Preparation and characterization of chitosan porous microcarriers for hepatocyte culture
2011-07-03XuBoWuChengHongPengFangHuangJieKuangSongLinYuYaDongDongandBaoSanHan
Xu-Bo Wu, Cheng-Hong Peng, Fang Huang, Jie Kuang, Song-Lin Yu, Ya-Dong Dong and Bao-San Han
Shanghai, China
Preparation and characterization of chitosan porous microcarriers for hepatocyte culture
Xu-Bo Wu, Cheng-Hong Peng, Fang Huang, Jie Kuang, Song-Lin Yu, Ya-Dong Dong and Bao-San Han
Shanghai, China
BACKGROUND:The bioartificial liver (BAL) is considered a possible alternative method for treating liver failure. The core of the BAL system is culturing liver cellsin vitrowith high density and activity. Microcarrier culture is a mode of high-density culture. We set out to prepare a novel porous microcarrier to improve the activity of liver cellsin vitro.
METHODS:Chitosan was used to prepare a novel porous spherical microcarrier with interconnected structure. The chitosan porous microcarriers (CPMs) were modified with gelatin to improve their biocompatibility. CPMs were co-cultured with liver cells, HL-7702 (L-02), to evaluate their effect on cell culture.
RESULTS:The average size of the CPMs was about 400 μm in diameter and their apertures were less than 30 μm. The pores of the microcarrier were interconnected. After fixation by sodium tripolyphosphate, the structure of the first freeze-dried CPMs was stable. To further improve the biocompatibility, the surface of CPMs was modified with gelatin through chemical crosslinking (GM-CPMs). Comparing the proliferation curves of L-02 cells cultured on simple CPMs, GM-CPMs and tissue culture polystyrene (TCPS, a mode of planar cell culture), the proliferation rates were similar in the first 5 days and the cells proliferated until day 8 in culture with microcarriers. The OD value of liver cells cultured on GM-CPMs was 1.97-fold higher than that on TCPS culture at day 8. Levels of urea and albumin in supernatants of cells cultured on GM-CPMs increased steadily for 8 days, and were clearly higher than those of cells cultured on TCPS (P<0.05).
CONCLUSIONS:The novel CPMs were promising microcarriers for hepatocyte culture and the GM-CPM seemed better. Porous microcarrier culture was beneficial for hepatocyte function and activity.
(Hepatobiliary Pancreat Dis Int 2011; 10: 509-515)
porous microcarrier; chitosan; gelatin, liver cells; cell culture
Introduction
Liver transplantation is still the most effective way for treating various end-stage liver diseases. However, clinicians have been looking for alternative approaches due to the donor shortage. The bioartificial liver (BAL) is considered a possible ideal method for treating acute liver failure. It can also serve as a bridge to liver transplantation for patients with types of liver failure.[1-3]
The core of the BAL system is culturing liver cells in vitro with a high density and activity.[4]Microcarrier culture provides a mode of high-density culture and a convenient means of transport.[5-7]But it is still a tough problem to retain and improve the function and activity of hepatocytes because primary hepatocytes dedifferentiate and lose specific hepatic functions rapidly in vitro. At present, primary hepatocytes are still the best cell source for the BAL. Hepatocytes are anchoragedependent cells. The structure of the matrix is very important for cell attachment and functional activity.[8]In vivo, hepatocytes grow in the niche of the extracellular matrix (ECM). The three-dimensional (3D) structure of the ECM is suitable for the growth and activity of hepatocytes. Culture techniques with 3D structures have been reported to be successful in maintenance of cell polarity and structure as well as in restoration of hepatocyte function.[9,10]
Chitosan, partially deacetylated chitin, is the only natural biological material with positive charge. It is a suitable biomaterial for scaffolds for various biomedical and pharmaceutical uses because it is biocompatible and biodegradable.[11-13]An additional reason for selecting chitosan as a scaffold for hepatocyte culture is that its structure is similar to glycosaminoglycans, which are components of the liver ECM. Gelatin is a collagen hydrolysis product with good biocompatibility. Many scaffolds acquire better biocompatibility after modification with gelatin.[14,15]
In this study, chitosan was used to manufacture a pilot spherical microcarrier with interconnected porous structure inside and outside. After modification with gelatin, the novel microcarriers were used to culture liver cells in vitro.
