Recurrent Patterns and Factors Involved in Node-negative Advanced Gastric Cancer
2010-01-08EnYiLiuMeiZuoZhongCaiGangLiuJinHuangWeiLiuShanZengBinLiJingChenLuJianHuangLiHaiRongJiang
En-Yi Liu,Mei-Zuo Zhong*,Cai-Gang Liu,Jin Huang,Wei Liu,Shan Zeng,Bin Li,Jing-Chen Lu,Jian-Huang Li,Hai-Rong Jiang
1Department of Oncology,Xiangya Hospital,Central South University,Changsha 410008,China;
2Department of Surgical Oncology,First Hospital,China Medial University,Shenyang 110001,China
INTRODUCTION
Although the survival rate of the patients with gastric cancer has been improved nowadays because of early detection,rational lymphadenectomy and several therapeutic modalities[1],gastric cancer still remains the second leading cause of cancer mortality in the world[2].The lymph node-negative gastric cancer has a better prognosis than the node-positive tumors,which might be explained by less aggressive biological behavior in these tumors[3].However,although curative surgery can be performed for advanced gastric cancers with negative node,recurrence can still occur through several patterns of dissemination including locoregional,hematogenous,and peritoneal seeding[4].
Currently,no effective therapy exists for the recurrent gastric carcinoma which is vulnerably resistant to chemotherapy[5-7].For this reason,estimation of the risk of recurrence in individual patients is important in clinical practice for applying adjuvant therapies after surgery,and also for planning an adequate follow-up program.Many studies have been devoted to investigating the prognostic factors of gastric cancer[8,9].However,clinicopathological predictive factors for tumor recurrence and recurrence patterns have not been well investigated in nodenegative advanced gastric adenocarcinoma after curative resection.
In this paper,an investigation relating to a large number of patients has been performed to find the recurrence patterns and factors associated with nodenegative advanced gastric cancer after curative resection,and also to evaluate the prognosis and to improve the treatment strategies.
PATIENTS AND METHODS
Patients’ Information
A total of 310 patients with lymph node-negative advanced gastric adenocarcinoma who received a curative resection between 2002 and 2006 were enrolled in the study.The male-to-female ratio of the 310 patients was 1:1.31,and the mean age was 57.82 years (range,25 to 77 years)at operation.Of the 310 cases,24 underwent less than D2 lymph node dissection,180 underwent D2 lymph node dissection,and 106 underwent more than D2 lymph node dissection.The selection criteria of the patients for this study are as follows: 1)the gastric tumor is deeper than submucosal invasion,2)a curative resection has been performed,3)an abdominal computed tomography (CT)scan or sonogram,and chest film have been performed to exclude distal metastasis before operation,4)lymph node metastasis have not been observed,and 5)the patient medical records are complete and available.
Lymph nodes were dissected from en bloc specimens,and classification of the dissected lymph nodes was verified by the surgeons who reviewed the excised specimens after surgery based on the classification (6th Edition)of the International Union Against Cancer (UICC)[10].The resected bloc specimens and lymph nodes were stained with hematoxylin and eosin,and examined by specialized pathologists.All patients were periodically followed up in inpatient or outpatient,or through mail or telephone interviews with the patients and their relatives.The last follow-up was Jan 1st,2010.The study protocol was approved by the Ethics Committee of Central South University and China Medical University.
The location of tumors was based on the classification (6th Edition)of the AJCC[10].The histological type and pN categories were also classified according to the 6th Edition of the AJCC[10],where the pN categories include pN0-3 (i.e.pN0: no node involved; pN1: 1-6 nodes involved; pN2: 7-15 nodes involved; and pN3: >15 nodes involved).
Statistical Methods
All the data were analyzed with SPSS 13.0 statistics software(Chicago,IL,USA).Clinicopathological characters among different groups were analyzed by chi-square tests or independent t tests.Logistic regression was used to select the independent predictors for the occurrence of the recurrence.Multivariate analysis was performed using the Cox proportional hazards model selected in forward stepwise.Hazard ratios and 95% confidence intervals(95% CI)were calculated.The Kaplan-Meier method and log rank test were adopted for the analysis of the survival rate comparison.A P value of less than 0.05 was considered statistically significant.
