Inhibition of Proliferation Induced by Cyclin D1 Gene Silence in Human Renal Carcinoma ACHN Cells
2010-01-08JiangWangFengjinGuoAnminChenCaihongYang
Jiang Wang,Feng-jin Guo,An-min Chen,Cai-hong Yang
Department of Orthopedics,Tongji Hospital,Tongji Medical College,Huazhong University of Science and Technology,Wuhan 430030,China
INTRODUCTION
In mammalian cell cycle, regulating division and differentiation takes place in the G1 phase and abnormal expression of cycle proteins in G1 phase are likely to cause disorder of cell cycle[1].CyclinD1 is the basic and important regulator in cell cycle conversion from G1 phase to S phase[2,3].The current research found that abnormal expression of CyclinD1 appeared in many human tumors[4],and was associated with tumor grade,clinical stage,and even metastasis,which have a direct impact on the prognosis of patients[5,6].In the study,we silenced the CyclinD1 gene by RNA interference[7-10]in order to observe apoptosis in renal cell carcinoma ACHN cells,and also to provide the basis that CyclinD1 works as one of metastasis related genes.
MATERIALS AND METHODS
Cell Line and Materials
The human renal carcinoma ACHN cells line were purchased from the typical species preservation center of Wuhan University.Pgenesil-1-U6 plasmid was purchased from Genesil Biotech Company.Trizol,Lipofectamine2000,and primers of GAPDH and CyclinD1 were provided by Invitrogen.Fetal bovine serum and MEM medium were purchased from GIBCO Company.RT-PCR reagent kits and related products were from the Promega Corporation.CyclinD1 rabbit anti-human polyclonal antibody and goat anti-rabbit IgG were Santa Cruz Co’s products.Restriction endonucleases and T4 ligase were JINGME BIOTECH company’s products.Matrigel artificial rubber was products from BD Co and Transwell chambers were from Costar Company.
shRNA Design
The CyclinD1 gene sequence was obtained from human gene pool (GenBank accession NO.NM-053056).We determined the interfere site with software of Dharmacon.Target sequence: TGAACA AGCTCAAGTGGAA(643 -661).After blast analysis,no homology was found.
Construct and Identification Recombinant Vector Pgenesil-CyclinD1-shRNA
CyclinD1 shRNA template was designed with BamHI and HindIII restriction sites at the end.After annealing,two single-strand shRNA DNA template(Genesil Co) became DNA double-strands,and then were connected by pGenesil-1-U6 empty vector which was digested by BamHI and HindIII.We named the product Pgenesil-CyclinD1-shRNA and identified it by enzyme cut and sequence.shRNA template’s sense strand: 5'-GATCCTGAAAAGCTCAAGTGGAATTC AAGACGTTCCACTTGAGCTTGTTCATTTTTTGC GACA-3'.Antisense strand: 3'-GACTTGTTCGAG TTCACCTTAAGTTCTGCAAGTAATCAACAAGTA AAAGCTGTTCGA-5'.Pgenesil-CyclinD1-shRNA for unrelated sequences worked as a negative control and was named Pgenesil-NC.
Cell Culture and Construction of Stable Transfective Cell Lines
ACHN cell culture used MEM medium containing 10% fetal calf serum and were placed in incubator with 5% CO2 at 37°C.ACHN cells were passaged to 24-well plate (1×105cells each well)at 24 hours before transfection.Pgenesil-NC and Pgenesil-CyclinD1-shRNA were transfected into ACHN cells.After 48 h,we singled out the monoclonal cells transfected successfully by limited dilution and cultured them in 96-well plates.
RT-PCR and Western-blotting
Cells in 6-well plates were collected and RNA was extracted.We got the cDNA by reverse transcription (Table 1).