Methods
Preparation of chitosan porous microcarrier (CPM)
Chitosan (MW: 150 000; 75%-85% deacetylated) was from Sigma, and chitosan solution was made by adding the powder into 1% acetic acid with stirring. Chitosan porous spherical scaffold with interconnected structure was fabricated by direct freezing in liquid nitrogen. In order to generate micro-droplets, we built a high-voltage electrostatic field with a microcapsulator (made in the University of Shanghai for Science and Technology).[16]
Chitosan solution was extruded from a 1 mL syringe (inner diameter of the needle: 0.45 mm), the microdroplets were formed in a high-voltage electrostatic field, and then instilled directly into liquid nitrogen.[17]We collected the frozen chitosan beads with a filter and put them into a pre-cooled vacuum freeze-dryer. Then, primary CPMs were prepared through freeze-drying for 24 hours. Different concentrations of the chitosan solution were prepared to make porous microcarriers (data not shown), and we found that 2% (W/V) chitosan solution was more stable in the production process including controlling the shape, size and aperture.
Structural fixation of primary CPMs
The lyophilized primary CPMs were soluble in neutral aqueous solution and the porous structure was destroyed. It was necessary to reduce the solubility and swelling ratio of chitosan to maintain the porous structure of the microcarriers. The primary CPMs were soaked in saturated sodium tripolyphosphate (STPP, Alfa Aesar, China) ethanol solution (85% ethanol). The ionic interactions between the positively-charged amino groups of the chitosan molecule and the negativelycharged counterion, tripolyphosphate were used to solidify the micro-structure of CPMs. After crosslinking for 24 hours, the porous micro-structure of CPMs was fixed but remained intact in neutral solution for cell culture.
Characteristics of the fixed CPMs
The shape, size and aperture are the main parameters for porous microcarriers. Lyophilized spherical CPMs were observed under a loupe and an optical microscope (Olympus IX71) and their diameters were measured by image analysis (ACT-2U V1.62). Scanning electron microscopy (SEM; Philips XL30 FEG) was used to examine their surface structure. Imaging was conducted at an accelerating voltage of 10 kV. The aperture of the CPM was estimated according to the bar length in the micrographs. The internal porous structure was observed in paraffin sections after hematoxylin-eosin (HE) staining.
CPMs modified with gelatin
Gelatin is a collagen hydrolysis product with good biocompatibility, which has been widely used in various types of surface modification of biomaterials. We applied gelatin to modify the simple CPMs for the improvement of biocompatibility. Gelatin was obtained from Fluka and a 2% solution was prepared by dissolution in distilled water. The hydrated simple CPMs were soaked in 2% gelatin and then the crosslinker, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC • HCl; Yuanju Bio-tech, Shanghai) and N-hydroxysuccinimide (NHS; Fluka, USA) were added into the solution. The final concentration of EDC was 10 mmol, and the ratio of EDC to NHS was 4:1. After cross-linking at 40 ℃ for 24 hours, 75% ethanol was used to wash the microcarriers twice, and to produce gelatin-modified CPMs.
Liver cells cultured with CPMs
Simple CPMs and gelatin-modified CPMs (GMCPMs) were used to culture the hepatocyte line, HL-7702 (L-02), and to evaluate their effects on cell culture. First, microcarriers were sterilized for 30 minutes with 75% ethanol, washed three times with sterile PBS solution, and then immersed in culture medium (RPMI 1640) containing 10% calf serum and antibiotics (100 units/mL penicillin, 100 units/mL streptomycin) for two days before culture. Normal L-02 liver cells were co-cultured with simple CPMs and GM-CPMs in an ordinary plastic Petri dish under oscillation in rotation.
