Decrease of Peripheral Blood CD8+/CD28- Suppressor T Cell Followed by Dentritic Cells Immunomodulation among Metastatic Breast Cancer Patients
2010-07-10GuohongSongJunRenLijunDiJingYuJieZhangBinShaoJunJiaWeiSun
Guo-hong Song, Jun Ren, Lijun Di, Jing Yu, Jie Zhang, Bin Shao, Jun Jia, Wei Sun
Key Laboratory of Carcinogenesis and Translational Research(Ministry of Education), Department of Medical Oncology, Peking University Cancer Hospital & Institute, Beijing 100142, China
Decrease of Peripheral Blood CD8+/CD28- Suppressor T Cell Followed by Dentritic Cells Immunomodulation among Metastatic Breast Cancer Patients
Guo-hong Song, Jun Ren*, Lijun Di, Jing Yu, Jie Zhang, Bin Shao, Jun Jia, Wei Sun
Key Laboratory of Carcinogenesis and Translational Research(Ministry of Education), Department of Medical Oncology, Peking University Cancer Hospital & Institute, Beijing 100142, China
Objective:To explore the effects of dentritic cells on the peripheral blood lymphocyte subpopulations of metastatic breast cancer patients treated with chemotherapy.
Methods:The current study involved 44 metastatic breast cancer patients treated with docetaxel-based chemotherapy. Among them, 25 cases were treated with dendritic cells derived from CD34+hematopoietic stem cells enriched autologous peripheral mononuclear cells after chemotherapy, and 19 cases received chemotherapy alone. Peripheral blood samples were collected from each patient before and after treatment, and lymphocyte subpopulations including CD3+, CD3+/CD4+, CD3+/CD8+, CD3-/CD16+56+, CD3+/CD16+56+, CD4+/CD25+, CD8+/CD28-, CD8+/CD28+, CD4/CD8, DC1, DC2 and DC1/DC2 were analysed by a 3-color flow cytometric analysis.
Results:The two treatment groups were well matched with regard to demographic and baseline disease characteristics. Comparing the changes of lymphocyte subpopulations between the two groups, it showed that the difference of the change of CD8+/CD28-lymphocyte had statistic significance. The percentage of CD8+/CD28-lymphocyte was lower in the chemotherapy+DC group, but higher in the chemotherapy-alone group.
Conclusion:As CD8+/CD28-lymphocyte represent a kind of suppressive T lymphocyte, we conclude that dentritic cell therapy can relieve immunosuppression to some extent.
Metastatic breast cancer; Dentritic cell; Lymphocyte subpopulations; Regulatory T cell
INTRODUCTION
The outcome for patients with metastatic breast cancer (MBC) is quite poor. Significant progress has been made in this field. New cytotoxic agents and target therapy have all shown enhanced therapeutic benefit for breast cancer patients[12]. Nonetheless, tumor relapse, the emergence of toxicities, and early death due to the emergence of resistant disease continue to pose great challenges for the clinical management of this disease. Hence, there is an urgent needed for novel intervention strategies capable ofsynergising with standard adjuvant therapies; maintaining activity against refractory tumors and avoiding further immune suppression. Immunosuppression may contribute to the disease progression. Complex interactions between the immune system and tumor exist in patients with metastatic cancer, nonspecific and systemic immune dysfunction is prevalent in advanced and metastatic cancer[3-5]. In previous studies significant changes have been demonstrated in the numbers and functions of peripheral blood lymphocyte subsets in patients with different malignant disorders[4,6-8]. So to achieve a better outcome for patients with MBC, immunotherapy is one potential treatment option.