RESULTS
Clinicopathological Characteristics of Advanced Gastric Cancers
Of 310 cases,300 were successfully followed up with a total follow-up rate of 96.8% (the median follow-up period was 67 months).The 5-year specific survival rate of 300 cases was 75.7%.Of the 300 patients, 15(5.0%) patients had locoregional recurrence,5(1.7%)had lymph node recurrence,27(9.0%)had peritoneal seeding recurrence,and 21(7.0%)had hematogenous metastasis including lung,liver,bone,and brain metastasis(Table 1).
Multivariate Analysis of Risk Factors of Recurrence
Using univariate analysis,larger tumor diameter(P=0.020),poorer differentiated tumors (P=0.018)and more cases of Borrmann IV cancer (P=0.024)were observed in patients with locoregional recurrence compared to patients without locoregional recurrence.In patients with lymph node recurrence,more tumors occupied the total stomach (P=0.001),there were poorer differentiated tumors (P=0.016),and more lymphovascular invasion (P=0.016)were found compared to cases without lymph node recurrence.In addition,more D1 dissection was found in patients with lymph node recurrence compared to cases without lymph node recurrence (P=0.001).In patients with peritoneal seeding recurrence,more multiple tumors (P=0.027),larger tumor diameter(P=0.028),deeper tumor invasion (P=0.001)and more cases of Borrmann IV cancer (P=0.033)were noted compared to patients without peritoneal seeding recurrence.Moreover,more lymphovascular invasion was observed in cases with hematogenous metastasis(P=0.001),while interestingly,there was a higher incidence rate of hematogenous metastasis in welldifferentiated tumors (P=0.001).
Using multivariate analysis,it was found that maximum tumor diameter(P=0.014),histological type(P=0.001)and Borrmann type(P=0.033)were independent factors involved in locoregional recurrence(Table 2).Lymph node recurrence was significantly affected by lymph node dissection(P=0.029)and lymphovascular invasion(P=0.004,Table 3).Clinicopathological factors predicting the peritoneal seeding recurrence were the depth of invasion(P=0.001)and Borrmann type(P=0.002,Table 4).In addition,lymphovascular invasion(P=0.013)and histological type(P=0.001)were significantly associated with hematogenous metastasis(Table 5).