Table 1.The primer and in the study
Amplification reaction program: After initial denaturation for 5 min at 94°C.Denaturation for 45s at 94°C,annealing for 1min at 50°C,extension for 90s at 72°C.Finally,experienced 32 times cycle,a extension was allowed for 10 min at 72°C.The products were analyzed with sequence and electrophoresis,meanwhile,the scanned images were analyzed quantitatively.Western blotting was used to analysed expression of CyclinD1 proteins.Added lysis buffer,cells were centrifuged for 5 min at 4°C under 15000g,and then collected supernatant,in which protein content was measured BCA method.The amount of 5µg total protein were electrophoresed in SDS-PAGE and transferred on a nitrocellulose fliter.After one hour block,the antibodies against Cyclin D1 and the second antibodies were added respectively.The immune reaction was visualized with western blot immunodetection kit.
Detection of Apoptosis by Flow Cytometry
Cells were collected and were centrifuged for 3mins,and then,washed twice with PBS.The density was adjusted to 106/ml by Binding buffer.Five µl AnnexinⅤ and PI were added into 100µl above cells.After incubated at room temperature for 15mins,the compound was mixed with 400µl buffer.Finally,samples were analyzed with Flow Cytometer.
Detection of Proliferation
Three groups cells(Pgenesil-NC,CyclinD1-shRNA and ACHN)were seeded in 96-well plates with the denisty of 1×103each well.Respectively,20µl MTT were poured into the well at 1th,2th,3th,4th,5th,6thday.After incubated at 37°C for 4 hours and added 150µl DMSO and shocked for 10 mins,the 96 well plates were putted on the Bio-Rad to measure absorbance (A)at 490-band.Take the mean for cell growth curve mapping.
Invasion Assays
For invasion assays,transwell polycarbonate filter was coated with 100µl of matrigel at a dilution of 1: 20 in serum-free medium and air-dried for 24 h.4×104cells in 200µl of serum-free medium were seeded into the upper chamber and 500µl medium containing 10% of serum were added into the lower one.After incubation with 5% CO2at 37°C for 36 h and 48h respectively,membranes were washed with PBS,fixed by xylene and stained with Trypan blue.With water rinsed,trans-membrane cells were observed and counted by microscopic.The experiment was performed thrice for three different cell lines.
Statistical Analysis
All the data were presented as ¯?±s and the differences among the groups were analyzed by One-Way ANOVA or SNK-q test with SPSS 10.0 statistical software.The criterion for statistical signaficance was determined at a level of 0.05.In comparing the differences of CyclinD1 mRNA expression,apoptotic ratio,absorbance value and quantity of trans-membrane cells among the groups,we used One-Way ANOVA method.The dates of CyclinD1 protein among the groups were analyzed by SNK-q test.
RESULTS
Identification of pGenesil-CyclinD1-shRNA
After enzyme cutting with and sequencing,it was confirmed that the shRNA for Cyclin D1 gene had be inserted into vector successfully.
Observation on Cells with Stable Transfection
After stable transfection,there was no significant loss of fluorescence intensity with contrastive observation in light microscopy and fluorescence microscopy.The floating cells and cells with pyknosis increased after 48h,furthermore,cells decreased rate of growth and extended the cycle(Figure 1).
Analysis of CyclinD1 Expression Inhibition
Results of RT-PCR showed that compared with negative and blank control groups,expression of CyclinD1 mRNA in cells transfected pGenesil-CyclinD1-shRNA has decreased significantly(P<0.05)(Figure 2).After assays of CyclinD1 protein with Western blotting,it was found that the level of CyclinD1 protein in cells stable expression CyclinD1-shRNA had declined distinctly,while those of other two control groups had no significant change(Figure 3).At the same time,in experimental group cells,expression of target protein were inhibited permanently after passaged and monoclone several times (Figure 4,5).