Immunotherapeutic strategies that successfully activate the immune system against tumor antigensare a prime alternative as they would probably be non-cross-resistant, specifically boost antitumor immunity and have non-overlapping toxicities. Dendritic cells (DCs) are antigen-presenting cells that are capable of stimulating antigen-specific naive and memory T cells. Ample evidences now indicate the existence of an immune response against breast cancer involving DC recruitment and activation within tumor tissue[9,10-12]. Despite the evidences, numerous studies have demonstrated severe phenotypic and functional impairment of DCs in patients with breast cancer[13,14]. Circulating DCs isolated from patients with breast cancer exhibited an impaired capacity to stimulate T lymphocyte proliferation and cytokine secretion[13]. Moreover, patients with metastatic breast cancer showed a large number of immature APCs with poor immunological function in the blood DC compartment[15]. Thus DC therapy is emerging as a crucial strategy to enhance anticancer activity for patients with this disease.
In this study we treated the metastatic breast cancer patients with mature dendritic cell derived from autologous peripheral CD34+hematopoietic stem cells enriched autologous peripheral mononuclear cells after chemotherapy, and try to explore the effects of dentritic cell on the immune function, such as the peripheral blood lymphocyte subpopulations of these patients.
MATERIALS AND METHODS
Patients and Treatment
The current study involved 44 patients with metastatic breast cancer who were admitted to the Department of Medical Oncology, Peking University Cancer Hospital & Institute between January 2008 and May 2009. All these patients were treated with docetaxel-based chemotherapy. Among these patients 25 cases were treated with dendritic cells derived from CD34+hematopoietic stem cells enriched autologous peripheral mononuclear cells after chemotherapy and 19 cases received chemotherapy alone. As it was a retrospective study, the case number of the two groups was not equal. DCs immunotherapy was approved by Beijing Health Administration and the Local Ethics Committee of Peking University Cancer Hospital & Institute. Written informed consent from each patient was obtained prior to study entry.
Hematopoietic Stem Cells (HSC) Mobilization and Collection
CD34+ HSCs were mobilized by docetaxel-based chemotherapy and G-CSF. After receiving chemotherapy, when the WBC count of peripherial blood decreased to ≤1.5×109/L, granulocyte colony stimulating factor (G-CSF) at a dose of 5µg/kg/day was given subcutaneously. Autologous peripheral blood mononuclear cells (PBMC) were isolated by leukapheresis when the WBC count increased to≥10.0×109/L. Leukapheresis was performed by the CBS200 Spectra cell separator and we analyzed the numbers of PBMC and CD34+HSC in the collections by flow cytometry.
Culture and Transfusion of Dendritic Cells
DCs were generated byin vitrostimulation with IL-4, GM-CSF and TNF-α. Human peripheral blood mononuclear cells (PBMC) were separated by Ficoll , and the adherent cells were grown in X-VIVO 15 (Cambrex Co, USA) with 100 μg/L of GM-CSF (Tebao bio Co., Xiamen China), 10μg/L of IL-4 (R&D Co, USA), and 2.5μg/L of TNF-α (R&D Co, USA). Cell counts and phenotype of DCs were analyzed 10 to14 days after culture. Molecules of CD1a, CD11c, CD80, CD86 and anti-HLA-DR were analyzed by flow cytometer. If the cells differentiated into typical matured dendritic cells with classical phenotype, venous transfusion were conducted immediately. DCs transfusion was conducted every three or four days for altogether 3 times, each time including 107~109dendritic cells.
Blood Samples
Heparinized peripheral blood samples were collected from each patient before chemotherapy. For the DC+chemotherapy group, blood samples were collected again after chemotherapy and the third DC infusion(about four weeks after the chemotherapy) and for the chemotherapy alone group, blood samples were collected again three or four weeks after the chemotherapy or just before the beginning of the next cycle of chemotherapy. All these fresh blood samples were analysed for T cell subpopulations, including CD3+, CD3+/CD4+, CD3+/CD8+, CD3-/CD16+56+, CD3+/CD16+56+, CD4+/CD25+, CD8+/CD28-, CD8+/CD28+, CD4/CD8, DC1, and DC2, DC1/DC2.