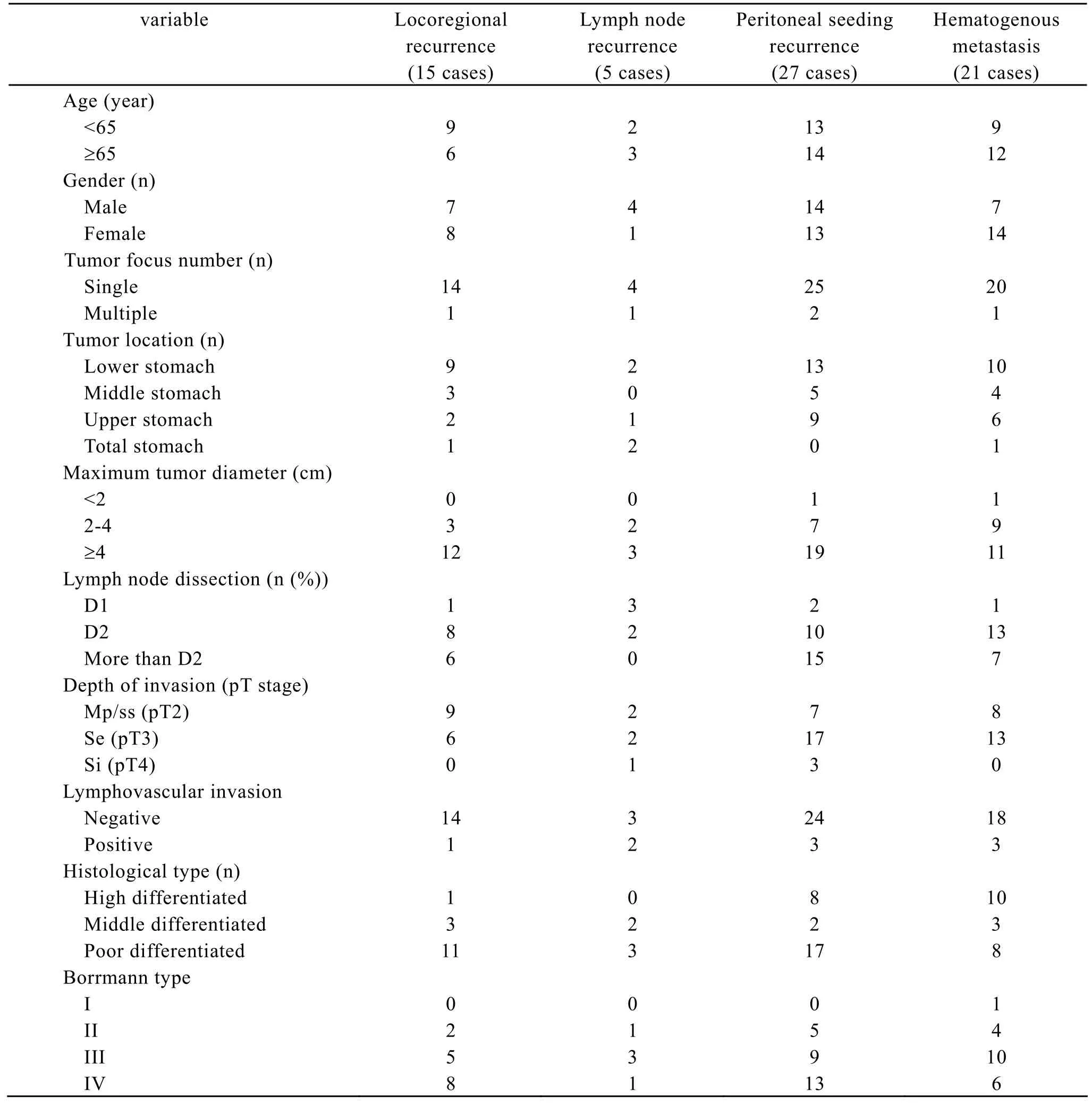
Table 1.Clinicopathological Characteristics of recurrence gastric cancers
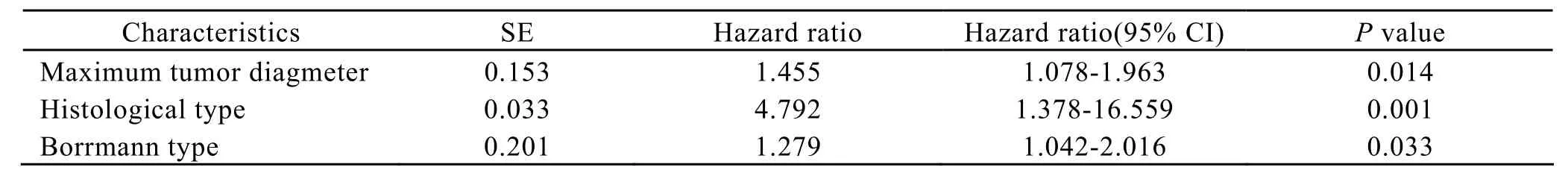
Table 2.Multivariate analysis of the factors involved in locoregional recurrence (n=15).

Table 3.Multivariate analysis of the factors involved in lymph node recurrence (n=5).

Table 4.Multivariate analysis of the factors involved in peritoneal seeding recurrence (n=27)

Table 5.Multivariate analysis fo the factors involved in hematogenous metastasis (n=21)
Multivariate Analysis of Prognostic Factors
Overall,the 5-year survival rate was not significantly different among the four groups with a different recurrence type(P=0.204, Figure 1).However,there was a significant difference in prognosis between the patients with postoperative recurrence and those without postoperative recurrence(P=0.001,Figure 2).Multivariate analysis using the Cox regression model,where the presence of postoperative recurrence with other potential prognostic factors such as age,gender,tumor focus number,tumor location,maximum tumor diameter,lymph node dissection,depth of invasion,lymphovascular invasion,histological type,Borrmann type and chemotherapy were included, identified postoperative recurrence(P=0.010),maximum tumor diameter(P=0.036),depth of invasion(P=0.002),histological type(P=0.029) and chemotherapy(P=0.010)as independent prognostic factors for advanced gastric cancers with negative nodes.In addition,of all 300 cases,46 cases received postoperative chemotherapy.After x2test,it was found that the cases who received post-operative chemotherapy got little hematogenous metastasis or lymph node recurrence compared to the patients who did not received post-operative chemotherapy (P=0.01,P=0.03).However,post-operative chemotherapy was not observed as a significant factor which influenced the locoregional recurrence and peritoneal seeding recurrence.
