survival and prognostic factors in the surgical treatment of mass-forming type cholangiocarcinoma. Surgery 2000;127:498-505.
18 Yamanaka N, Yasui C, Yamanaka J, Ando T, Kuroda N, Maeda S, et al. Left hemihepatectomy with microsurgical reconstruction of the right-sided hepatic vasculature. A strategy for preserving hepatic function in patients with proximal bile duct cancer. Langenbecks Arch Surg 2001;386: 364-368.
19 Kondo S, Hirano S, Ambo Y, Tanaka E, Kubota T, Katoh H. Arterioportal shunting as an alternative to microvascular reconstruction after hepatic artery resection. Br J Surg 2004;91: 248-251.
Received October 7, 2010
Accepted after revision January 29, 2011
Author Affiliations: Department of Hepatobiliary Surgery (Guo SH, Li CH, Chen YL, Song JN, Zhang AQ and Zhou C) and Department of Surgical Oncology (Guo SH), General Hospital of PLA, Beijing 100853, China
Yong-Liang Chen, MD, Department of Hepatobiliary Surgery, General Hospital of PLA, Beijing 100853, China (Tel: 86-10-66938030; Fax: 86-10-66938030; Email: chenyongl301@yahoo.com.cn)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(11)60090-8
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Protective effect of clodronate-containing liposomes on intestinal mucosal injury in rats with severe acute pancreatitis
- Collagen proportionate area of liver tissue determined by digital image analysis in patients with HBV-related decompensated cirrhosis
- Evaluation outcomes of donors in living donor liver transplantation: a single-center analysis of 132 donors
- Salvianolic acid B modulates the expression of drug-metabolizing enzymes in HepG2 cells
- Protective effect of probiotics on intestinal barrier function in malnourished rats after liver transplantation