Cell Isolation
Heparinized peripheral blood was obtained, 100μl whole blood was used per tube and they were stained with antibodies, and incubated in the dark at 4°C for 15 min. After hemolysis 10 and gradients Centrifugation, the cells were washed twice in PBSand subjected to flow cytometric analysis. The following antibodies, anti-CD4(FITC/CD8(PE) /CD3(PerCP)(Becton-Dickinson, BD), anti-CD4-fluorescein isothiocyanate (FITC) (Beckman-Coulter), anti-CD25-phycoerythrin (PE) (Beckman-Coulter), anti-CD28-fluorescein (Beckman-Coulter), anti-CD8-phycoerythrin (PE) (Beckman-Coulter), CD3(FITC/CD16+56(PE) (Becton-Dickinson, BD), anti-CD19-PC5(Beckman-Coulter) were used.
Flow Cytometric Analysis
A 3-color flow cytometric analysis protocol was performed to determine cell phenotype. Flow cytometry was performed on Epics XL (Beckman-Coulter), and Expo32 ADC software (Beckman-Coulter) was used for analysis. Lymphocytes were gated by forward scatter versus side scatter. T lymphocytes were further gated on anti-CD3(PerCP) positive cells in the lymphocyte gate. B lymphocytes were further gated on anti-CD19(PerCP) positive cells in the lymphocyte gate. NK lymphocytes were further gated on anti-CD16+56(PE) positive cells in the lymphocyte gate. Analysis was set to stop when 5000 events had been analyzed.
Statistical Methods
Statistical analyses of data were carried out using SPSS software Version 15.0. APvalue of less than 0.05 was regarded as statistically significant. Quantitative factors were compared by Pcarson’sχ2contingency Table analysis. Results of the relative numbers of all lymphocyte subpopulations were presented as¯x±s deviations. Statistical analyses were performed using the Student'st-test to compare the changes of lymphocyte subpopulations between chemotherapy+DC group and chemotherapy alone group.
RESULTS
Study Population
A total of 44 patients were enrolled, 25 in chemotherapy DC+ group, 19 in chemotherapy alone group. The treatment groups were well matched with regard to demographic and baseline disease characteristics. The detailed results are shown in Table 1.
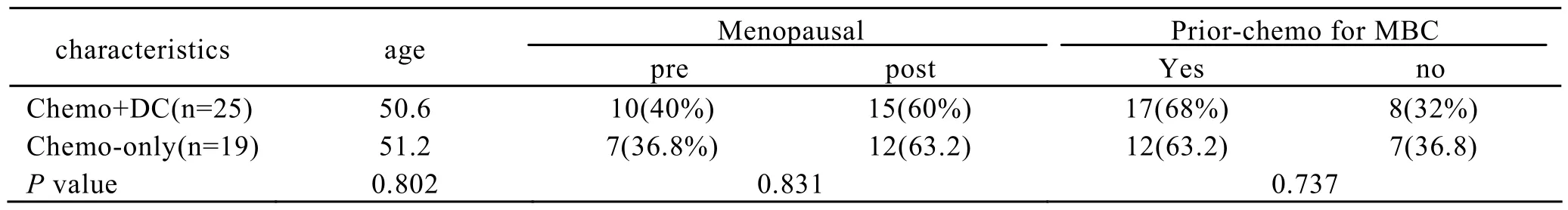
Table 1.Demographic and Baseline Disease Characteristics of Patients
Comparison of the Changes of Lymphocyte Subpopulations between Chemotherapy+DC Group and Chemotherapy-alone Group
Only the difference of the change of CD8+/CD28- lymphocyte between the two groups had statistic significance(P=0.018). It showed that the percentage of CD8+/CD28- lymphocyte was lower in the chemotherapy+DC group, and higher in the chemotherapy-alone group. The results are shown in Table 2.