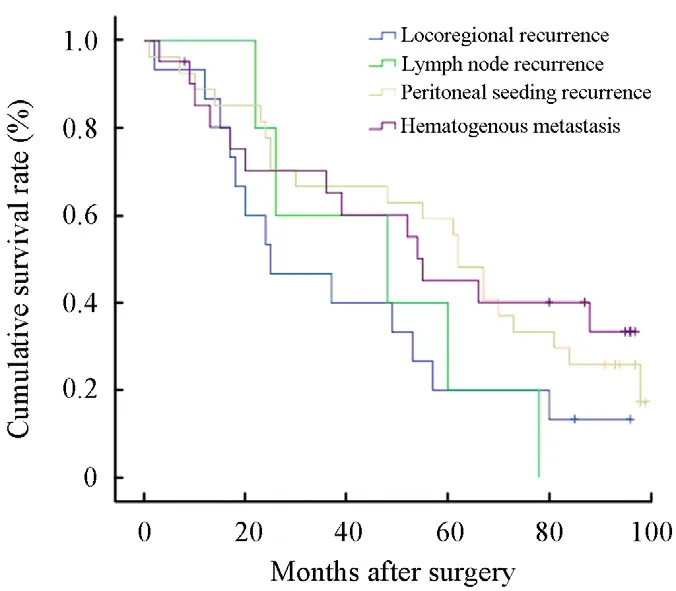
Figure 1.The 5-year survival rate was not significantly different among the four groups with a different recurrence type (P=0.204).
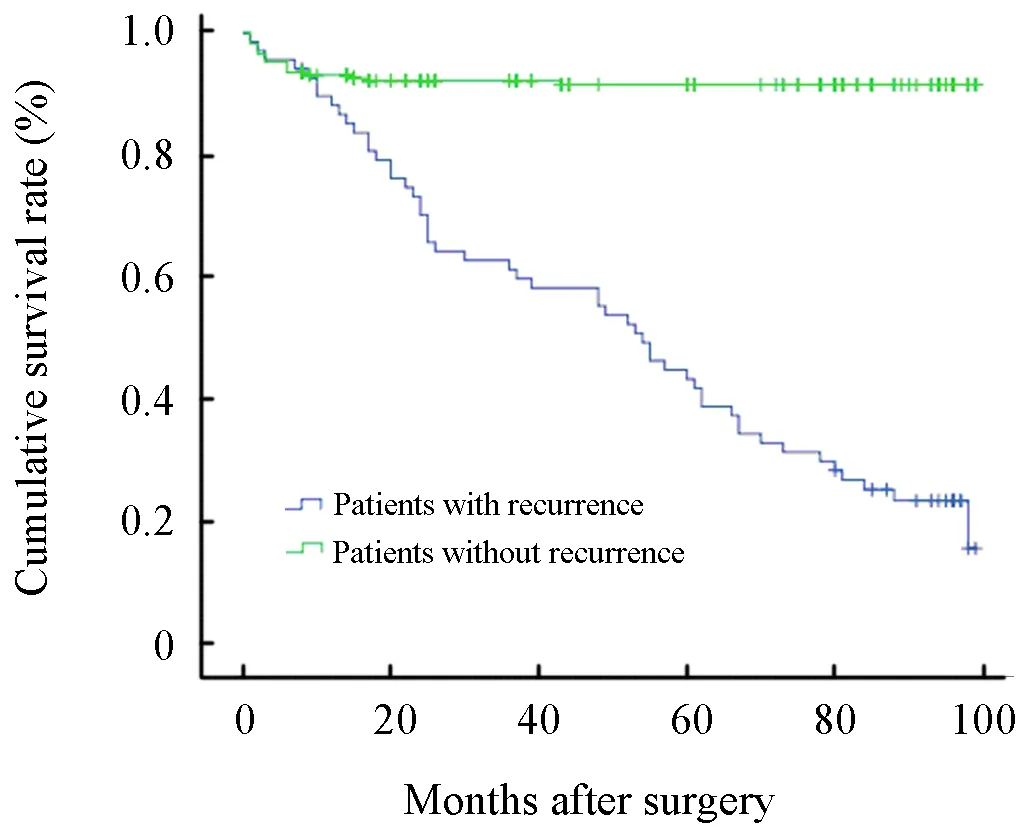
Figure 2.Difference in prognosis between the patients with postoperative recurrence and those without postoperative recurrence (P=0.001)
DISCUSSION
Gastric cancer is one of the major causes of the cancer death worldwide.Survival of the patients with gastric cancer is still not optimistic,although the 5-year survival rate has been improved due to early detection,rational lymphadenectomy and several therapeutic modalities[11-13].At present,the value of programs to detect recurrence of a previously operated gastric cancer is still a controversial subject.It was acknowledged that lots of patients suffered postoperative recurrence though they received curative surgery.It is generally accepted that tumor recurrence can occur through several major recurrent routes including lymph node metastasis,hematogenous spreading,peritoneal seeding and locoregional recurrence.Curative surgery has been performed for advanced gastric cancers with negative nodes.However,most of the studies have widely discussed prognostic factors for node-negative gastric cancer that has undergone curative resection,while recurrent patterns and factors involved in gastric adenocarcinoma are still not well known[14,15].
Huang et al[16]reported that node-negative advanced gastric cancer has a high amount of peritoneal seeding and locoregional recurrence after analyzing the recurrent sites and correlation of clinicopathological factors of 372 patients with lymph node-negative advanced gastric cancer who underwent R0 resection.In the present study,peritoneal seeding recurrence and hematogenous metastasis were predominant in node-negative advanced gastric cancer.Moreover,Huang et al[16]reported that the factors predicting the peritoneal seeding was serosal exposure,lymphovascular invasion,Lauren's diffuse type differentiation and scirrhous stromal reaction.Serosal exposure,tumor size,and microscopic infiltrating growth type were considered as the influencing factors for locoregional recurrence.Meanwhile,the cases with lymphovascular invasion were prone