Cell morphology on CPMs was observed underan optical microscope every day. Because the CPMs were opaque, only cells growing on the surface of the microcarrier could be seen under the microscope. A fluorescent dye, acridine orange (AO), was used to stain cells for assessing the growth morphology of liver cells on the CPMs by fluorescence microscopy. The cellseeded CPMs were collected, washed with PBS, and then incubated in PBS with 0.5 mg/L AO for 15 minutes before observation.
Scanning electron microscope (SEM, FEI Quanta 200) was used to examine the morphology of cell-seeded CPMs. Briefly, glutaraldehyde-preserved (2.5%, 4 ℃) cellladen microcarriers were pre-treated with 1% OsO4for 2 hours, then dehydrated in gradient ethanol solutions and further dried by critical point drying in isopentyl acetate. Platinum was sputtered for SEM contrast.
In order to observe the internal structure of microcarriers and cells cultured on them, the cell-laden microcarriers were first fixed in 2.5% glutaraldehyde (GA) at 4 ℃, and then processed routinely and embedded in paraffin. Serial sections of the microcarriers were cut and stained with HE, and then the internal morphology and the cell distribution inside and outside the CPMs were observed under an optical microscope.
Cell proliferation test
L-02 cells (5×103) were co-cultured with a specific amount of simple CPMs and GM-CPMs in 96-well suspension culture plates (Greiner, Germany). The proliferation of cells on microcarriers was tested using the MTT assay with Cell Counting Kit-8 (Dojindo, Tokyo, Japan) according to the manufacturer's instructions. Briefly, samples of cell-seeded microcarriers were cultured in 200 μL culture medium, 20 μL of WST-8 reagent was added every day for 8 days, and followed by incubation at 37 ℃ under 5% CO2for 2 hours. Then the absorbance at 450 nm was measured with a microplate reader. The control group was L-02 cells cultured in 96-well tissue culture plates with tissue culture polystyrene (TCPS, Corning).
Functional assays
L-02 cells (5×105) were co-cultured with sterilized GM-CPMs in ordinary plastic petri dishes (ø: 35 mm), and the co-culture system was rotated on a shaker for the first 12 hours. We collected the supernatant every 48 hours. The supernatant samples were analyzed, and human albumin was tested by ELISA assay with corresponding kits according to the manufacturer's guidance (Bethyl Laboratories, USA). Urea was tested by an automatic biochemical analyzer. The control group was L-02 cells cultured in 6-well tissue culture plates with TCPS.
Statistical analysis
All data analyses were performed using Statistical Package for the Social Sciences (SPSS) version 13.0 software (SPSS, Inc., Chicago, IL). Student's t test was used to compare the means. P<0.05 was considered statistically significant.
Results
Characteristics of CPMs
We used 2% (W/V) chitosan solution to prepare the porous scaffold because of the stable production process and larger surface aperture. The first freezedried samples, primary CPMs, partially dissolved in neutral aqueous solution. In order to preserve the porous structure and reduce the solution rate of CPMs, STPP was used to fix the primary CPMs by crosslinking between the chitosan molecular chains.
After fixation by STPP, the porous structure of CPM was preserved and became more stable. We observed the structure by loupe, optical microscope and SEM (Fig. 1). The CPMs were spherical and opaque, and their average diameter was less than 400 μm. We saw the porous structure on the surface of CPMs under the SEM, and the pore size was less than 30 μm. More importantly, the pores of the CPMs were interconnected from outside toinside. This was a real 3D spherical scaffold. The CPMs were soft and tough. After modification by crosslinking with gelatin, we saw the gelatin fixed on the surface of simple CPMs under the SEM (Fig. 2).

Fig. 1. CPMs under a loupe (A, original magnification ×16), optical microscope (B, original magnification ×40; bar, 200 μm) and SEM (C, original magnification ×250; bar, 100 μm; D, original magnification ×500; bar, 50 μm).
Morphology of L-02 cells cultured on CPMs
L-02 cells were co-cultured with CPMs in an ordinary plastic petri dish with the condition of oscillation in rotation. After 6 hours, some cells adhered to the surface of the microcarriers (Fig. 3A). More and more cells attached to the surface of the CPMs with time, and the majority of cells had adhered to the GMCPMs after co-culture for 48 hours (Fig. 3B, C). The living cells on the CPMs were identified with a green color under a fluorescence microscope after AO staining. Fluorescence staining revealed the morphology of cells on microcarriers, and simultaneously, green staining with AO indicated cells with high activity.