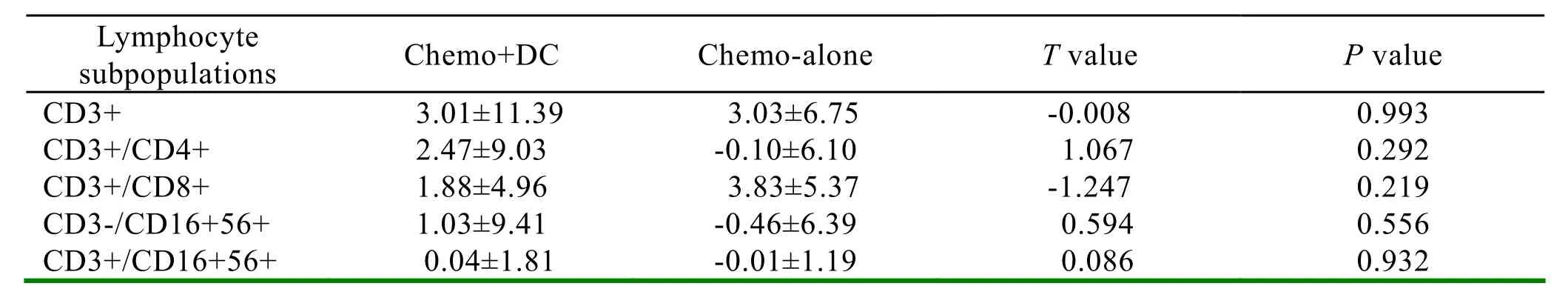
Table 2.Comparison of the changes of lymphocyte subpopulations between the chemotherapy+DC group and chemotherapy-alone group (t-test,α=0.05)
DC: dendritic cell; Chemo: chemotherapy
DISCUSSION
Successful treatment of advanced breast cancer is a significant clinical problem. Metastatic breast cancer patients have impaired immune functions. Immunosuppression may contribute to the progression of disease. Complex interactions between the immune system and tumor exist in patients with metastatic cancer. Multiple cellular and molecular layers of the suppressive network have been imposed in the tumor microenvironment in patients with cancers[16]. Chemotherapy is the most actively used modality in the treatment of advanced-stage cancer. It is well established that it is associated with significant immunosuppression[17,18]. Defectis in DC in cancer patients have also been considered as a reason for tumor-induced immune suppression in hosts. There is evidence to suggest that cancer patients may have DC that do not express co-stimulatory molecules and remain immature; thus, directly influencing the tumor-induced immune response and contributing to immune suppression[12,19]. Moreover a number of studies have demonstrated that tumor-infiltrating lymphocytes in 45-60% of breast cancers produce immunoregulatory cytokines, such as IL-4, IL-10 and/or transforming growth factor (TGF)-β[20,1], thus negatively influencing the generation of protective antitumor responses. Regulatory T cell (Treg)-mediated immune suppression has also been suggested[22]. The frequency of Tregs in peripheral blood of patients with breast cancer has been found to be significantly higher than in healthy volunteers, and such accumulation appears to have an impact on the efficiency of the immune response[22,23].
Regulatory T cells exist both within the CD4 (CD4+CD25+T cells)[24,25]and CD8 subsets. CD8+CD28- T cells represent a unique subset of regulatory T cells which is major histocompatibility complex (MHC) class I restricted, T suppressor (Ts) cells. They inhibit both proliferative and cytotoxic T cell responses[26-29]. CD8+CD28- Ts cells acquire and exert their functions via direct interaction with APC, recognize MHC peptide complexes on the cell surface of APC and trigger the upregulation of inhibitory receptors and down- regulation of costimulatory molecules, rendering the APC tolerogenic[30,31].
It becomes increasingly clear that neither cytotoxic therapy nor immunotherapy alone would be able to successfully solve the problem to acquire a satisfactory result. Chemotherapy frequently fails because of the development of drug resistance[32], and immunotherapy alone is not able to maintain effective antitumor immune response in the presence of bulky tumor because of the effects of tumor-derived immunosuppressive factors[33]. It appears that only a combination of different modalities may provide the necessary breakthrough in the treatment of this group of patients.