to hematogenous spreading[16].In our study,it was identified that maximum tumor diameter,histological type and Borrmann type were independent factors involved in locoregional recurrence.Lymph node recurrence was significantly affected by lymph node dissection and lymphovascular invasion.Clinicopathological factors which predicting peritoneal seeding recurrence were the depth of invasion and Borrmann type.In addition,lymphovascular invasion and hitological type were associated with hematogenous metastasis.The outcomes might give us some suggestions when we recommend examinations in post-operative check-up for the patients with node-negative gastric adenocarcinoma after curative resection.
Interestingly,it was observed that welldifferentiated tumors were more prone to hematogenous metastasis compared to poor differentiated tumors.This finding is not consistent with other studies[17,18].However,we have not found the proper explanation for this phenomenon.More clinical investigation and basic experiments addressing this topic well be expected.In addition,it was found that post-operative chemotherapy was the positive factor for hematogenous metastasis and lymph node recurrence.Using Cox regression analysis,it was identified that postoperative recurrence,maximum tumor diagmeter,depth of invasion and histological type were independent prognostic factors for advanced gastric cancer with negative nodes.Lee et al[19]reported that lymphovascular invasion and depth of cancer invasion were two independent but correlated survival predictors for node-negative gastric cancers after evaluating the clinicopathologicaol data of 689 gastric cancer patients who underwent curative resection.
In conclusion,this research demonstrated that the most common recurrent site of node-negative gastric cancer was peritoneal seeding and hematogenous metastasis.We also found that each different recurrence pattern had specific predictive factors that affected the recurrence.Our findings will hopefully improve the early detection of recurrence in patients with node-negative gastric adenocarcinoma after curative resection.
[1]Adchi Y,Shiraishi N,Kitano S.Modern treatment of early gastric cancer: review of the Japanese experience[J].Dig Surg 2002; 19: 333-9.