We collected cell-seeded CPMs which had been cocultured for 5 days to observe the morphology of cells by SEM and paraffin sections. SEM showed that the cells grew well and were partially embedded in the pores of the microcarriers with a flat shape as seen from the outside (Fig. 4). This is different from the form of cells cultured on carriers a flat surface (cytodex-3). This mode of 3D culture may benefit cell function, especially in primary hepatocytes.

Fig. 2. Gelatin-modified CPM (SEM, A, original magnification ×250; B, original magnification ×500).

Fig. 3. L-02 cells adhered to the surface of GM-CPMs at 6 hours (A, optical microscope, original magnification ×40) and 48 hours (B, optical microscope; C, fluorescence microscope) (original magnification ×40).
The CPM showed an internal porous structure in paraffin sections (Fig. 5). All cells were on the surface of the CPM, and no cells were in the internal pores although the size of the pore was larger than the size of a single cell. Next, we want to further expand the pore size. Microcarriers with larger pores may be better for cells growing into the internal pores, and that could further improve the cell density and signal transduction between cells.
Proliferation of cells cultured with CPMs
Cells grew well and proliferated quickly on the novel microcarriers. Comparing the proliferation curves of L-02 cells cultured on simple CPMs, GM-CPMs and TCPS (Fig. 6), we found that the proliferation rates were similar in the first 5 days. Cells cultured with microcarriers proliferated until day 8, while the cells cultured with TCPS arrested, and even began to die after day 5. On day 8, the OD value of liver cellscultured on GM-CPMs was 1.97-fold higher than that on TCPS culture. Although this was mainly due to the larger surface area of microcarriers in the same volume, it indeed showed that the novel CPMs had good biocompatibility and no cytotoxicity.
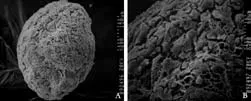
Fig. 4. L-02 cells grown on the surface of simple CPMs for 5 days (SEM, A, original magnification ×200; B, original magnification ×500).
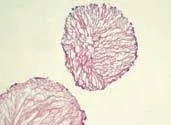
Fig. 5. Paraffin section of cell-laden GM-CPMs co-cultured for 5 days (HE staining, original magnification ×100).
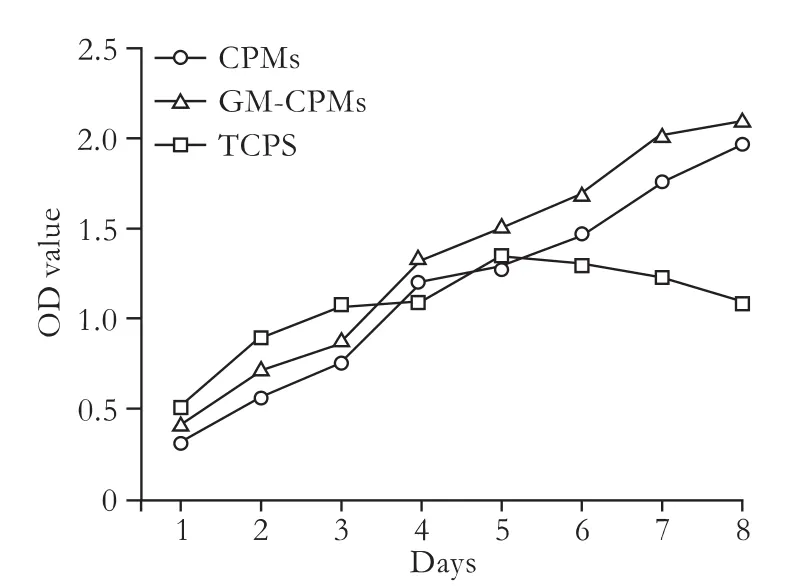
Fig. 6. Proliferation curves of L-02 cells cultured on CPMs, GMCPMs and TCPS.