In this study we treated the metastatic breast cancer patients with mature dendritic cells derived from autologous peripheral CD34+hematopoietic stem cells enriched autologous peripheral mononuclear cells after chemotherapy, and performed a detailed analysis of peripheral blood lymphocyte subpopulations during chemo-immune therapy. It showed that the percentage of CD8+/CD28- lymphocytes was lower in the chemotherapy+DC group, but higher in the chemotherapy-alone group. The difference of the change of CD8+/CD28- lymphocyte between the two groups has statistic significance. This means that chemotherapy has immunosuppressive effect, while combined with DC the immunosuppression can be somewhat relieved.
Our approach is based on the findings that DCs are able to take up apoptotic cells and to process and present antigens associated with these cells[34,35]. A number of studies show that dendritic cells are initiators of immune responses, it can induce T-cell responses, enhance T-cell reactivity and stimulate tumor-specific CTLs, capable of lysing autologous tumor cells[36-39]. Also some reports show that DC therapy can induce an increase of tumor-specific cytotoxic T lymphocytes in the peripheral blood[37,40,41]. But there was few reports about the effect of DC on the peripherial blood regulatory T cells, especially the CD8+/CD28- suppressor T cells. Ahn’s study[42]suggests for the first time that Dendritic cells partially abrogate the regulatory activity of CD4+CD25+T cells present in the human peripheral blood.
In our study, it showed that chemotherapy is associated with immunosuppression (higher percentage of CD8+/CD28- Ts cells), the CD8+/ CD28- suppressor T cells decrease when combined therapy. It seems that DC downregulates the suppressor T cells, enhances the immune reconstruction. This effect may be critical for the success of combined treatment.
Thus, we can conclude that besides the activation of T cell response, the relief of immunosuppression via the downregulation of CD8+/CD28- suppressor T cells also plays an important role in the DC immunotherapy.
REFERENCES
[1] Therasse P, Piccart M, van de Velde CJ, et al. The EORTC Breast Cancer Group: 40 years of research contributing to improve breast cancer management[J]. Eur J Cancer 2002; 38(Suppl 4): S39-43.
[2] Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2[J]. N Engl J Med 2001; 344: 783-92.
[3] Kiessling R, Wasserman K, Horiguchi S, et al. Tumor-induced immune dysfunction[J]. Cancer Immunol Immunother 1999; 48: 353-62.
[4] Melichar B, Touskova M, Solichova D, et al. CD4+ T-lymphocytopenia and systemic immune activation in patients with primary and secondary liver tumours[J]. Scand J Clin Lab Invest 2001; 61: 363-70.
[5] Lissoni P, Brivio F, Ferrante R, et al. Circulating immature and mature dendritic cells in relation to lymphocyte subsets in patients with gastrointestinal tract cancer[J].Int J Biol Markers 2000; 15: 22-5.
[6] Melichar B, Jandik P, Krejsek J, et al. Mitogeninduced lymphocyte proliferation and systemic immune activation in cancer patients[J]. Tumori 1996; 82: 218-20.
[7] Okita R, Saeki T, Takashima S, et al. CD4+CD25+regulatory T cells in the peripheral blood of patients with breast cancer and non-small cell lung cancer[J]. Oncol Rep 2005;14:1269-73.
[8] Meloni F, Morosini M, Solari N, et al. Foxp3 expressing CD4+CD25+and CD8+CD28−T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma[J]. Human Immunology 2006; 67: 1-12.
[9] Shortman K, Caux C. Dendritic cell development: multiple pathways to nature’s adjuvants[J]. Stem Cells 1997; 15: 409–19.
[10] Lespagnard L, Gancberg D,Rouas G, et al. Tumorinfiltrating dendritic cells in adenocarcinomas of the breast: a study of 143 neoplasms with a correlation to usual prognostic factors and to clinical outcome[J]. Int J Cancer 1999; 84: 309-14.