[2]Ohtsu A,Yoshida S,Sajio N.Disparities in gastric cancer chemotherapy between the East and West[J].J Clin Oncol 2006; 24: 2188-96.
[3]Jackson C,Cunningham D,Oliveira J,et al.Gastric cancer: ESMO clinical recommendations for diagnosis,treatment and follow-up[J].Ann Oncol 2009; 20 Suppl 4: 34-6.
[4]Lai JF,Kim S,Kim K,et al.Prediction of recurrence of early gastric cancer after curative resection[J].Ann Surg Oncol 2009; 16:1896-902.
[5]Hijioka S,Chin K,Seto Y,et al.Eight-year survival after advanced gastric cancer treated with S-1 followed by surgery[J].World J Gastroenterol 2010;16: 2824-7.
[6]D’Ugo D,Persiani R,Zoccali M,et al.Surgical issues after neoadjuvant treatment for gastric cancer[J].Eur Rev Med harmacol Sci 2010; 14: 315-9.
[7]Scartozzi M,Pistelli M,Bittoni A,et al.Novel perspectives for the treatment of gastric cancer: from a global approach to a personalized strategy[J].Curr Oncol Rep 2010; 12: 175-85.
[8]Yoshino S,Watanabe S,Imano M,et al.Improvement of QOL and prognosis by treatment of superfine dispersed lentinan in patients with advanced gastric cancer[J].Hepatogastroenterology 2010; 57: 172-7.
[9]Songun I,Putter H,Kranenbarg EM,et al.Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial[J].Lancet Oncol 2010; 11: 439-49.
[10]Greene FL,Page DL,Fleming ID,et al.AJCC Cancer Staging Manual[M].6th Edition.New York: Springer;2002.
[11]Wong R,Cunningham D.Optimising treatment regimens for the management of advanced gastric cancer[J].Ann Oncol 2009; 20: 605-8.
[12]Asaka M,Kato M,Graham DY.Prevention of gastric cancer by Helicobacter pylori eradication[J].Intern Med 2010; 49: 633-6.
[13]Dikken JL,Jansen EP,Cats A,et al.Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer[J].J Clin Oncol 2010; 28: 2430-6.
[14]Yokota T,Ishiyama S,Saito T,et al.Lymph node metastasis as a significant prognostic factor in gastric cancer: a multiple logistic regression analysis[J].Scand J Gastroenterol 2004; 39: 380-4.
[15]Hyung WJ,Lee JH,Choi SH,et al.Prognostic impact of lymphatic and/or blood vessel invasion in patients with node-negative advanced gastric cancer[J].Ann Surg Oncol 2002; 9: 562-7.
[16]Huang KH,Chen JH,Wu CW,et al.Factors affecting recurrence in node-negative advanced gastric cancer[J].J Gastroenterol Hepatol 2009; 24:1522-6.
[17]Hwang SE,Yang DH,Kim CY.Prognostic factors for survival in patients with hepatic recurrence after curative resection of gastric cancer[J].World J Surg 2009; 33:1468-72.
[18]Tiberio GA,Coniglio A,Marchet A,et al.Metachronous hepatic metastases from gastric carcinoma: a multicentric survey[J].Eur J Surg Oncol 2009; 35: 486-91.
[19]Lee CC,Wu CW,Lo SS,et al.Survival predictors in patients with node-negative gastric carcinoma[J].J Gastroenterol Hepatol 2007;22:1014-8.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Decrease of Peripheral Blood CD8+/CD28- Suppressor T Cell Followed by Dentritic Cells Immunomodulation among Metastatic Breast Cancer Patients
- Chinese Journal of Cancer Research Guidelines for Authors
- Inhibition of Proliferation Induced by Cyclin D1 Gene Silence in Human Renal Carcinoma ACHN Cells
- Interleukin-18 Suppresses Angiogenesis and Lymphangiogenesis in Implanted Lewis Lung Cancer
- High Expression of ERCC1 Is a Poor Prognostic Factor in Chinese Patients with Non-small Cell Lung Cancer Receiving Cisplatin-based Therapy
- Association of Lysosome Associated Protein Transmembrane 4 Beta Gene Polymorphism with the Risk of Pancreatic Cancer