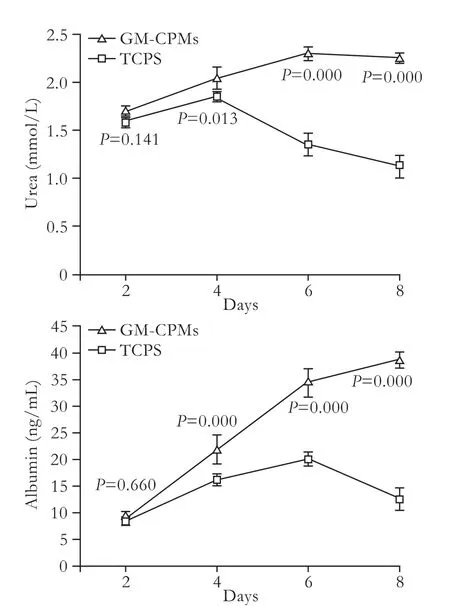
Fig. 7. Levels of urea and albumin in supernatants of L-02 cells cultured on GM-CPMs and TCPS.
In addition, liver cells cultured with GM-CPMs showed better proliferative ability than cells cultured with simple CPMs. The P values from day 1 to day 8 were 0.000, 0.000, 0.085, 0.137, 0.018, 0.002, 0.025 and 0.144. Usually, more cells were cultured on GM-CPMs than on simple CPMs, especially in the first two days (P=0.000). This indicates that gelatin modification is a good method for improving the biocompatibility of an engineered scaffold.
Hepatocyte function in culture with GM-CPMs
Synthesis of urea and secretion of albumin are important signs of good function in liver cells and reflect their activity. Levels of urea and albumin in supernatant samples were measured every 48 hours to evaluate the effect of GM-CPMs compared with TCPS culture (Fig. 7). Levels of urea and albumin in supernatants of GM-CPMs culture increased steadily over 8 days, and were clearly higher than those of TCPS culture (P<0.05). However, the number of cells in GM-CPM culture was not greater than in TCPS culture for the first 4 days, according to the proliferation curve. This indicated better activity of liver cells cultured on the GM-CPMs.
Discussion
Currently, liver transplantation is still the only curative treatment for liver failure due to end-stage liver diseases. Donor organ shortage, high cost, and the need for immunosuppressive medications are still the major limitations in the field of liver transplantation. Thus, alternative cell-based therapeutic approaches to liver failure have been proposed and liver tissue engineering is under investigation aiming at creating an artificial liver tissue. The BAL is a simple means of establishing cell-based therapeutic methods of treating liver failure. Liver cells with high activity are the core components of such an extracorporeal liver support system. Function and differentiation of liver cells are influenced by the 3D organ architecture, so researchers believe that such structure contributes to maintaining the function and activity of liver cells in vitro. The use of polymeric matrices with 3D structure for liver cells has been studied in the fields of culture and differentiation.[18-20]
Chitosan is considered to be an optimal biological material for various biomedical and pharmaceutical uses because of its biocompatibility and biodegradability. To prepare a novel porous spherical microcarrier with interconnected structure, a real 3D spherical scaffold, directly frozen chitosan microcarriers were prepared by extruding droplets of different concentrations of chitosan solution from a 26-gauge needle through a highvoltage electrostatic field built with a microcapsulator and collecting the droplets in liquid nitrogen for vacuum freeze-drying.[11]The strength of the electrostatic field influences the size of droplets and the concentration of chitosan solution influences the aperture of the freezedried microcarrier. We only report the establishment of a mode of manufacturing a porous micro-spherical scaffold with chitosan by direct freezing in liquid nitrogen.
In our study the lyophilized primary CPMs dissolvedin neutral aqueous solution, although the raw material, chitosan, does not dissolve in this situation. We suspected that deep hypothermia or the fast freezing process might damage the structure of molecular chains of chitosan. We designed a process to fix the structure of the primary CPMs with STPP, which is a non-toxic crosslinker which reacts with the amino groups of chitosan. The type of chitosan, STPP concentration, and crosslinking time could affect the stability of CPMs.