Figure 1.Three groups’ cells observed with optical and fluorescence microscope(×400)A:Cyclin D1-shRNA group; B:Pgenesil-NC group;C:ACHN group
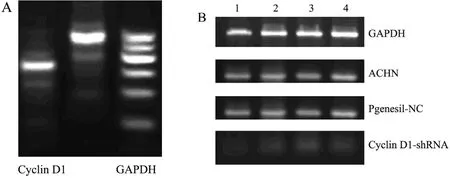
Figure 2.Cyclin D1 mRNA expression detected by RT-PCR(electrophoretogram).A:Cyclin D1 mRNA in ACHN cells B:Cyclin D1 mRNA in three groups Lane1-4:Repeated wells

Figure 3.Western-blot analysis of Cyclin D1 protein in three groups.Lane 1-3: Cyclin D1-shRNA group,Pgenesil-NC group,ACHN group

Figure 4.expression of Cyclin D1 protein in monoclonal cell from cells with stable transfection.Lane 1-4:down-regulated expression of Cyclin D1 protein at different periods
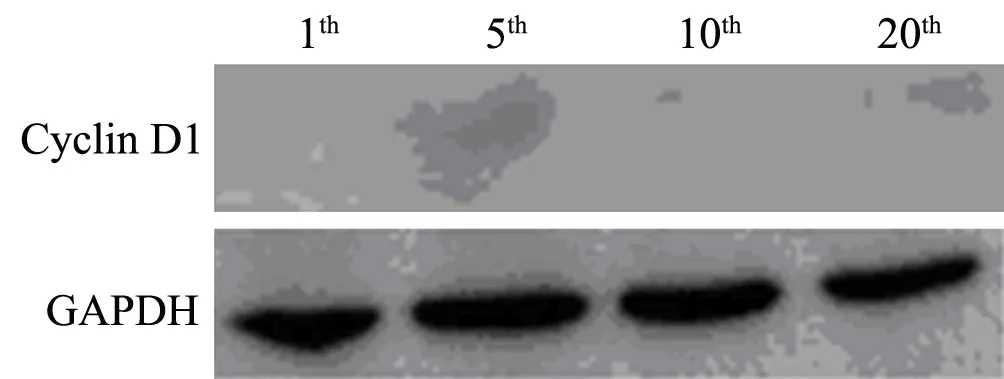
Figure 5.After several passages,down-regulated expression of Cyclin D1 protein can be detected by Western Blotting in Cyclin D1-shRNA group cells.
Effect of RNA Interference on Apoptosis
Results of FCM analysis showed that early apoptotic cells accounted for (1.52±0.20)% of total cells in the negative group and (2.04±0.50)% in non-transfected group.While early apoptotic rate reached (19.03±0.30)% in CyclinD1-shRNA group.By comparison,Late apoptotic ratio in CyclinD1-shRNA group cells reached (18.23 ± 0.30)% and the other two ratios of Pgenesil-NC group cells and ACHN group cells were only (3.10±0.20)% and(3.91±0.70)%.There was a significant difference(Figure 6).
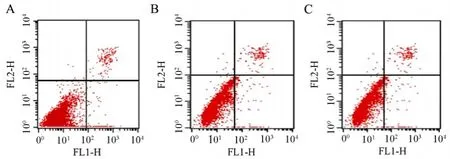
Figure 6.Analysis of apoptotic cells by flow cytometry in three groups.A:Cyclin D1-shRNA group;B:Pgenesil-NC group;C:ACHN group
Impact of Cyclin D1 Down-regulated on Cell Proliferation
With absorbance value as vertical ordinate and time as abscissa,cell growth curve was drawn (Figure 7).From the figure,the growth curve of cells transfected with pGenesil-CyclinD1-shRNA was found to be flat and has no obvious proliferative phase.That means RNA interference to CyclinD1 can slower the proliferation rate of ACHN cells significantly.
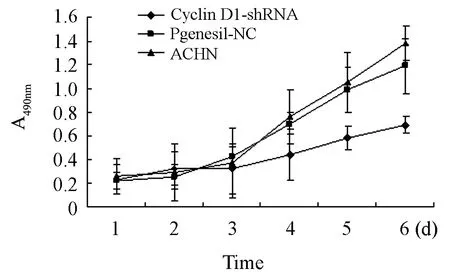
Figure 7.Growth curve of three groups’ cells
Changes of Cells Invasion
After building chambers for 36h and 48h,the number of trans-membrane cells in CyclinD1-shRNA group were far less than the number of negative group and blank group(P<0.05,Figure 8).Obviously,CyclinD1 gene silencing decreased the ability of ACHN cells invasion.