[11] Iwamoto M, Shinohara H, Miyamoto A, et al. Prognostic value of tumor-infiltrating dendritic cells expressing CD83 in human breast carcinomas[J]. Int J Cancer 2003; 104: 92-7.
[12] Bell D, Chomarat P, Broyles D, et al. In breast carcinoma tissue, immature dendritic cells reside within the tumor,whereas mature dendritic cells are located in peritumoral areas[J]. J Exp Med 1999; 190: 1417-26.
[13] Gabrilovich DI, Corak J, Ciernik IF, et al. Decreased antigen presentation by dendritic cells in patients with breast cancer[J]. Clin Cancer Res 1997; 3: 483-90.
[14] Satthaporn S, Robins A, Vassanasiri W, et al. Dendritic cells are dysfunctional in patients with operable breast cancer[J]. Cancer Immunol Immunother 2004; 53: 510-8.
[15] Pinzon-charry A, Ho CS, Laherty R, et al. A population of HLA-DR+ immature cells accumulate in the blood dendritic cell compartment of patients with different types of cancer[J]. Neoplasia 2005; 7: 1112-22.
[16] Zou W. Immunosuppressive networks in the tumor environment and their therapeutic relevance[J]. Nat Rev Cancer 2005; 5: 263-74.
[17] Mokyr MB, Dray S. Interplay between the toxic effects of anticancer drugs and host antitumor immunity in cancer therapy[J]. Cancer Invest 1987; 5: 31-8.
[18] Oliver RT, Nouri AM. T cell immune response to cancer in humans and its relevance for immunodiagnosis and therapy[J]. Cancer Surv 1992; 13: 173-204.
[19] Almand B, Resser JR, Lindman B, et al. Clinical significance of defective dendritic cell differentiation in cancer[J]. Clin Cancer Res 2000; 6: 1755–66.
[20] Camp BJ, Dyhrman ST,Memoli VA, et al.In situcytokine production by breast cancer tumorinfiltrating lymphocytes[J]. Ann Surg Oncol 1996; 3: 176-84.
[21] Venetsanakos E, Beckman I, Bradley J, et al. High incidence of interleukin 10 mRNA but not interleukin 2 mRNA detected in human breast tumors[J]. Br J Cancer 1997; 75: 1826-30.
[22] Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma[J]. J Immunol 2002; 169: 2756-61.
[23] Wolf AM, Wolf D, Steurer M, et al. Increase of regulatory T cells in the peripheral blood of cancer patients[J]. Clin Cancer Res 2003; 9: 606-12.
[24] Sakaguchi S, Takagushi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chain (CD25) Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases[J]. J Immunol 1995; 155: 1151-64.
[25] Groux H, O'Garra A, Bigler M, et al. A CD4+ T cell subset inhibits antigen-specific T cell responses and prevents colitis[J].Nature 1997; 389: 737-42.
[26] Cortesini R, LeMaoult J, Ciubotariu R, et al. CD8+CD28- T suppressor cells and the induction of antigen-specific, antigen-presenting cell-mediated suppression of Th reactivity[J]. Immunol Rev 2001; 182: 201-6.
[27] Scotto L, Naiyer AJ, Galluzzo S, et al. Overlap Between Molecular Markers Expressed by Naturally Occurring CD4+CD25- Regulatory T Cells and Antigen Specific CD4+CD25- and CD8+CD28- T Suppressor Cells[J]. Human Immunology 2004; 65: 1297-306.
[28] Filaci G, Fravega M, Fenoglio D, et al. Non-antigen specific CD8+ T supporessor lymphocytes[J]. Clin Exp Med 2004; 4: 86-92.
[29] Filaci G, Fenoglio D, Fravega M, et al. CD8+CD28-T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers[J]. J Immunol 2007; 179: 4323-34.