Gelatin is a collagen hydrolysis product that has been widely used in the surface modification of biomaterials. It was reported that the surface of chitosan contains numerous amino groups which might influence cell proliferation.[21]Simultaneously, we applied gelatin to modify the simple CPMs using the crosslinker, EDC and NHS in order to improve the biocompatibility of the CPMs. We observed the micro-structure of GMCPMs under SEM. Of course, it is better to measure the quantity of gelatin on the surface of GM-CPMs after immersion in culture medium.
After fixation by STPP (CPMs) or modification with gelatin (GM-CPMs) in our study, the porous spherical scaffolds were used to culture cells. We observed that the liver cells grew well on the simple CPMs and were partly embedded in the pores of the microcarriers. Although the cells did not grow into the pores, a larger contact area between the cells and microcarriers due to the pores could assist cell attachment and growth. Comparing the growth morphology and proliferation curves of L-02 cells cultured on simple CPMs, GM-CPMs and TCPS, we found that the novel CPMs were suitable for hepatocyte culture, especially the GM-CPMs, but it was difficult to distinguish from morphology because we used L-02 cells for culture and they had strong proliferative capacity on the simple CPMs or GM-CPMs. Moreover, when primary hepatocytes were used for culture, differences in growth morphology and function could be distinguished.
Comparing the hepatocyte function of L-02 cells cultured on GM-CPMs and TCPS showed that cells cultured on GM-CPMs had higher activity than those in TCPS culture. Mature primary hepatocytes with liver function are cubic or round in vitro, so it is difficult for them to attach to flat culture plates or microcarriers with a flat surface. A porous microcarrier might increase the contact area to improve the cell attachment. This is very important for hepatocytes, which are anchoragedependent cells.
In conclusion, we manufactured a novel porous microcarrier with chitosan, which provides a 3D spherical scaffold for cultured cells. This porous microcarrier is stable and safe for liver cells in culture by preliminary tests. This is a good device for culturing hepatocytes at high density and high activity for BAL. Other characteristics of the new microcarrier will be evaluated by experiments in vitro and in vivo, including the effect of 3D structure on the functional aspects of primary hepatocytes. In addition, the microcarrier structure itself needs further improvement.
Funding:This study was supported by grants from the National Natural Science Foundation of China (30672043, 30772105) and the National 863 program of China (2008AA02Z417).
Ethical approval:Not needed.
Contributors:WXB, PCH and HBS designed the research. WXB, PCH and HF analyzed the data and wrote the paper. All authors contributed to the design and interpretation of the study and to further drafts. PCH is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Maddrey WC. Bioartificial liver in the treatment of hepatic failure. Liver Transpl 2000;(4 Suppl 1):S27-30.
2 Rozga J, Morsiani E, Lepage E, Moscioni AD, Demetriou AA, Giorgio T. Isolated hepatocytes in a bioartificial liver: A single group view and experience. Biotechnol Bioeng 1994;43:645-653.
3 Lee PJ, Hung PJ, Lee LP. An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnol Bioeng 2007;97:1340-1346.
4 Kanematsu T, Eguchi S. Hepatocyte-based bioartificial liver support: past, present and future. Surg Today 1998;28:483-486.
5 Wu C, Pan J, Bao Z, Yu Y. Fabrication and characterization of chitosan microcarrier for hepatocyte culture. J Mater Sci Mater Med 2007;18:2211-2214.
6 Xiao C, Huang Z, Li W, Hu X, Qu W, Gao L, et al. High density and scale-up cultivation of recombinant CHO cell line and hybridomas with porous microcarrier Cytopore. Cytotechnology 1999;30:143-147.
7 Wang C, Gong Y, Lin Y, Shen J, Wang DA. A novel gellan gelbased microcarrier for anchorage-dependent cell delivery. Acta Biomater 2008;4:1226-1234.