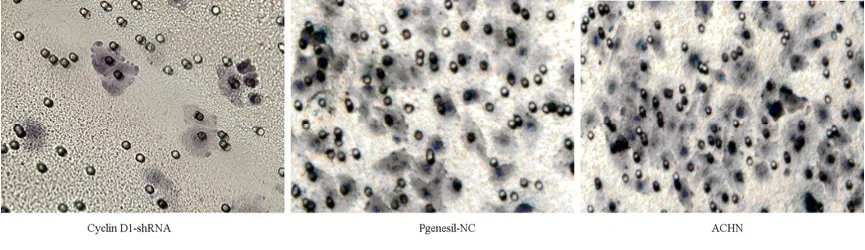
Figure 8.Invasion of three groups’ cells observed by Tanswell chamber method(×200)
DISCUSSION
The growth of cells relies on the balanced control by cycle regulatory factors.Any disorder among them will result in abnormal cells proliferation and tumorigenesis.CyclinD1 gene is an important proto-oncogene proposed recently.CyclinD1 protein always plays an important role in the cell cycle G1-S conversion.When CyclinD1 combined with cyclin-dependent kinase 4 (CDK4),the complex will accelerate cells transformation from G1 phase to enter S phase and shorten the cycle.On normal condition,the protein of CyclinD1 will be degrad rapidly when cells enter into S phase.However,when the gene over-activated,CyclinD1 will be expressed with a high level and cycle will be disturbed[1-10].
Researches on CyclinD1 with RNA interference technology have been reported currently[11].People mostly used small fragments like dsRNA or siRNA to transfect cells directly.Under this circumstance,the RNA is susceptible to be decomposed and difficult to be maintained a long time.Efficiency of transfection is low and only transient expression can be detected[12].In our study,plasmids carrying shRNA were synthesized and then were transfected with lipofectin.Entering the cells,shRNA were identified by RNA enzymes and loop was cut out,so the shRNA formed.Intracellular expression of shRNA was maintained with high-copy volume and target gene was silenced persistently.The effect was superior to that of siRNA in vitro[13,14].
At present,CyclinD1 has been proved to be an important indicator for prognosis of various tumors.Compared 47 patients with pancreatic cancer,12 patients with ductal carcinoma and 10 cases with normal pancreatic tissue,it was found that CyclinD1 expression had a direct impact on cell’s proliferation and differentiation in human pancreatic cancer.While size of tumor,ratio of metastasis and one-year survival rate has no significant correlation with it.Li YJ considered that CyclinD1 might be used to assess the prognosis for tumor[15].Having detecting CyclinD1 expression of astrocytoma grade from II to IV in situ hybridization and immunohistochemistry,Sallinen[16]found that CyclinD1 over-expressed in different grades and the expressional level of grade IV was the highest.Dimova I[17]collected more than 1000 cases with ovarian tumors including malignant,low-grade malignant and benign samples,then used method of fluorescence in situ hybridization to find that CyclinD1 amplification rate attained 8.46% in malignant groups and amplification rate of low-grade malignant tumors was 8.11%,while no amplification was found in different grades and stages of benign tumors.Finally,he draws a conclusion that the amplication of CyclinD1 gene was an early event in ovarian cancer.People detected CyclinD1 expression in patients with papillary thyroid cancer and found that 20 patients among 22 cases with metastasis had an over-expression of CyclinD1 protein.By contrast,the ratio was only 8.8% in cases without metastasis.At the same time,the results of PCR analysis showed that there was no gene amplification in cases with CyclinD1 over-expression.Therefore,they speculated that abnormal expression of CyclinD1 would be used to predict metastasis and have no relationship with gene amplification[18].Through blot hybridization,Some people[19,20]found that the expression level of CyclinD1 in cervical cancer group was higher than that in atypical hyperplasia group and the difference was significant which explained,for early cervical cancer,CyclinD1 was a good marker for evaluating prognosis.After studing nasopharyn- geal carcinoma,reseachers thought that CyclinD1 expression depended on cell cycle.In G0/G1 phase,CyclinD1 expression attained the highest level.In S phase and G2/M phase,expression decreased.When CyclinD1 gene had been knocked out,proliferation of cells were inhibited significantly and stagnated in S phase.The same results were found in renal cell carcinoma.Amplification and polymorphism of CyclinD1 gene may be involved in tumor’s proliferation,invasion,grade and stage which can be used as marker for differentiation in renal cell carcinoma[21-26].These outcomes showed that over- expression of CyclinD1 was closely related to proliferation and invasion of tumor and could be looked as an indicator to determine prognosis of renal cell carcinoma.