[30] Chang CC, Ciubotariu R, Manavalan JS, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4[J]. Nat Immunol 2002; 3: 237-43.
[31] Manavalan JS, Rossi PC, Vlad G, et al. High expression of ILT3 and ILT4 is a general feature of tolerogenic dendritic cells[J]. Transpl Immunol 2003; 11: 245-58.
[32] Shah MA, Schwartz GK. Cell cycle-mediated drug resistance: an emerging concept in cancer therapy[J]. Clin Cancer Res 2001; 7: 2168-81.
[33] Keilholz U, Weber J, Finke JH, et al. Immunologic monitoring of cancer vaccine therapy: results of a workshop sponsored by the society for biologic therapy[J]. J Immunother 2002; 25: 97-138.
[34] Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs[J]. Nature 1998; 392: 86-9.
[35] Nouri-Shirazi M, Banchereau J, Bell D, et al. Dendritic cells capture killed tumor cells and present their antigens to elicit tumor-specific immune responses[J]. J Immunol 2000; 165: 3797-803.
[36] Gong J, Avigan D, Chen D, et al. Activation of antitumor cytotoxic T lymphocytes by fusions of human dendritic cells and breast carcinoma cells[J]. Proc Natl Acad Sci USA 2000; 97:2715-18.
[37] Neidhardt-berard EM, Berard F, Banchereau J, et al. Dendritic cells loaded with killed breast cancer cells induce differentiation of tumor-specific cytotoxic T lymphocytes[J]. Breast Cancer Res 2004; 6: R322-8.
[38] Svane IM, Pedersen AE, Johnsen HE, et al. Vaccination with p53-peptide-pulsed dendritic cells, of patients with advanced breast cancer: report from a Phase I study[J]. Cancer Immunol Immunother 2004; 53: 633-41.
[39] Loveland BE, Zhao A, White S,et al. Mannan-MUC1-pulsed dendritic cell immunotherapy: a Phase I trial in patients with adenocarcinoma[J]. Clin Cancer Res 2006; 12: 869-77.
[40] Titzer S, Christensen O, Manzke O, et al. Vaccination of multiple myeloma patients with idiotype-pulsed dendritic cells: immunological and clinical aspects[J]. Br J Haematol 2000; 108: 805-16.
[41] Morse MA, Clay TM, Colling K, et al. HER2 dendritic cell vaccines[J]. Clin Breast Cancer 2003; 3(Suppl 4): S164-72.
[42] Ahn JS, Krishnadas DK, Agrawal B. Dendritic cells partially abrogate the regulatory activity of CD4+CD25+T cells present in the human peripheral blood[J]. Int Immunol 2007; 19: 227-37.
R737.9 Document code: A Article ID: 1000-9604(2010)04-0310-06
10.1007/s11670-010-0310-6
2010−03−21;Accepted2010−08−17
This work was supported by a grant from the Beijing Capital Development Foundation for Medical Sciences(No. 2007-2053).
*Corresponding author.
E-mail: renjun@bjcancer.org
© Chinese Anti-Cancer Association and Springer-Verlag Berlin Heidelberg 2010
杂志排行
Chinese Journal of Cancer Research的其它文章
- Chinese Journal of Cancer Research Guidelines for Authors
- Inhibition of Proliferation Induced by Cyclin D1 Gene Silence in Human Renal Carcinoma ACHN Cells
- Interleukin-18 Suppresses Angiogenesis and Lymphangiogenesis in Implanted Lewis Lung Cancer
- High Expression of ERCC1 Is a Poor Prognostic Factor in Chinese Patients with Non-small Cell Lung Cancer Receiving Cisplatin-based Therapy
- Association of Lysosome Associated Protein Transmembrane 4 Beta Gene Polymorphism with the Risk of Pancreatic Cancer
- Recurrent Patterns and Factors Involved in Node-negative Advanced Gastric Cancer