8 Hammond JS, Beckingham IJ, Shakesheff KM. Scaffolds for liver tissue engineering. Expert Rev Med Devices 2006;3:21-27.
9 Yang P, Wang C, Shi Z, Huang X, Dang X, Xu S, et al. Prefabrication of vascularized porous three-dimensional scaffold induced from rhVEGF(165): a preliminary study in rats. Cells Tissues Organs 2009;189:327-337.
10 Wang S, Nagrath D, Chen PC, Berthiaume F, Yarmush ML. Three-dimensional primary hepatocyte culture in synthetic self-assembling peptide hydrogel. Tissue Eng Part A 2008;14:227-236.
11 Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999;20:1133-1142.
12 Hsieh WC, Chang CP, Lin SM. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissueengineering. Colloids Surf B Biointerfaces 2007;57:250-255.
13 Wan Y, Fang Y, Wu H, Cao X. Porous polylactide/chitosan scaffolds for tissue engineering. J Biomed Mater Res A 2007; 80:776-789.
14 Kawase M, Michibayashi N, Nakashima Y, Kurikawa N, Yagi K, Mizoguchi T. Application of glutaraldehyde-crosslinked chitosan as a scaffold for hepatocyte attachment. Biol Pharm Bull 1997;20:708-710.
15 Yagi K, Michibayashi N, Kurikawa N, Nakashima Y, Mizoguchi T, Harada A, et al. Effectiveness of fructosemodified chitosan as a scaffold for hepatocyte attachment. Biol Pharm Bull 1997;20:1290-1294.
16 Haque T, Chen H, Ouyang W, Martoni C, Lawuyi B, Urbanska AM, et al.In vitrostudy of alginate-chitosan microcapsules: an alternative to liver cell transplants for the treatment of liver failure. Biotechnol Lett 2005;27:317-322.
17 Ho MH, Kuo PY, Hsieh HJ, Hsien TY, Hou LT, Lai JY, et al. Preparation of porous scaffolds by using freeze-extraction and freeze-gelation methods. Biomaterials 2004;25:129-138.
18 Fiegel HC, Kaufmann PM, Bruns H, Kluth D, Horch RE, Vacanti JP, et al. Hepatic tissue engineering: from transplantation to customized cell-based liver directed therapies from the laboratory. J Cell Mol Med 2008;12:56-66.
19 Xing Q, Zhao F, Chen S, McNamara J, Decoster MA, Lvov YM. Porous biocompatible three-dimensional scaffolds of cellulose microfiber/gelatin composites for cell culture. Acta Biomater 2010;6:2132-2139.
20 Hu X, Xiao C, Huang Z, Guo Z, Zhang Z, Li Z. Pilot production of u-PA with porous microcarrier cell culture. Cytotechnology 2000;33:13-19.
21 Li G, Wang YJ, Ren L, Leng YX, Gao QY, Ge J. Preparation and characterization of gelatin-immobilized chitosan film. J Funct Materials 2008;39:1929-1932.
Received September 30, 2010
Accepted after revision March 28, 2011
All my life, as down an abyss without a bottom,ihave been pouring vanloads of information into that vacancy of oblivionicall my mind.
— Logan Pearsall Smith
Author Affiliations: Center of Organ Transplantation, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200025, China (Wu XB, Peng CH, Huang F, Kuang J, Yu SL, Dong YD and Han BS); Department of General Surgery, Central Hospital, Minhang District, Shanghai 201100, China (Wu XB)
Cheng-Hong Peng, MD, Center of Organ Transplantation, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200025, China (Tel: 86-21-64370045ext360509; Fax: 86-21-64373909; Email: chhpeng@188.com)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(11)60086-6
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Protective effect of clodronate-containing liposomes on intestinal mucosal injury in rats with severe acute pancreatitis
- Collagen proportionate area of liver tissue determined by digital image analysis in patients with HBV-related decompensated cirrhosis
- Evaluation outcomes of donors in living donor liver transplantation: a single-center analysis of 132 donors
- Salvianolic acid B modulates the expression of drug-metabolizing enzymes in HepG2 cells
- Protective effect of probiotics on intestinal barrier function in malnourished rats after liver transplantation