In our researches,RNA interference technology was used.We constructed the CyclinD1shRNA expression plasmid vector successfully and transferred it into ACHN cells.Cells initially were screened with G418 and then,using the limited dilution method and under fluorescence microscopy,we established the ACHN cells stably expressing shRNA with monoclonal expansion.Breathe through many times passages and cryopreservation,the cell lines still remained stable in characters and CyclinD1 mRNA and protein expression significantly decreased.It is more important that inhibition of CyclinD1 expression caused ACHN cells to slow down the speed of proliferation,weaken capacity of migration and induce apoptosis.The observations showed that floating cells and cells with pyknosis increased and rate of growth decreased.
[1]Sherlock G,Rosamond J.Starting to cycle: G1 controls regulating cell division in budding yeast[J].J Gen Microbiol 1993; 139: 2531-41.
[2]Witzel II,Koh LF,Perkins ND.Regulation of cyclin D1 gene expression[J].Biochem Soc Trans 2010; 38:217-22.
[3]Lukas J,Muller H,Bartkova J,et al.DNA tumor virus oncoproteins and retinoblastoma gene mutations share the ability to relieve the cell's requirement for cyclin D1 function in G1[J].J Cell Biol 1994; 125: 625-38.
[4]Biliran H Jr.,Wang Y,Banerjee S,et al.Overexpression of cyclin D1 promotes tumor cell growth and confers resistance to cisplatin-mediated apoptosis in an elastase-myc transgene-expressing pancreatic tumor cell line[J].Clin Cancer Res 2005; 11:6075-86.
[5]George E,Polissar NL,Wick M.Immunohistochemical evaluation of p16INK4A,E-cadherin,and cyclin D1 expression in melanoma and Spitz tumors[J].Am J Clin Pathol 2010; 133: 370-9.
[6]Lopez-Beltran A,Luque RJ,Alvarez-Kindelan J,et al.Prognostic factors in survival of patients with stage Ta and T1 bladder urothelial tumors: the role of G1-S modulators (p53,p21Waf1,p27Kip1,cyclin D1,and cyclin D3),proliferation index,and clinicopathologic parameters[J].Am J Clin Pathol 2004; 122:444-52.
[7]Molenaar JJ,Ebus ME,Koster J,et al.Cyclin D1 and CDK4 activity contribute to the undifferentiated phenotype in neuroblastoma[J].Cancer Res 2008; 68:2599-609.
[8]Shen FH,Fan XY,Liu BC.Decrease of cyclin D1 and CDK4 protein and their related factors induced by quartz in human embryonic lung fibroblasts[J].Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2008; 26: 391-4.
[9]Schiewer MJ,Morey LM,Burd CJ,et al.Cyclin D1 repressor domain mediates proliferation and survival in prostate cancer[J].Oncogene 2009; 28: 1016-27.
[10]Warenius H,Howarth A,Seabra L,et al.Dynamic heterogeneity of proteomic expression in human cancer cells does not affect Cdk1/Cdk4 coexpression[J].J Exp Ther Oncol 2008; 7: 237-54.
[11]Peer D,Park EJ,Morishita Y,et al.Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target[J].Science 2008;319: 627-30.
[12]Wu YM,Chen SM.Recent advances on RNA interference[J].Chin J Biochem Mol Biol 2003; 19:411-7.
[13]Paul CP,Good PD,Winer I,et al.Effective expression of small interfering RNA in human cells[J].Nat Biotechnol 2002; 20: 505-8.
[14]Brummelkamp TR,Bernards R,Agami R.A system for stable expression of short interfering RNAs in mammalian cells[J].Science 2002; 296: 550-3.
[15]Li YJ,Wei ZM,Meng YX,et al.Beta-catenin up-regulates the expression of Cyclin D1,c-myc and MMP-7 in human pancreatic cancer: relationships with carcinogenesis and metastasis[J].World J Gastroenterol 2005; 11: 2117-23.
[16]Sallinen SL,Sallinen PK,Kononen JT,et al.Cyclin D1 expression in as rocytomas is asso ciated with cell proliferation activit and patient prognosis[J].Pathology 1999; 188: 289-93.
[17]Dimova I,Zaharieva B,Raicheva S,et al.Association of Cyclin D1 copy number changes with histological type in ovarian tumors[J].Acta Oncol 2004; 43:675-9.
[18]Shao J,Teraishi F,Katsuda K,et al.p53 inhibits adriamycin induced down-regulation of Cyclin D1 expression in human cancer cells[J].Biochem Biophys Res Commun 2002; 290: 1101-7.
[19]Cai FG,Xiao JS,Ye QF,et al.Effects of ischemic preconditioning on Cyclin D1 expression during early ischemic reperfusion in rats[J].World J Gastroenterol 2006; 12: 2936-40.
[20]Lu S,Zhang BH,Wang ZH.Expression of Survivin,Cyclin D1,p21WAF1,Caspase23 in Cervical Cancer and Its Relation with Prognosis[J].J Huazhong Univ Sci Techn [Med Sci]2005; 25: 78-81.
[21]Sukov WR,Ketterling RP,Lager DJ,et al.CCND1 rearrangements and cyclin D1 over expression in renal oncocytomas: frequency,clinicopathologic features,and utility in differentiation from chromophobe renal cell carcinoma[J].Hum Pathol 2009; 40: 1296-303.
[22]Zhang T,Zheng J,Jiang N,et al.Overexpression of DLC-1 induces cell apoptosis and proliferation inhibition in the renal cell carcinoma [J].Cancer Lett 2009; 283: 59-67.
[23]Lin BT,Brynes RK,Gelb AB,et al.Cyclin D1 expression in renal carcinomas and oncocytomas: an immunohistochemical study[J].Mod Pathol 1998; 11:1075-81.
[24]Barroca H,Castedo S,Vieira J,et al.Altered expression of key cell cycle regulators in renal cell carcinoma associated with Xp11.2 translocation[J].Pathol Res Pract 2009; 205: 466-72.
[25]Shigeru K,Yasuyoshi M,Hiroshi K,et al.Downregulation of the c-Fes protein-tyrosine kinase inhibits the proliferation of human renal carcinoma cells[J].Int J Oncol 2009; 34: 89-96.
[26]Zhang JH,Wang XF,Ye HY,et al.Expression of p27kipl protein and cyclinD1 protein in renal cell carcinoma[J].J Chin J Urol 2001; 22: 264-6.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Decrease of Peripheral Blood CD8+/CD28- Suppressor T Cell Followed by Dentritic Cells Immunomodulation among Metastatic Breast Cancer Patients
- Chinese Journal of Cancer Research Guidelines for Authors
- Interleukin-18 Suppresses Angiogenesis and Lymphangiogenesis in Implanted Lewis Lung Cancer
- High Expression of ERCC1 Is a Poor Prognostic Factor in Chinese Patients with Non-small Cell Lung Cancer Receiving Cisplatin-based Therapy
- Association of Lysosome Associated Protein Transmembrane 4 Beta Gene Polymorphism with the Risk of Pancreatic Cancer
- Recurrent Patterns and Factors Involved in Node-negative Advanced Gastric Cancer
